Pressure- and Temperature-Dependent Crystallization Kinetics of Isotactic Polypropylene under Process Relevant Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Differential Scanning Calorimetry (DSC)
2.3. Fast Scanning Chip Calorimetry (FSC)
2.4. Pressure-Volume-Temperature Measurement (pvT)
3. Results and Discussion
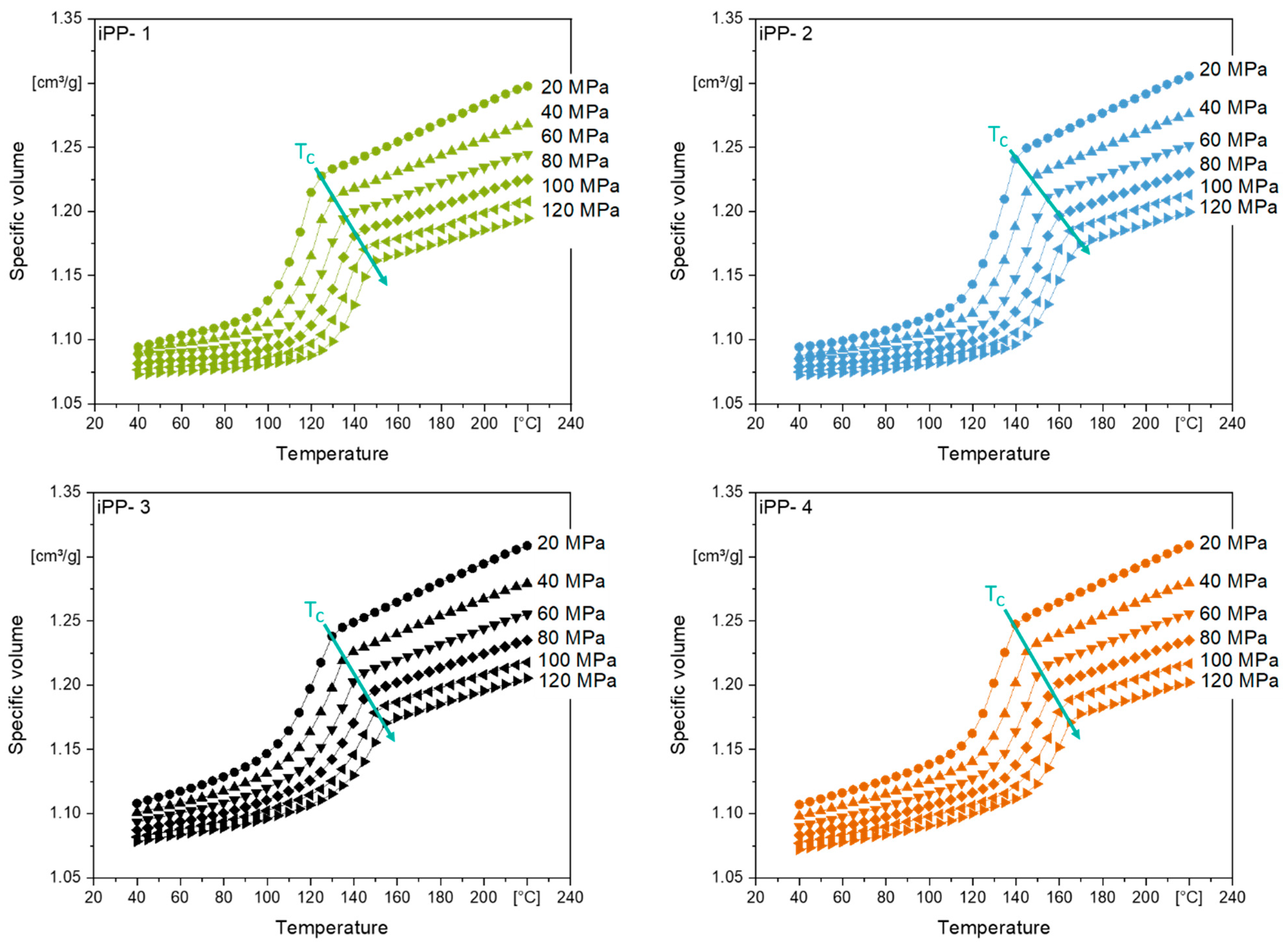
3.1. Crystallization Temperature as Function of Pressure
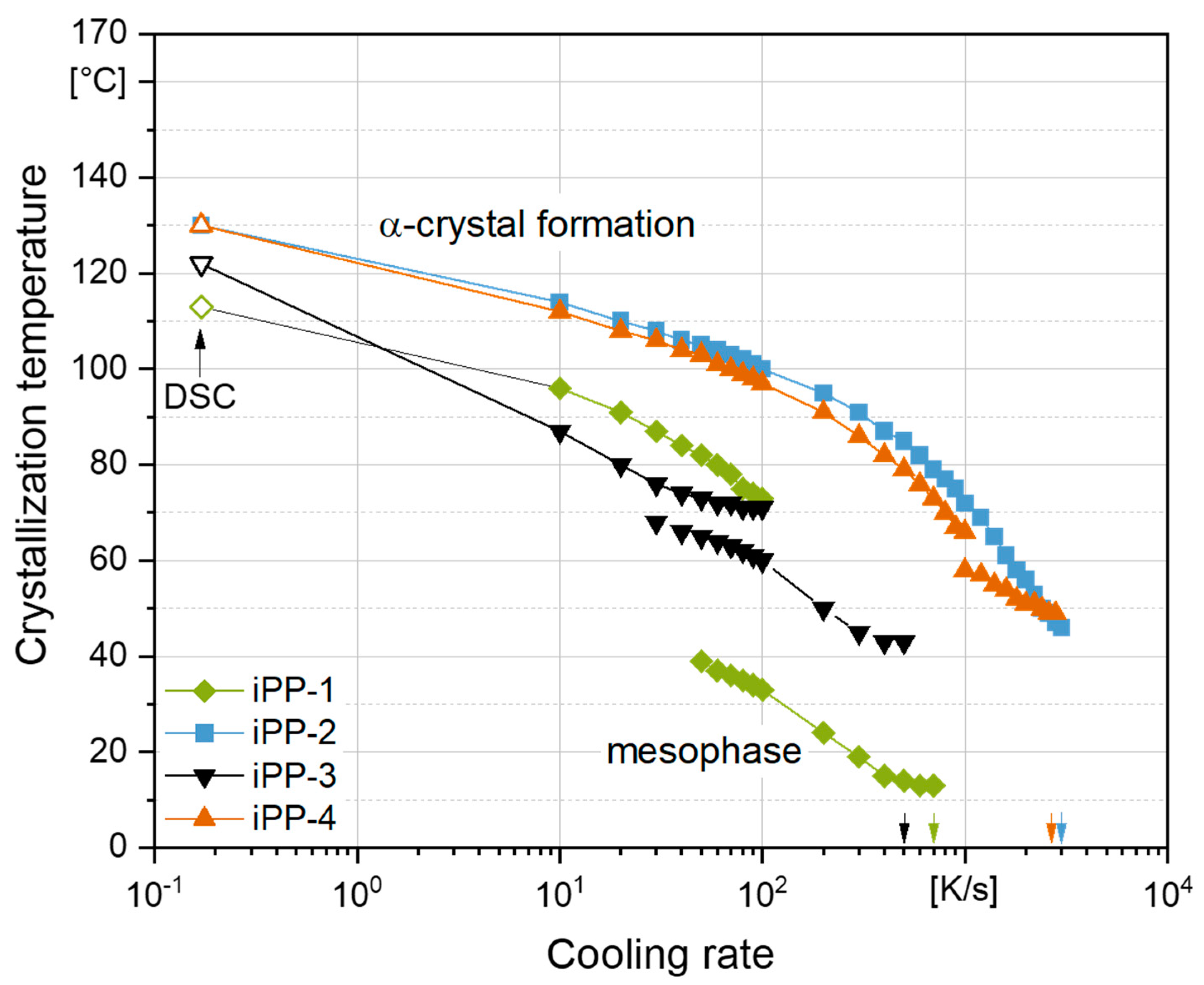
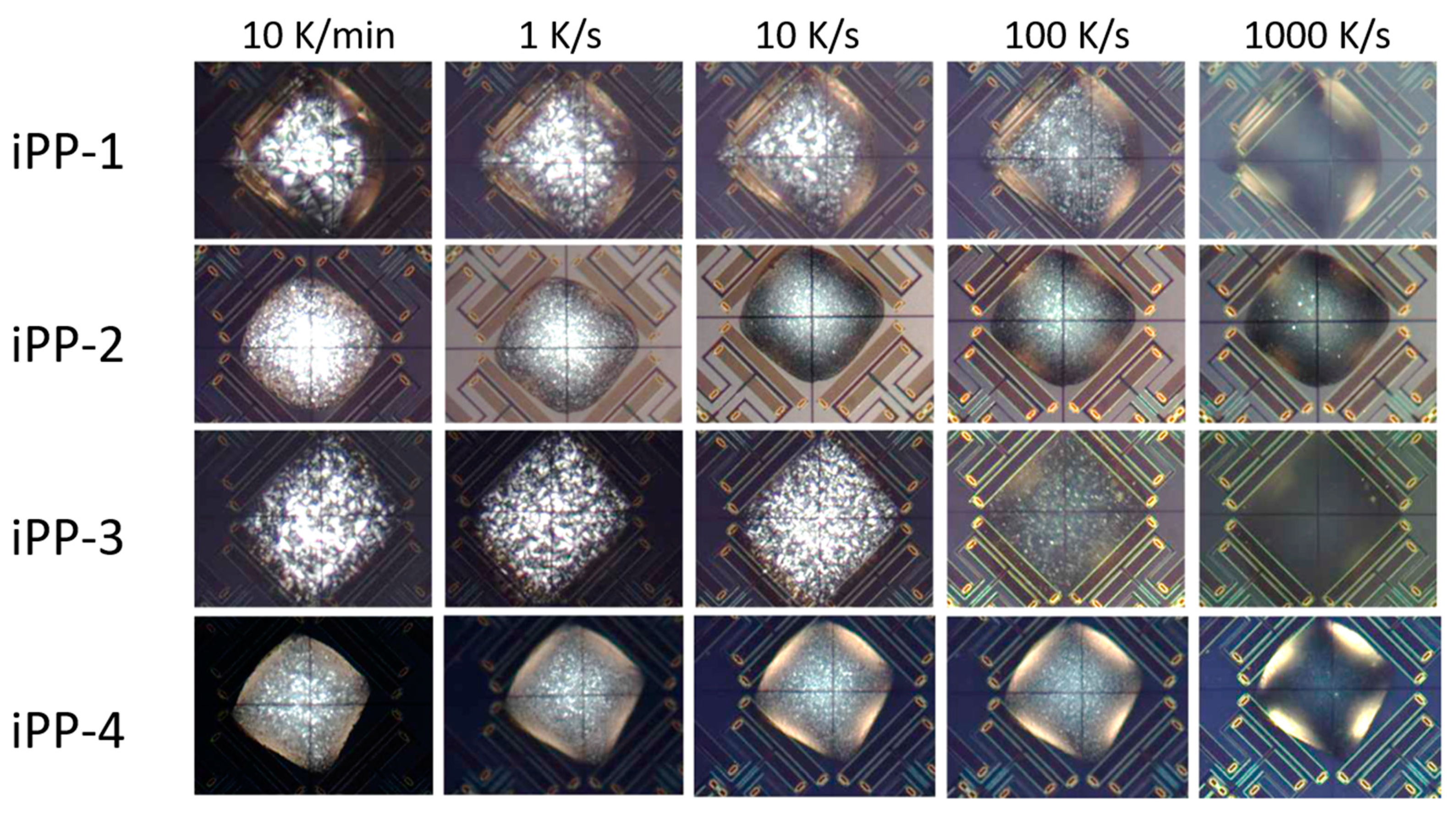
3.2. Crystallization Temperature as a Function of Cooling Rate
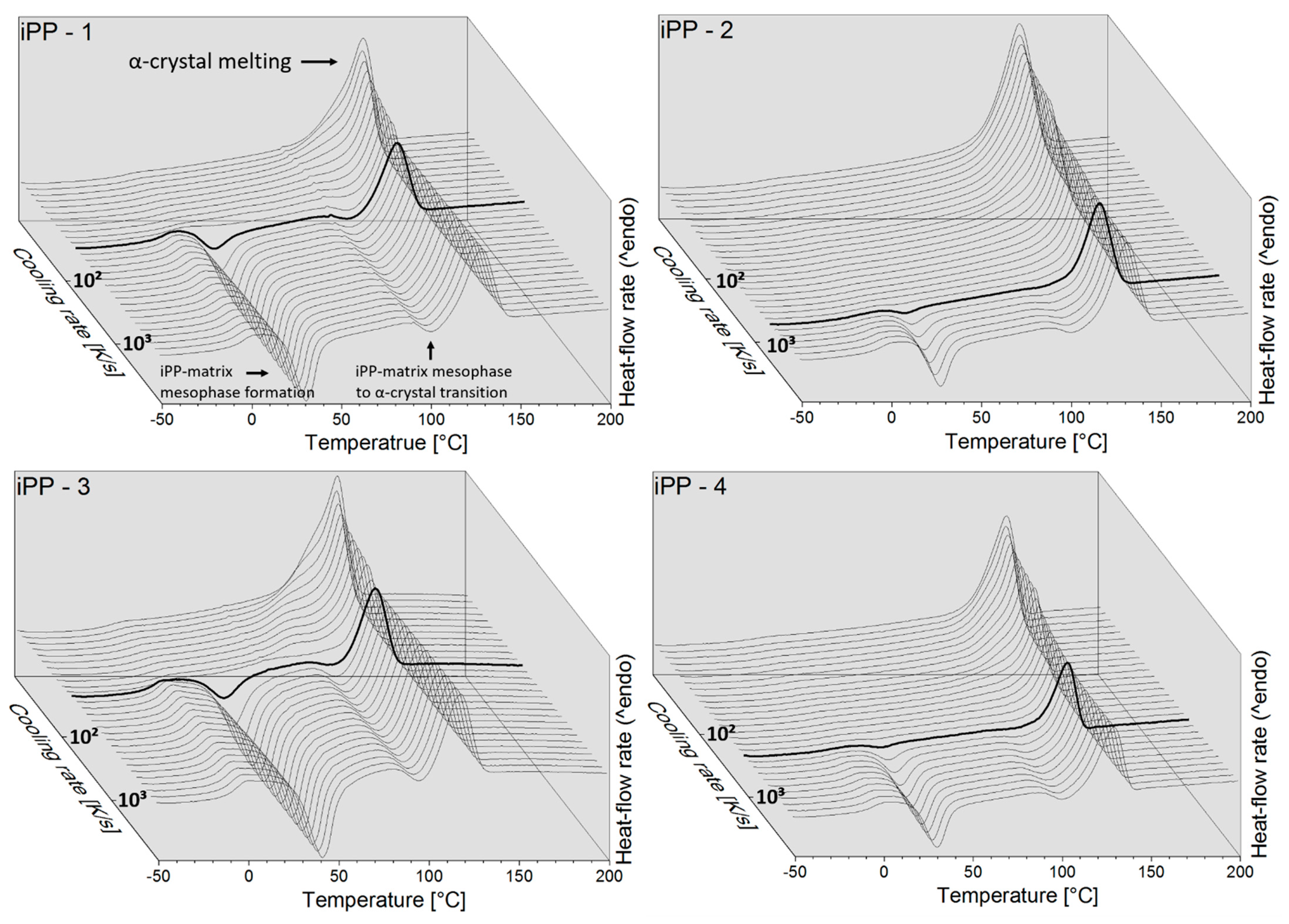
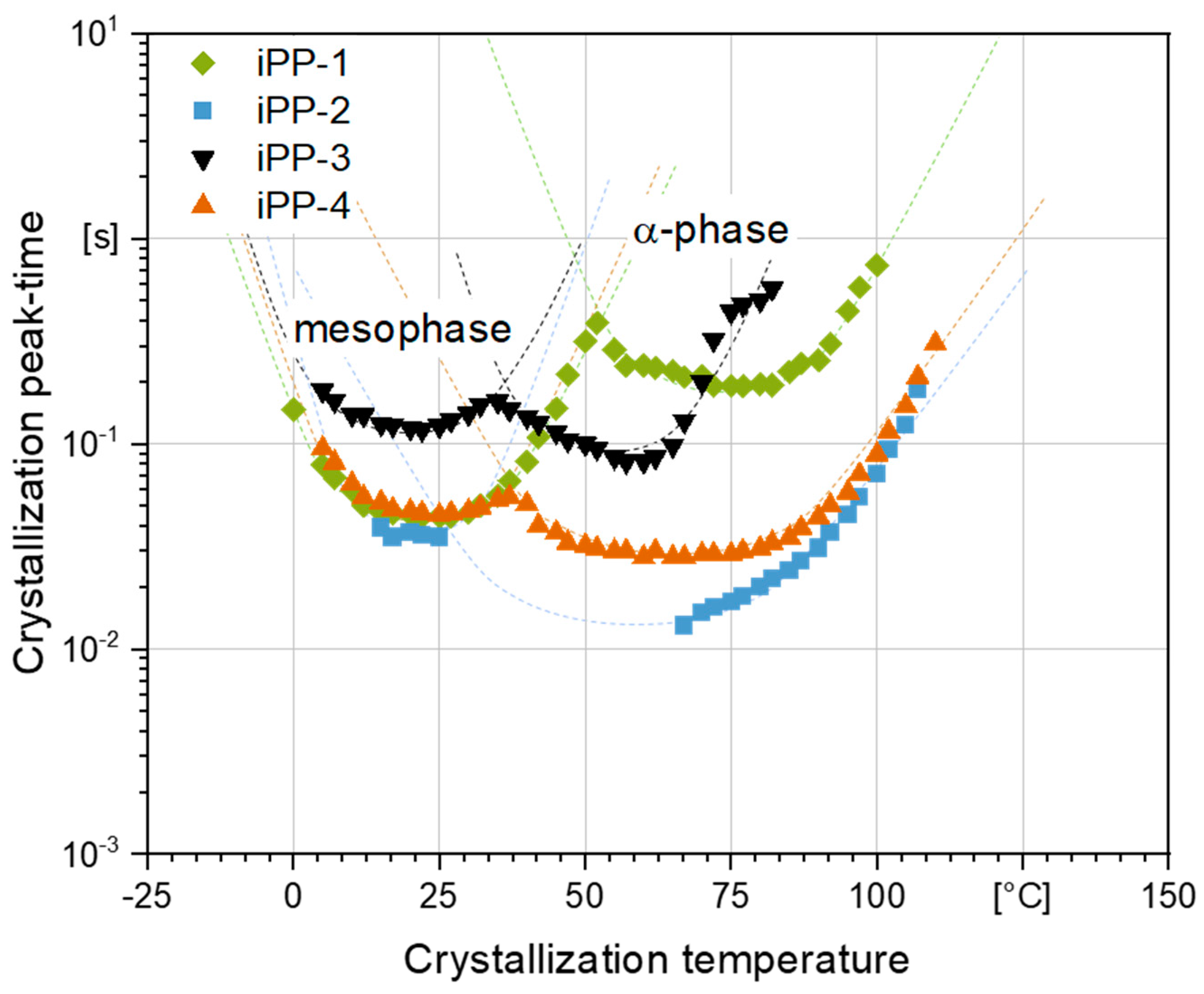
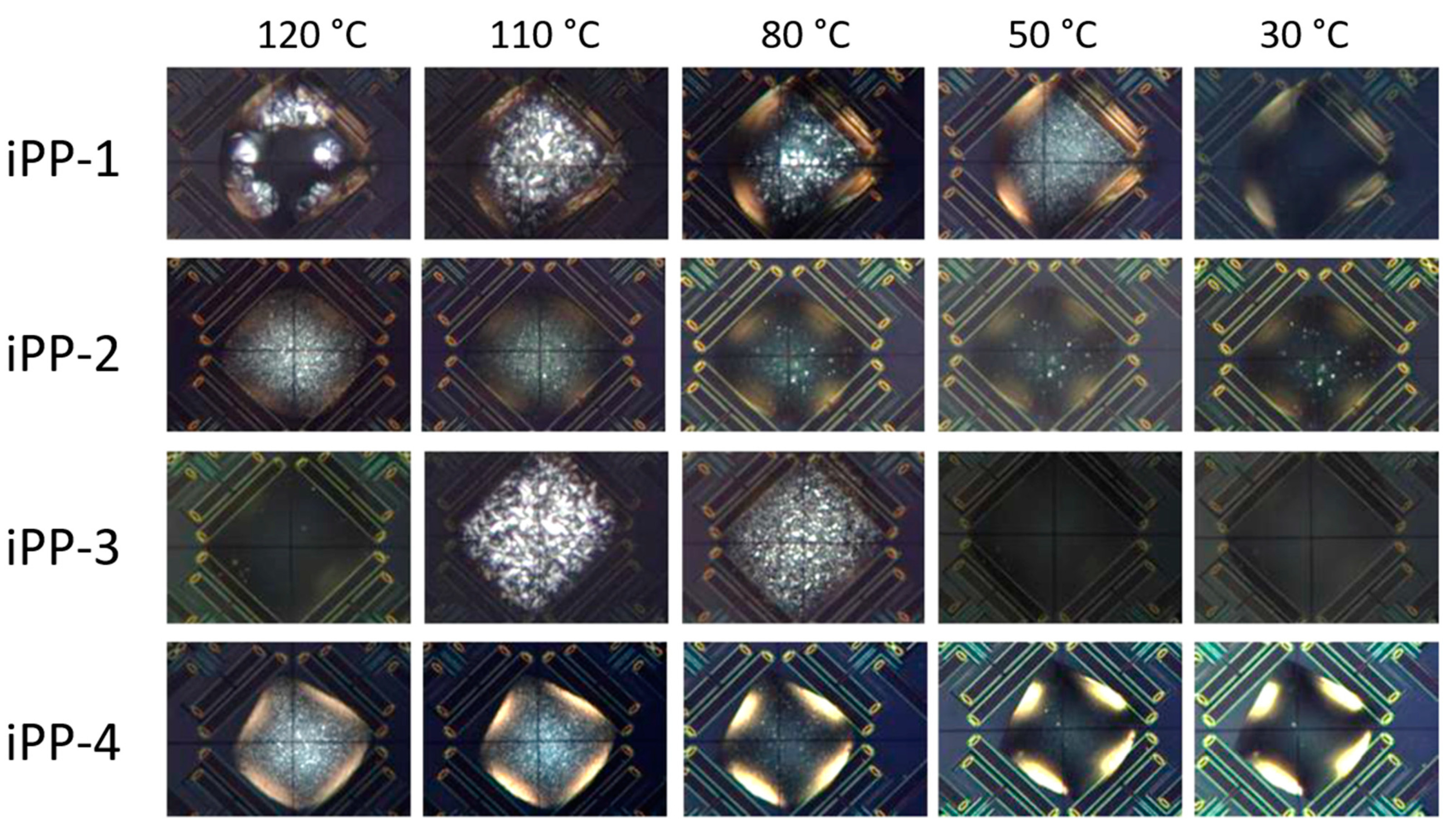
3.3. Crystallization Rate as Function of Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Addink, E.J.; Beintema, J. Polymorphism of crystalline polypropylene. Polymer 1961, 2, 185–193. [Google Scholar] [CrossRef]
- Jones, A.T.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Natta, G.; Corradini, P. Structure and Properties of Isotactic Polypropylene. Nuovo Cim. 1960, 15, 12. [Google Scholar] [CrossRef]
- Auriemma, F.; de Ballesteros, O.R.; De Rosa, C.; Corradini, P. Structural disorder in the alpha form of isotactic polypropylene. Macromolecules 2000, 33, 8764–8774. [Google Scholar] [CrossRef]
- Keith, H.D.; Padden, F.J.; Walter, N.M.; Wyckoff, H.W. Evidence for a second crystal form of polypropylene. J. Appl. Phys. 1959, 30, 1485–1488. [Google Scholar] [CrossRef]
- Padden, F.J.; Keith, H.D. Spherulitic crystallization in polypropylene. J. Appl. Phys. 1959, 30, 1479–1484. [Google Scholar] [CrossRef]
- Turnerjones, A. Development of gamma-crystal form in random copolymers of propylene and their analysis by dsc and X-ray methods. Polymer 1971, 12, 487–508. [Google Scholar] [CrossRef]
- Zia, Q.; Radusch, H.J.; Androsch, R. Deformation behavior of isotactic polypropylene crystallized via a mesophase. Polym. Bull. 2009, 63, 755–771. [Google Scholar] [CrossRef]
- Zia, Q.; Androsch, R.; Radusch, H.J. Effect of the Structure at the Micrometer and Nanometer Scales on the Light Transmission of Isotactic Polypropylene. J. Appl. Polym. Sci. 2010, 117, 1013–1020. [Google Scholar] [CrossRef]
- Mileva, D.; Wang, J.B.; Gahleitner, M.; Jariyavidyanont, K.; Androsch, R. New Insights into Crystallization of Heterophasic Isotactic Polypropylene by Fast Scanning Chip Calorimetry. Polymers 2020, 12, 1683. [Google Scholar] [CrossRef]
- Gahleitner, M.; Bachner, C.; Ratajski, E.; Rohaczek, G.; Neissl, W. Effects of the catalyst system on the crystallization of polypropylene. J. Appl. Polym. Sci. 1999, 73, 2507–2515. [Google Scholar] [CrossRef]
- Gahleitner, M.; Jaaskelainen, P.; Ratajski, E.; Paulik, C.; Reussner, J.; Wolfschwenger, J.; Neissl, W. Propylene-ethylene random copolymers: Comonomer effects on crystallinity and application properties. J. Appl. Polym. Sci. 2005, 95, 1073–1081. [Google Scholar] [CrossRef]
- Laihonen, S.; Gedde, U.W.; Werner, P.E.; Westdahl, M.; Jaaskelainen, P.; MartinezSalazar, J. Crystal structure and morphology of melt-crystalized poly(propylene-stat-ethylene) fractions. Polymer 1997, 38, 371–377. [Google Scholar] [CrossRef]
- Laihonen, S.; Gedde, U.W.; Werner, P.E.; MartinezSalazar, J. Crystallization kinetics and morphology of poly(propylene-stat-ethylene) fractions. Polymer 1997, 38, 361–369. [Google Scholar] [CrossRef]
- Cavallo, D.; Gardella, L.; Alfonso, G.C.; Mileva, D.; Androsch, R. Effect of comonomer partitioning on the kinetics of mesophase formation in random copolymers of propene and higher alpha-olefins. Polymer 2012, 53, 4429–4437. [Google Scholar] [CrossRef]
- Chen, J.Y.; Cao, Y.; Kang, J.A.; Li, H.L. Effect of Temperature and Comonomer Content on Thermal Behavior and Crystallization Property of the Propylene-Ethylene Random Copolymers. J. Macromol. Sci. Part B 2011, 50, 248–265. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Ricco, T. Crystallization and morphology of reactor-made blends of isotactic polypropylene and ethylene-propylene rubber. J. Appl. Polym. Sci. 1997, 66, 1007–1014. [Google Scholar] [CrossRef]
- Doshev, P.; Lohse, G.; Henning, S.; Krumova, M.; Heuvelsland, A.; Michler, G.; Radusch, H.J. Phase interactions and structure evolution of heterophasic ethylene-propylene copolymers as a function of system composition. J. Appl. Polym. Sci. 2006, 101, 2825–2837. [Google Scholar] [CrossRef]
- Gahleitner, M.; Tranninger, C.; Doshev, P. Heterophasic copolymers of polypropylene: Development, design principles, and future challenges. J. Appl. Polym. Sci. 2013, 130, 3028–3037. [Google Scholar] [CrossRef]
- Cavallo, D.; Azzurri, F.; Floris, R.; Alfonso, G.C.; Balzano, L.; Peters, G.W. Continuous Cooling Curves Diagrams of Propene/Ethylene Random Copolymers. The Role of Ethylene Counits in Mesophase Development. Macromolecules 2010, 43, 2890–2896. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R. Nucleation-controlled semicrystalline morphology of bulk polymers. Polym. Cryst. 2018, 1, 15. [Google Scholar] [CrossRef]
- Foresta, T.; Piccarolo, S.; Goldbeck-Wood, G. Competition between alpha and gamma phases in isotactic polypropylene: Effects of ethylene content and nucleating agents at different cooling rates. Polymer 2001, 42, 1167–1176. [Google Scholar] [CrossRef]
- De Santis, F.; Adamovsky, S.; Titomanlio, G.; Schick, C. Isothermal nanocalorimetry of isotactic polypropylene. Macromolecules 2007, 40, 9026–9031. [Google Scholar] [CrossRef]
- De Santis, F.; Adamovsky, S.; Titomanlio, G.; Schick, C. Scanning nanocalorimetry at high cooling rate of isotactic polypropylene. Macromolecules 2006, 39, 2562–2567. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R.; Cavallo, D.; Alfonso, G.C. Structure formation of random isotactic copolymers of propylene and 1-hexene or 1-octene at rapid cooling. Eur. Polym. J. 2012, 48, 1082–1092. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R. Effect of co-unit type in random propylene copolymers on the kinetics of mesophase formation and crystallization. Colloid Polym. Sci. 2012, 290, 465–471. [Google Scholar] [CrossRef]
- Mileva, D.; Wang, J.B.; Gahleitner, M.; Doshev, P.; Androsch, R. Crystallization behaviour of heterophasic propylene-ethylene copolymer at rapid cooling conditions. Polymer 2016, 102, 214–220. [Google Scholar] [CrossRef]
- Forstner, R.; Peters, G.W.M.; Meijer, H.E.H. A Novel Dilatometer for PVT Measurements of Polymers at High Cooling—And Shear Rates. Int. Polym. Process. 2009, 24, 114–121. [Google Scholar] [CrossRef]
- van Drongelen, M.; Roozemond, P.C.; Peters, G.W.M. Non-isothermal Crystallization of Semi-Crystalline Polymers: The Influence of Cooling Rate and Pressure. In Polymer Crystallization Ii: From Chain Microstructure to Processing; Auriemma, F., Alfonso, G.C., DeRosa, C., Eds.; Springer: Berlin, Germany, 2017; Volume 277, pp. 207–242. [Google Scholar] [CrossRef]
- van Drongelen, M.; van Erp, T.B.; Peters, G.W.M. Quantification of non-isothermal, multi-phase crystallization of isotactic polypropylene: The influence of cooling rate and pressure. Polymer 2012, 53, 4758–4769. [Google Scholar] [CrossRef]
- Van der Beek, M.H.E.; Peters, G.W.M.; Meijer, H.E.H. The influence of cooling rate on the specific volume of isotactic poly(propylene) at elevated pressures. Macromol. Mater. Eng. 2005, 290, 443–455. [Google Scholar] [CrossRef]
- Zuidema, H.; Peters, G.W.M.; Meijer, H.E.H. Influence of cooling rate on pVT-data of semicrystalline polymers. J. Appl. Polym. Sci. 2001, 82, 1170–1186. [Google Scholar] [CrossRef] [Green Version]
- Karl, V.H.; Asmussen, F.; Ueberreiter, K. Pressure-dependence of viscoelastic and physicochemical properties of polymers.1. description of a high-pressure dilatometer and some applications. Angew. Makromol. Chem. 1977, 62, 145–161. [Google Scholar] [CrossRef]
- Karl, V.H.; Asmussen, F.; Ueberreiter, K. Pressure-dependence of viscoelastic and physicochemical properties of polymers.4. isothermic and nonisothermic crystallization. Eur. Polym. J. 1977, 13, 1025–1031. [Google Scholar] [CrossRef]
- Zhang, L.; Van Drongelen, M.; Alfonso, G.C.; Peters, G.W.M. The effect of pressure pulses on isotactic polypropylene crystallization. Eur. Polym. J. 2015, 71, 185–195. [Google Scholar] [CrossRef]
- Bruckner, S.; Phillips, P.J.; Mezghani, K.; Meille, S.V. On the crystallization of gamma-isotactic polypropylene: A high pressure study. Macromol. Rapid Commun. 1997, 18, 1–7. [Google Scholar] [CrossRef]
- Schick, C.; Mathot, V. Fast Scanning Calorimetry, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 6–13. [Google Scholar] [CrossRef]
- Wang, J.; Hopmann, C.; Schmitz, M.; Hohlweck, T.; Wipperfurth, J. Modeling of pvT behavior of semi-crystalline polymer based on the two-domain Tait equation of state for injection molding. Mater. Des. 2019, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Zoller, P. Crystallization of polypropylene, nylon-66 and poly(ethylene-terephthalate) at pressures to 200 mpa—Kinetics and characterization of products. J. Polym. Sci. Part B-Polym. Phys. 1994, 32, 1049–1067. [Google Scholar] [CrossRef]
- Sorrentino, A.; Pantani, R. Determination of the effect of pressure on viscosity of an isotactic polypropylene. Polym. Bull. 2013, 70, 2005–2014. [Google Scholar] [CrossRef]
- Luye, J.F.; Regnier, G.; Le Bot, P.; Delaunay, D.; Fulchiron, R. PVT measurement methodology for semicrystalline polymers to simulate injection-molding process. J. Appl. Polym. Sci. 2001, 79, 302–311. [Google Scholar] [CrossRef]
- Nakafuku, C. High-pressure dta study on the melting and crystallization of isotactic polypropylene. Polymer 1981, 22, 1673–1676. [Google Scholar] [CrossRef]
- Leute, U.; Dollhopf, W.; Liska, E. Dilatometric study on melting of polypropylene at elevated pressure. Colloid Polym. Sci. 1978, 256, 914–922. [Google Scholar] [CrossRef]
- Gahleitner, M.; Mileva, D.; Gloger, D.; Androsch, R.; Tranchida, D. Polymer Structure Effects on Crystallization and Properties in Polypropylene Film Casting. In Proceedings of Pps-32: The 32nd International Conference of the Polymer Processing Society; Maazouz, A., Ed.; American Institute of Physics: Melville, NY, USA, 2017; p. 1914. [Google Scholar] [CrossRef] [Green Version]
- Androsch, R.; Monami, A.; Kucera, J. Effect of an alpha-phase nucleating agent on the crystallization kinetics of a propylene/ethylene random copolymer at largely different supercoiling. J. Cryst. Growth 2014, 408, 91–96. [Google Scholar] [CrossRef]
- Schawe, J.E.K.; Vermeulen, P.A.; van Drongelen, M. Two processes of alpha-phase formation in polypropylene at high supercoiling. Thermochim. Acta 2015, 616, 87–91. [Google Scholar] [CrossRef]
- Cai, J.; Luo, R.Q.; Lv, R.H.; He, Y.C.; Zhou, D.S.; Hu, W.B. Crystallization kinetics of ethylene-co-propylene rubber/isotactic polypropylene blend investigated via chip-calorimeter measurement. Eur. Polym. J. 2017, 96, 79–86. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R.; Zhuravlev, E.; Schick, C. Temperature of Melting of the Mesophase of Isotactic Polypropylene. Macromolecules 2009, 42, 7275–7278. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S.; Duraccio, D.; Schick, C. Isothermal crystallization of isotactic poly(propylene) studied by superfast calorimetry. Macromol. Rapid Commun. 2007, 28, 875–881. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Mesophases in polyethylene, polypropylene, and poly(1-butene). Polymer 2010, 51, 4639–4662. [Google Scholar] [CrossRef] [Green Version]
- Mileva, D.; Androsch, R.; Zhuravlev, E.; Schick, C.; Wunderlich, B. Formation and Reorganization of the Mesophase of Isotactic Polypropylene. Mol. Cryst. Liq. Cryst. 2012, 556, 74–83. [Google Scholar] [CrossRef]


| Material | Grade | Density [g/cm3] | Nucleated Yes/No | MFR [g/10 min] | Yield Stress [MPa] | Tmelt * [°C] |
|---|---|---|---|---|---|---|
| iPP-1 | HP | 0.900 | no | 19 | n.s. | 163 |
| iPP-2 | HP | 0.905 | yes | 20 | 40 | 166 |
| iPP-3 | RACO | 0.900 | no | 23 | 29 | 150 |
| iPP-4 | HECO | 0.905 | yes | 15 | 26 | 164 |
| Material | [g/mol] | Cooling Rate [K/s] | Pressure Shift Factor [K/MPa] | R-Value | ||
|---|---|---|---|---|---|---|
| iPP-1 | iPP HP | 238 × 103 | 2.1 | 0.11 | 0.25 | 0.996 |
| iPP-2 | iPP HP | 260 × 103 | 3.2 | 0.11 | 0.27 | 0.996 |
| iPP-3 | iPP RACO | 213 × 103 | 2.5 | 0.11 | 0.24 | 0.996 |
| iPP-4 | iPP HECO | 238 × 103 | 2.6 | 0.11 | 0.27 | 0.997 |
| Reference | ||||||
| [31] | iPP HP | 365 × 103 | 5.2 | - | 0.27 | - |
| [39] | iPP HP | 300 × 103 | 6.4 | 0.042 | 0.2287 | - |
| [41] | iPP HP | - | - | 0.083 | 0.2625 | - |
| [42] | iPP HP | - | - | 0.10 | 0.2536 | - |
| [43] | iPP HP | different fractions | 0.012 | 0.38 | - | |
| [32] | iPP HP | 365 × 103 | 5.4 | 40 | 0.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoerer, Y.; Boldt, R.; Androsch, R.; Kuehnert, I. Pressure- and Temperature-Dependent Crystallization Kinetics of Isotactic Polypropylene under Process Relevant Conditions. Crystals 2021, 11, 1138. https://doi.org/10.3390/cryst11091138
Spoerer Y, Boldt R, Androsch R, Kuehnert I. Pressure- and Temperature-Dependent Crystallization Kinetics of Isotactic Polypropylene under Process Relevant Conditions. Crystals. 2021; 11(9):1138. https://doi.org/10.3390/cryst11091138
Chicago/Turabian StyleSpoerer, Yvonne, Regine Boldt, René Androsch, and Ines Kuehnert. 2021. "Pressure- and Temperature-Dependent Crystallization Kinetics of Isotactic Polypropylene under Process Relevant Conditions" Crystals 11, no. 9: 1138. https://doi.org/10.3390/cryst11091138
APA StyleSpoerer, Y., Boldt, R., Androsch, R., & Kuehnert, I. (2021). Pressure- and Temperature-Dependent Crystallization Kinetics of Isotactic Polypropylene under Process Relevant Conditions. Crystals, 11(9), 1138. https://doi.org/10.3390/cryst11091138






