Effect of Erosion Behavior of FeO-CaO-SiO2-MgO-Al2O3 Blast Furnace Primary Slag on Al2O3 Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
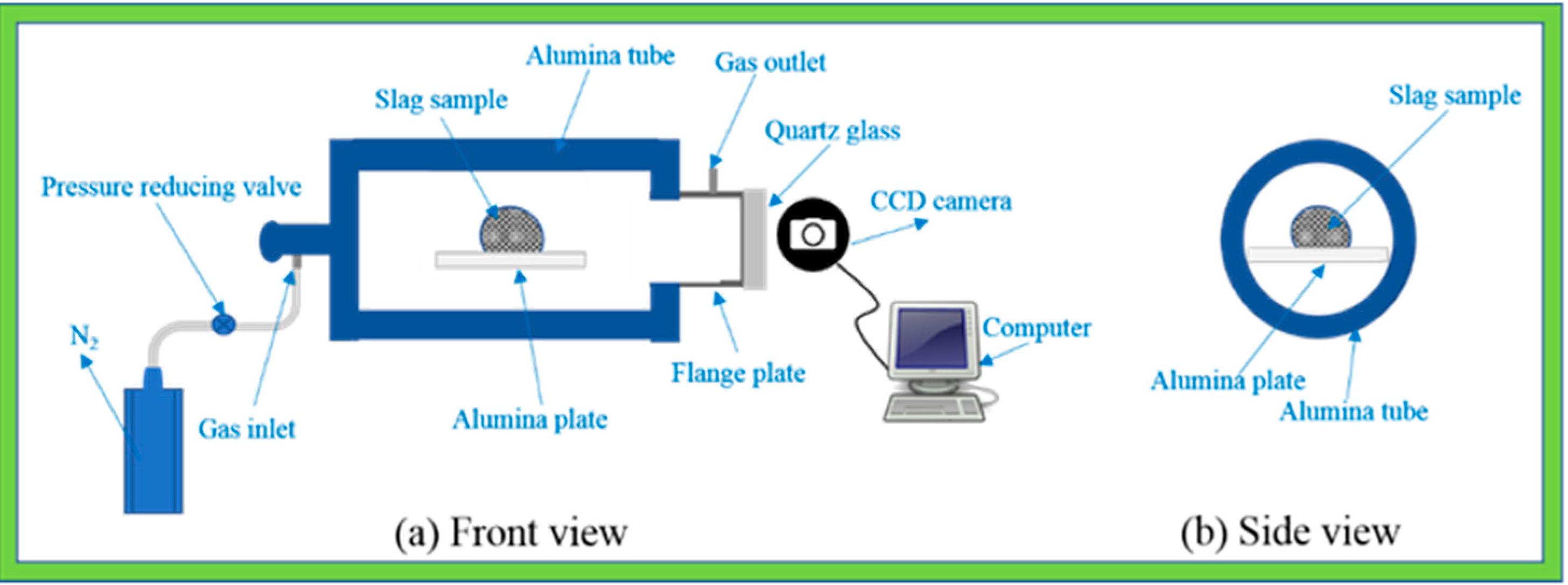
2.2. Experimental Setup and Methods
3. Results and Discussion
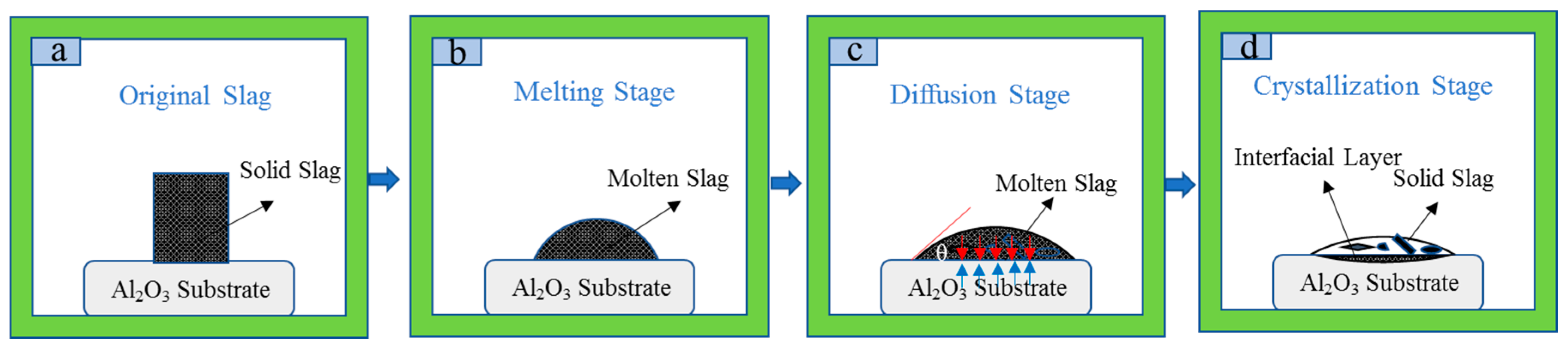
3.1. Wetting Behavior of Slag and Al2O3 Substrate
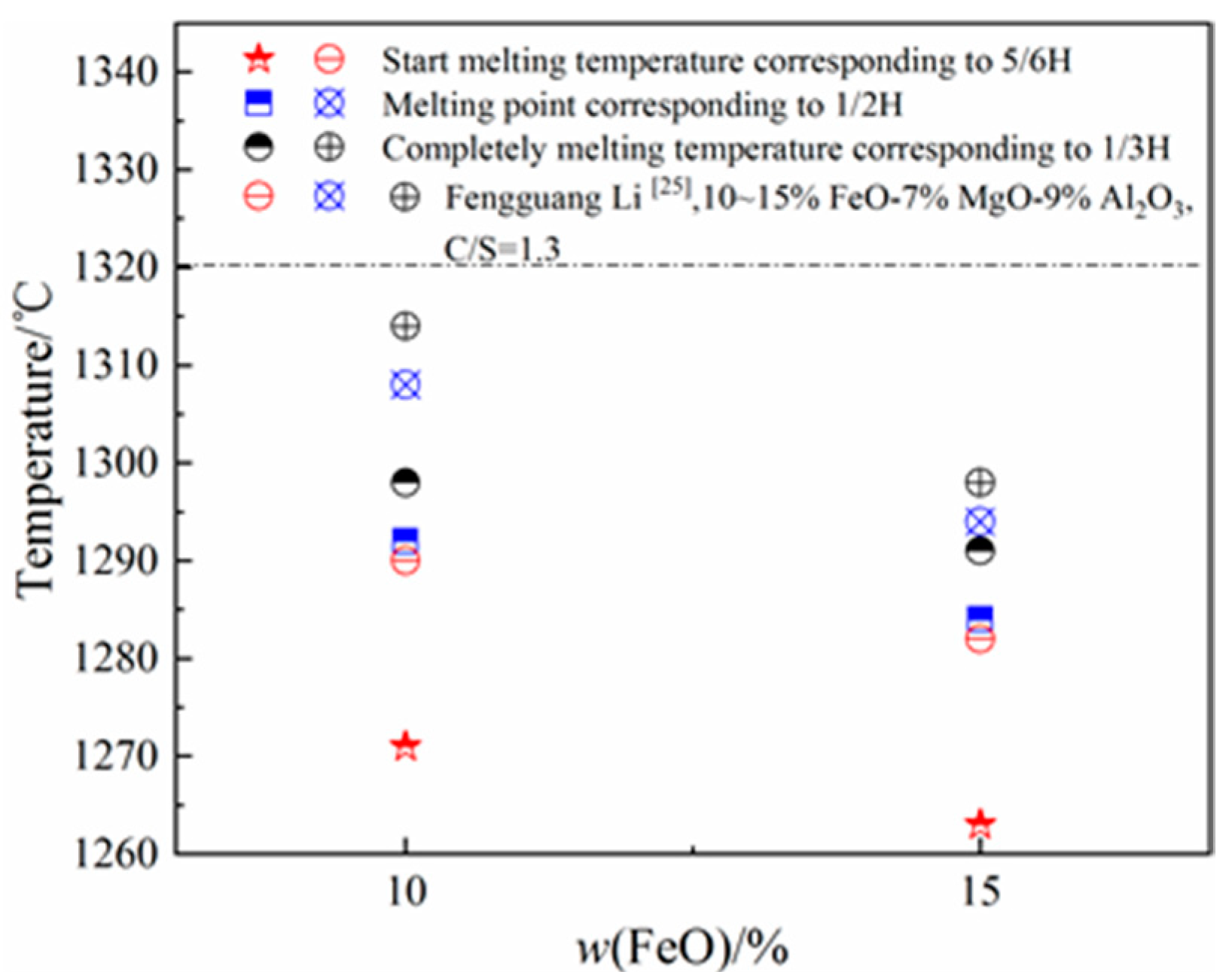
3.2. Effect of w(FeO) on Melting Temperature
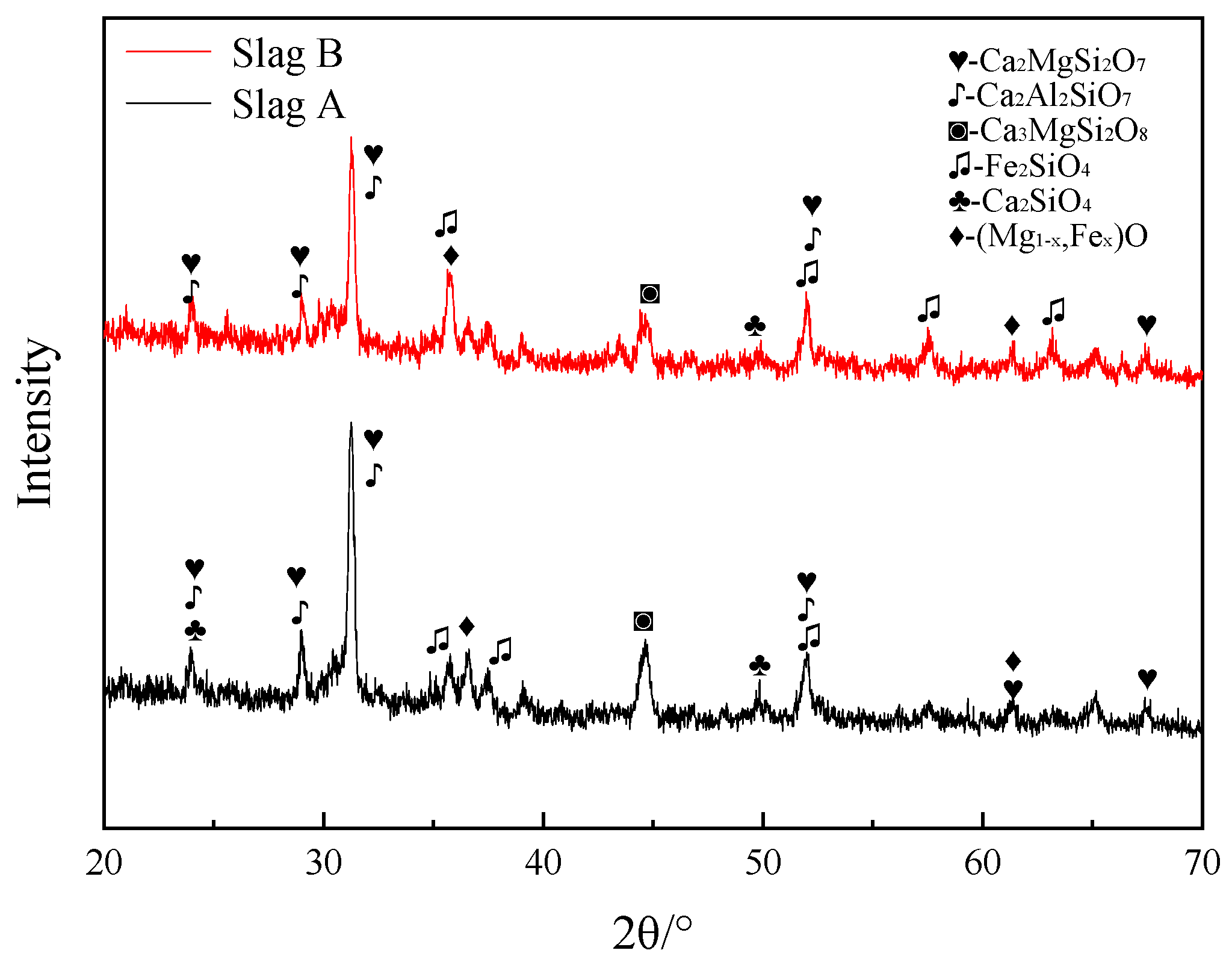
3.3. Mineral Composition of Slags
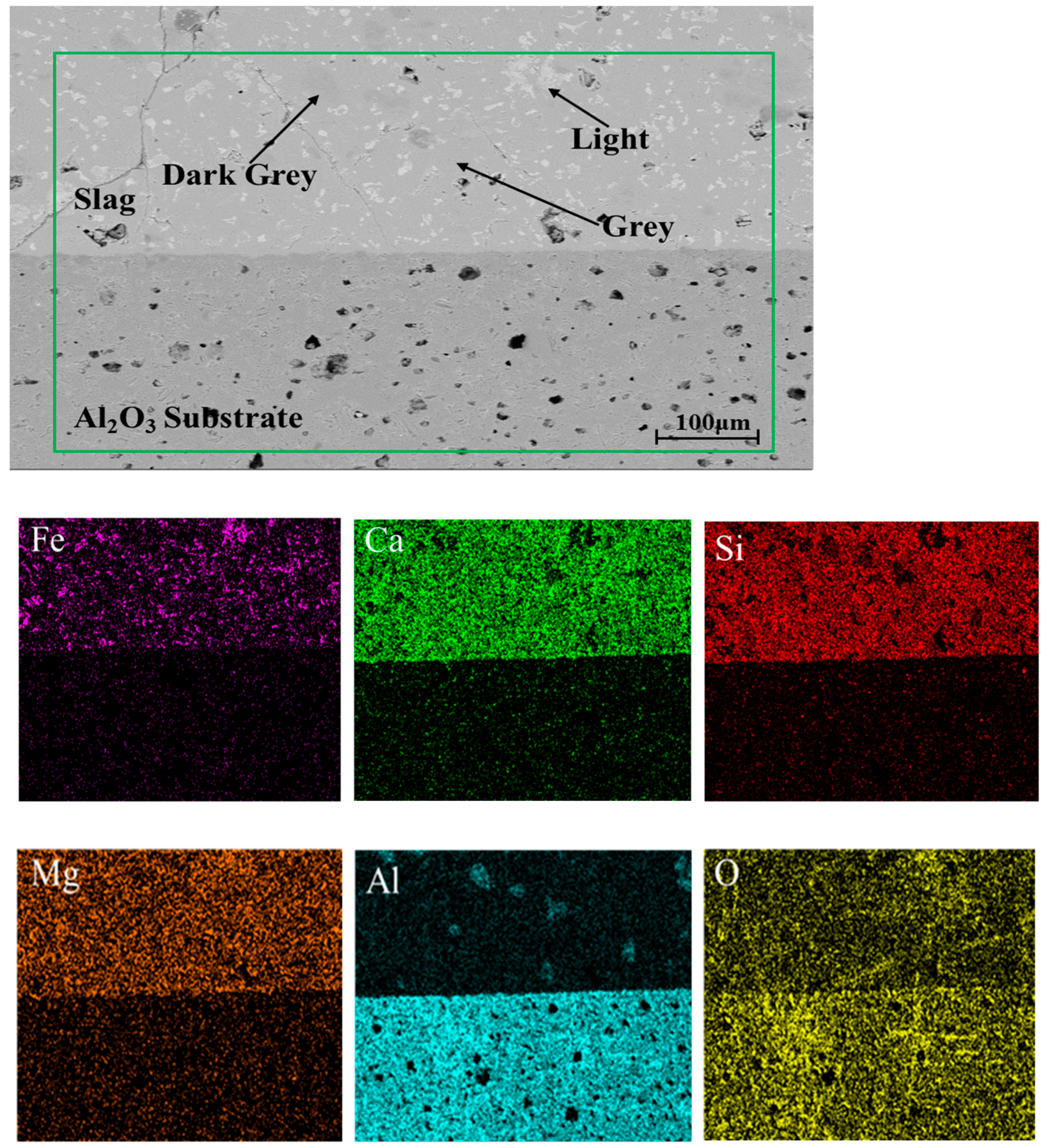
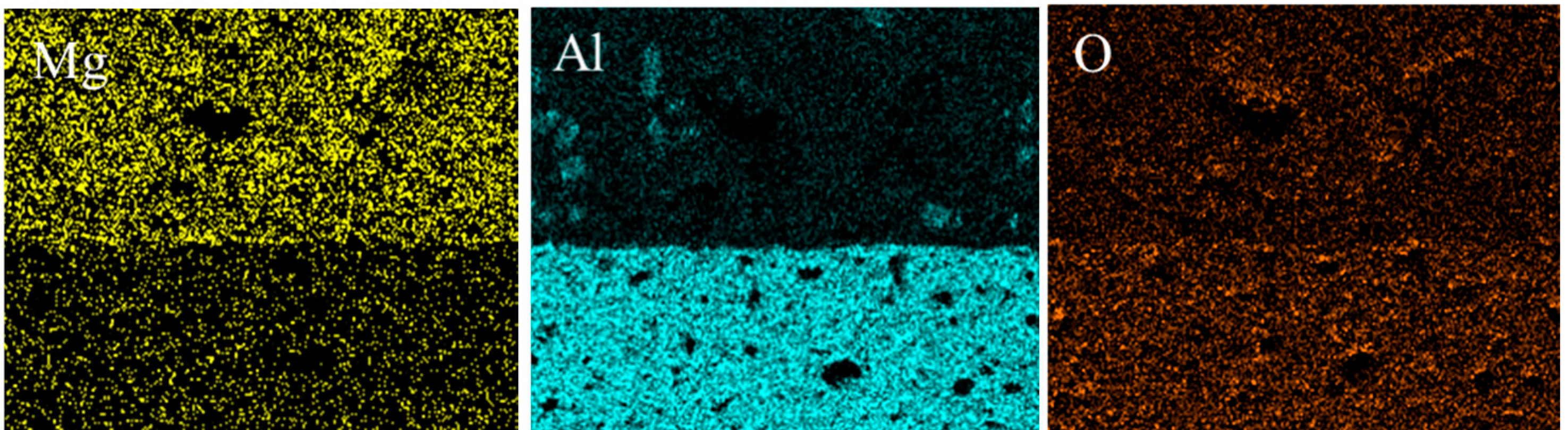
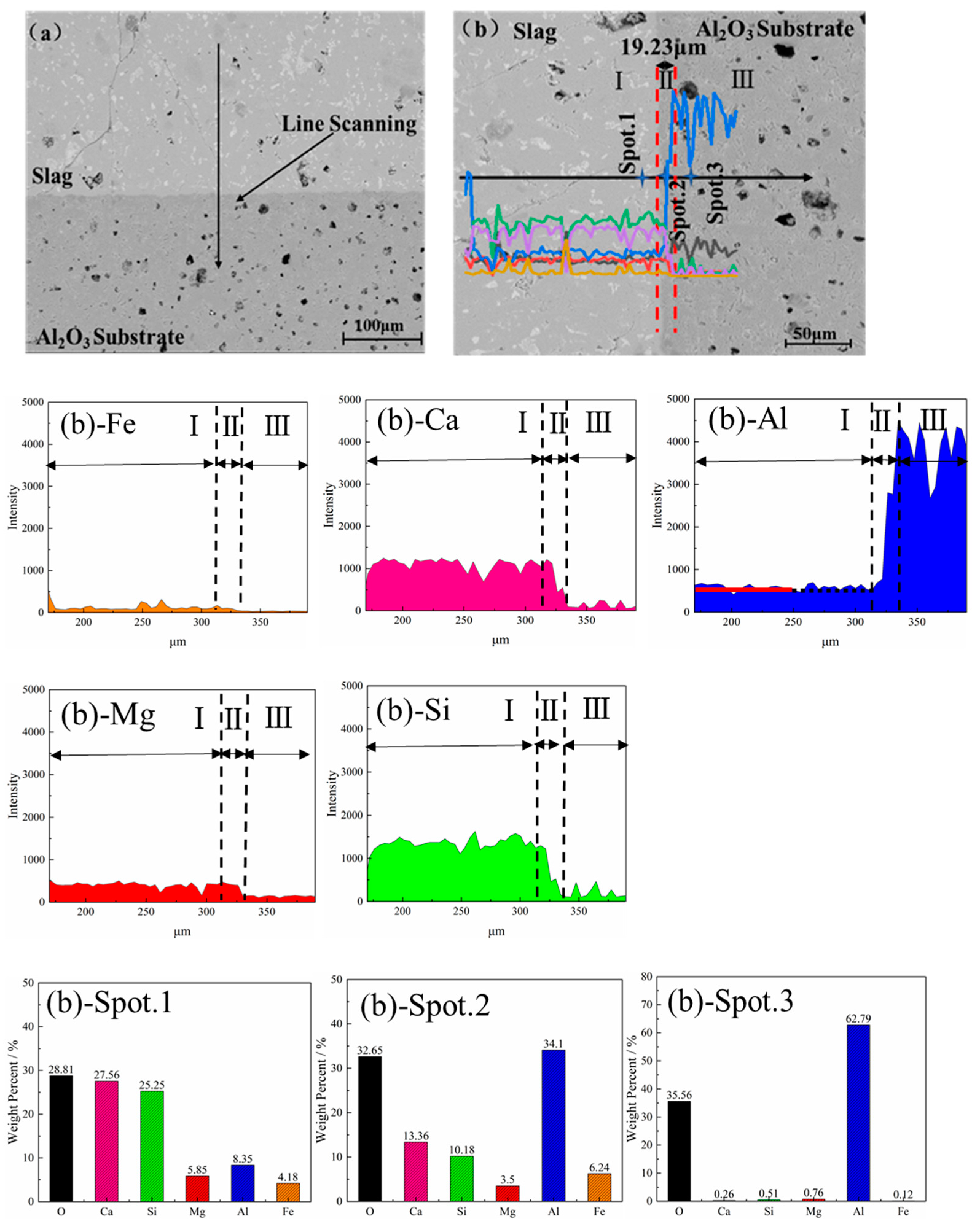
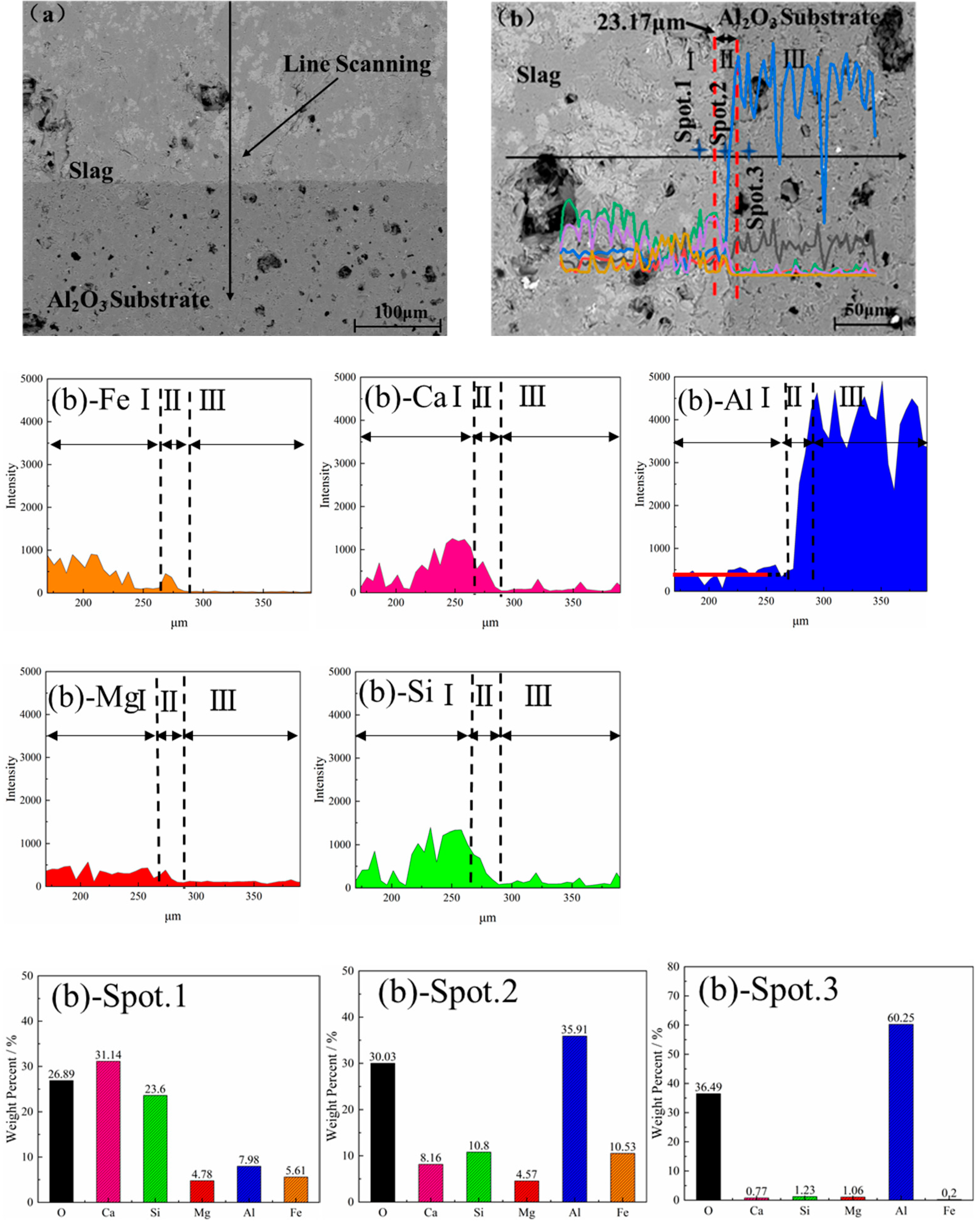
3.4. Element Distribution and Microstructure
3.5. Erosion Thickness of Slag and Al2O3 Substrate
3.6. Corrosion Mechanism
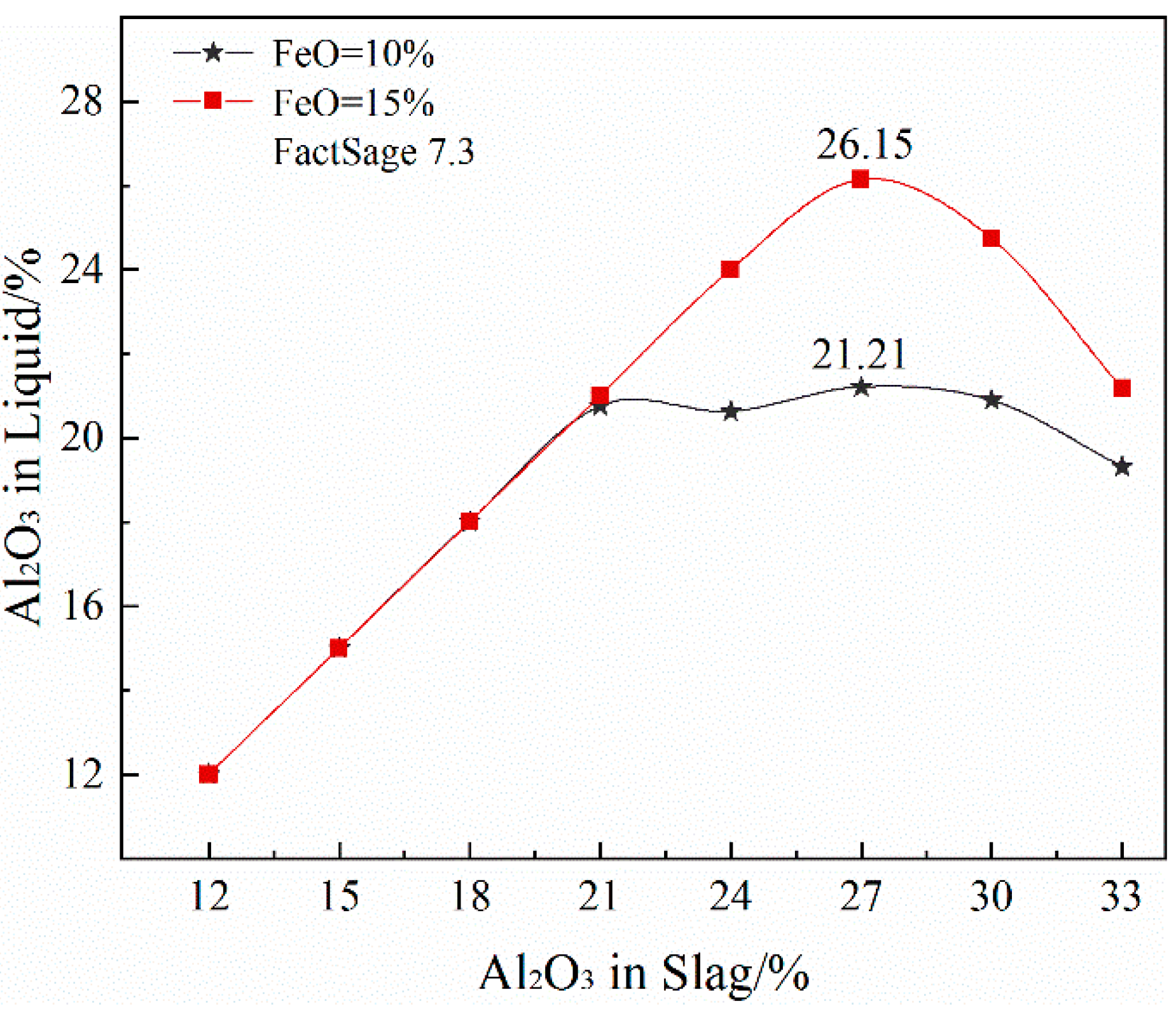
3.6.1. Solubility of Al2O3 in the Liquid Phase
3.6.2. Crystallization Process of the Interface Layer
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, K.O.; Ma, X.; Zhu, J.; Xu, H.; Wang, G.; Zhao, B. Phase equilibria in the system CaO-SiO2-Al2O3-MgO-15 and 20wt% “FeO” with CaO/SiO2 ratio of 1.3. ISIJ Int. 2016, 56, 1728–1737. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, G.; Pelton, A. Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 systems. Metal. Trans. B 1993, 24, 807–816. [Google Scholar] [CrossRef]
- Mao, H.; Hillert, M.; Selleby, M.; Sundman, B. Thermodynamic assessment of the CaO-Al2O3-SiO2 system. J. Am. Ceram. Soc. 2006, 89, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.; Torrecillas, R.; Aza, A.; Pena, P. Effect of spinel Content on slag attack resistance of high alumina refractory castables. J. Eur. Ceram. Soc. 2007, 27, 4623–4631. [Google Scholar] [CrossRef]
- Yi, S. Bosh slag chemistry control for high PCR and low slag volume blast furnace operation. Steel Res. Int. 2003, 74, 413–417. [Google Scholar] [CrossRef]
- Jiao, K.; Zhang, J.; Chen, C.; Liu, Y. Effect of TiO2 and FeO on the Viscosity and Structure of Blast Furnace Primary Slags. Steel Res. Int. 2017, 88, 1600296. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, B.; Zhang, D.; Zhi, X.; Evans, T. Phase Equilibria Studies in the CaO-SiO2-Al2O3-MgO System with CaO/SiO2 Ratio of 1.10. ISIJ Int. 2016, 56, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Wang, G.; Wu, S.; Zhu, J.; Zhao, B. Phase Equilibria in the CaO-SiO2-Al2O3-MgO System with CaO/SiO2 Ratio of 1.3 Relevant to Iron Blast Furnace Slags. ISIJ Int. 2015, 55, 2310–2317. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.O.; Ma, X.; Zhu, J.; Xu, H.; Wang, G.; Zhao, B. Phase Equilibria in the System “FeO”-CaO-SiO2-Al2O3-MgO with CaO/SiO2 1.3. ISIJ Int. 2016, 56, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Liu, Q.; Zhang, J.; Chen, M.; Ma, X.; Zhao, B. Laboratory study of phase transitions and mechanism of reduction of FeO from high Ti-bearing blast furnace primary slag by graphite. Ironmak. Steelmak. 2015, 42, 117–125. [Google Scholar] [CrossRef]
- Pal, J.; Ghorai, S.; Goswami, M.; Ghosh, D.; Bandyopadhyay, D.; Ghosh, S. Behavior of fluxed lime iron oxide pellets in hot metal bath during melting and refining. Int. J. Miner. Metall. Mater. 2013, 20, 329–337. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, H.; Wu, S.; Liu, Y. Effects of combined pre-straining and pre-aging on natural aging and bakehardening response of an Al-Mg-Si alloy. Acta Metall. Sin. 2013, 26, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Shen, F.; Jiang, X.; Wu, G.; Wei, G.; Li, X.; Shen, Y. Proper MgO Addition in Blast Furnace Operation. ISIJ Int. 2006, 46, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ma, K.; Xu, Y.; Xu, J.; Wen, L. Influences of CaO/SiO2/MgO/Al2O3 on the Formation Behavior of FeO-Bearing Primary-Slags in Blast Furnace; Springer International Publishing: New York, NY, USA, 2017; pp. 251–258. [Google Scholar]
- Sako, E.; Braulio, M.; Luz, A.; Zinngrebe, E.; Pandolfelli, V. Slag resistance of Al2O3-MgO refractory castables in different environmental conditions. J. Am. Ceram. Soc. 2013, 96, 3252–3257. [Google Scholar] [CrossRef]
- Blond, E.; Schmitt, N.; Hild, F.; Blumenfeld, P.; Poirier, J. Effect of slag impregnation on thermal degradations in refractories. J. Am. Ceram. Soc. 2007, 90, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Cheremisina, E.; Schenk, J.; Nocke, L.; Paul, A.; Wimmer, G. Kinetics and Mechanisms of Dolime Dissolution in Steelmaking Slag. Metall. Mater. Trans. B 2019, 50, 1269–1276. [Google Scholar] [CrossRef]
- Cooper, A.R. Kinetics of refractory corrosion. In Ceramic Engineering and Science Proceedings; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1981; pp. 1063–1089. [Google Scholar]
- Afshar, S.; Allaire, C. The corrosion of refractory aggregates by molten aluminum. JOM 2000, 52, 43–46. [Google Scholar] [CrossRef]
- Monaghan, B.; Abdeyazdan, H.; Dogan, N.; Rhamdhani, M.; Longbottom, R.; Chapman, M. Effect of slag composition on wettability of oxide inclusions. ISIJ Int. 2015, 55, 1834–1840. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Liu, Y.; Lu, X.; You, Z. Corrosion behavior of Al2O3 substrate by SiO2-MgO-FeO-CaO-Al2O3 slag. J. Mater. Res. Technol. 2019, 9, 314–321. [Google Scholar] [CrossRef]
- Sarkar, R.; Sohn, H. Interaction of ferrous oxide with alumina refractory under flash ironmaking conditions. Ceram. Int. 2019, 45, 15417–15428. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Zhang, L. Transient interaction between molten iron, alumina lining refractory and slag. J. Manuf. Sci. Prod. 2013, 13, 133–143. [Google Scholar]
- Choi, J.; Lee, H.; Kim, J. Dissolution rate of Al2O3 into molten CaO-SiO2-Al2O3 slags. ISIJ Int. 2002, 42, 852–860. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H. Wetting of solid Al2O3 with molten CaO-Al2O3-SiO2. ISIJ Int. 2003, 43, 1348–1355. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, G.; Ding, Z.; Dai, Q.; Li, B. Effect of additives on melting point of LATS refining ladle slag. J. Iron Steel Res. Int. 2007, 14, 25–29. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Li, B.; Zhang, X.; Yan, Y. Effect of B2O3 on melting temperature of CaO-based ladle refining slag. J. Iron Steel Res. Int. 2010, 17, 18–22. [Google Scholar] [CrossRef]
- Li, F.; Qi, C.; Wang, Y. Effect of FeO on softening characteristics of primary slag under different alkalinity. Iron Steel 2018, 53, 26–32. (In Chinese) [Google Scholar]
- Sarkar, R.; Hong, Y. Interaction of pure alumina refractory with FeO-SiO2 and FeO-SiO2-CaO slags relevant to the novel flash ironmaking technology. Steel Res. Int. 2019, 90, 1900104. [Google Scholar] [CrossRef]



| NO | C/S* | Slag Composition | ||||
|---|---|---|---|---|---|---|
| FeO | CaO | SiO2 | MgO | Al2O3 | ||
| Slag A | 1.4 | 10 | 40.83 | 29.17 | 8 | 12 |
| Slag B | 1.4 | 15 | 37.92 | 27.08 | 8 | 12 |
| NOi | Locations | Composition (wt%) | ||||
|---|---|---|---|---|---|---|
| FeO | CaO | SiO2 | MgO | Al2O3 | ||
| Slag A | Light | 77.65 | 1.04 | 0.15 | 9.56 | 11.60 |
| Grey | 4.42 | 40.19 | 36.43 | 7.90 | 11.06 | |
| Dark grey | 3.30 | 34.01 | 14.64 | 2.29 | 45.76 | |
| Slag B | Light | 71.75 | 1.89 | 1.62 | 11.78 | 12.96 |
| Grey | 5.92 | 40.67 | 34.46 | 6.76 | 12.19 | |
| Dark grey | 3.63 | 30.74 | 17.25 | 0.56 | 47.82 | |
| NOi | Locations | Composition/(wt%) | ||||
|---|---|---|---|---|---|---|
| FeO | CaO | SiO2 | MgO | Al2O3 | ||
| Slag A | Spot.1 | 4.35 | 31.22 | 43.78 | 7.89 | 12.76 |
| Spot.2 | 6.76 | 15.75 | 18.36 | 4.91 | 54.22 | |
| Spot.3 | 0.14 | 0.29 | 0.90 | 1.04 | 97.63 | |
| Slag B | Spot.1 | 5.79 | 35.04 | 40.65 | 6.41 | 12.11 |
| Spot.2 | 10.96 | 9.25 | 18.73 | 6.16 | 54.90 | |
| Spot.3 | 0.22 | 0.91 | 2.20 | 1.48 | 95.19 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Shen, F.; Gao, Q.; Jiang, X.; Zheng, H. Effect of Erosion Behavior of FeO-CaO-SiO2-MgO-Al2O3 Blast Furnace Primary Slag on Al2O3 Substrate. Crystals 2021, 11, 957. https://doi.org/10.3390/cryst11080957
Pan X, Shen F, Gao Q, Jiang X, Zheng H. Effect of Erosion Behavior of FeO-CaO-SiO2-MgO-Al2O3 Blast Furnace Primary Slag on Al2O3 Substrate. Crystals. 2021; 11(8):957. https://doi.org/10.3390/cryst11080957
Chicago/Turabian StylePan, Xiangyang, Fengman Shen, Qiangjian Gao, Xin Jiang, and Haiyan Zheng. 2021. "Effect of Erosion Behavior of FeO-CaO-SiO2-MgO-Al2O3 Blast Furnace Primary Slag on Al2O3 Substrate" Crystals 11, no. 8: 957. https://doi.org/10.3390/cryst11080957
APA StylePan, X., Shen, F., Gao, Q., Jiang, X., & Zheng, H. (2021). Effect of Erosion Behavior of FeO-CaO-SiO2-MgO-Al2O3 Blast Furnace Primary Slag on Al2O3 Substrate. Crystals, 11(8), 957. https://doi.org/10.3390/cryst11080957






