Synthetic BiOBr/Bi2S3/CdS Crystalline Material and Its Degradation of Dye under Visible Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Preparation of BiOBr/Bi2S3/CdS Composite
2.2. Photocatalytic Degradation Studies
3. Results and Discussion
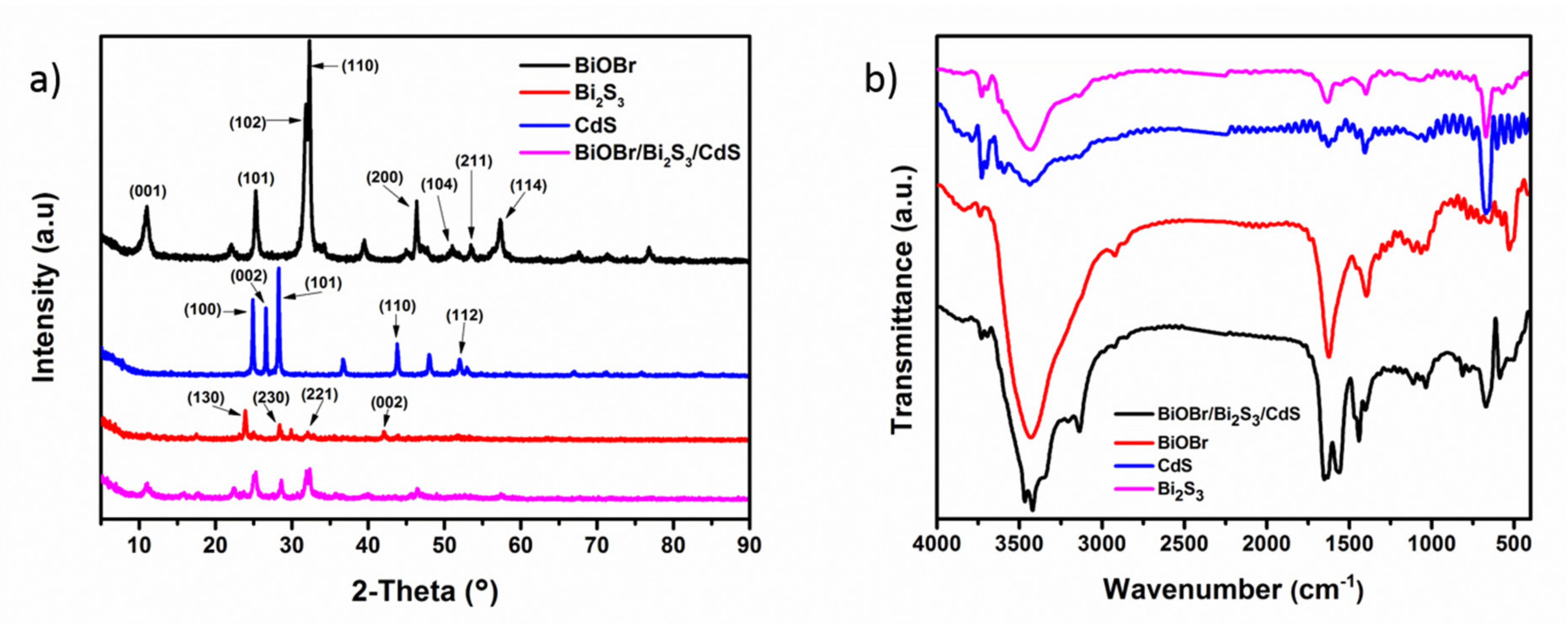
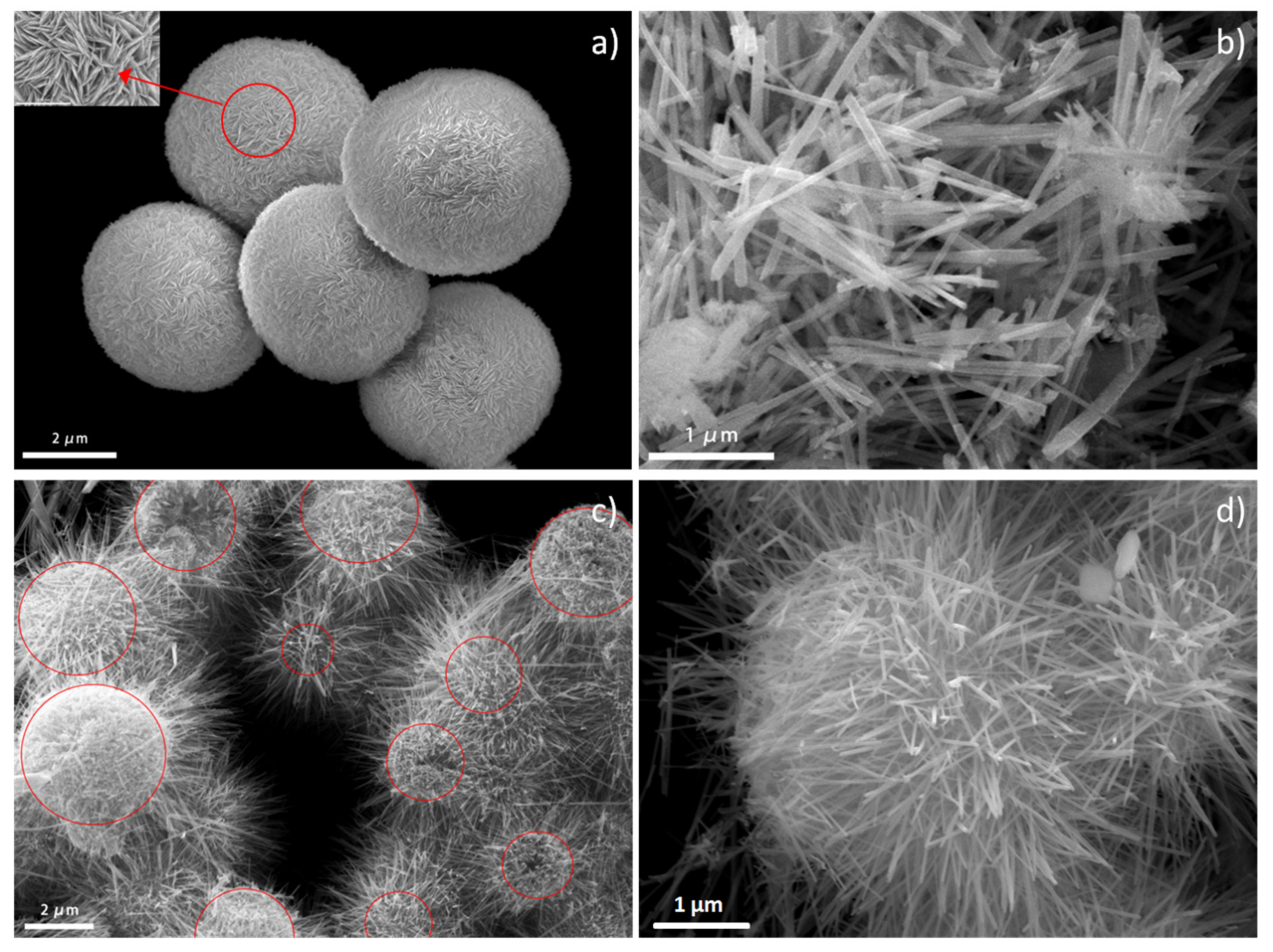
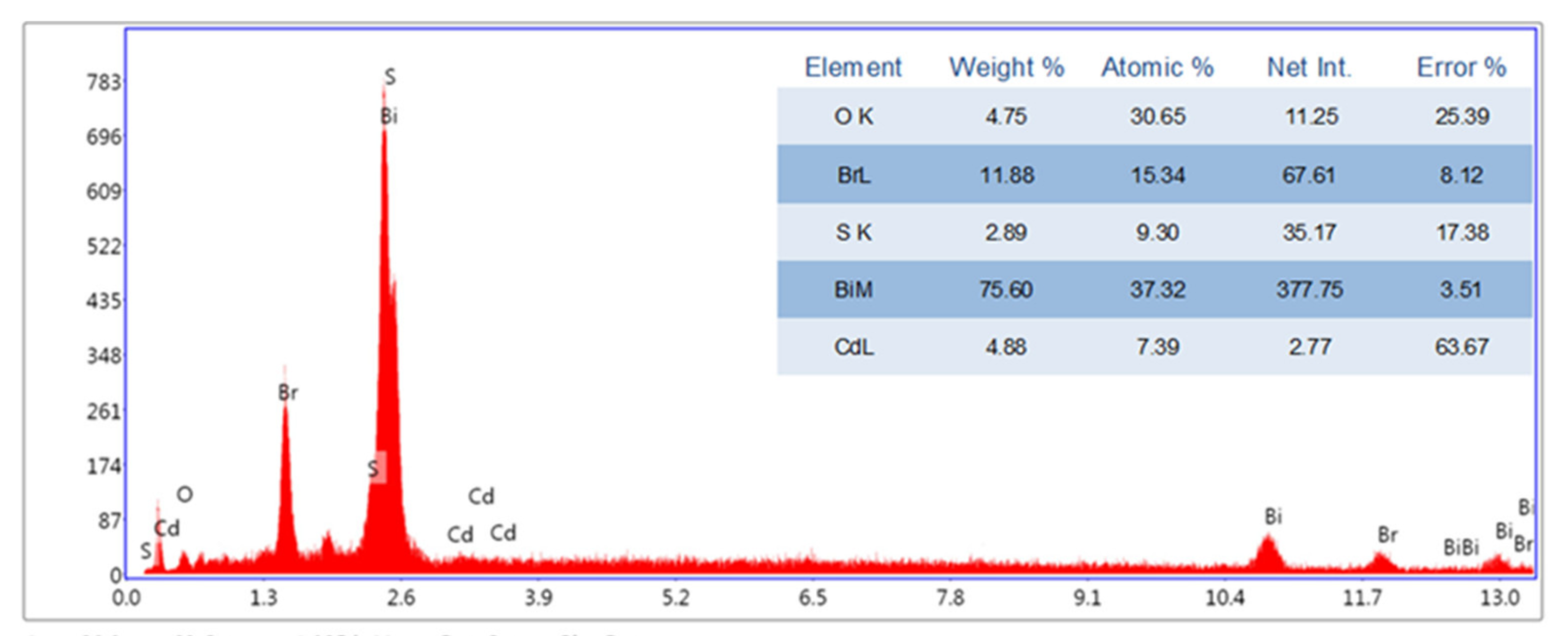
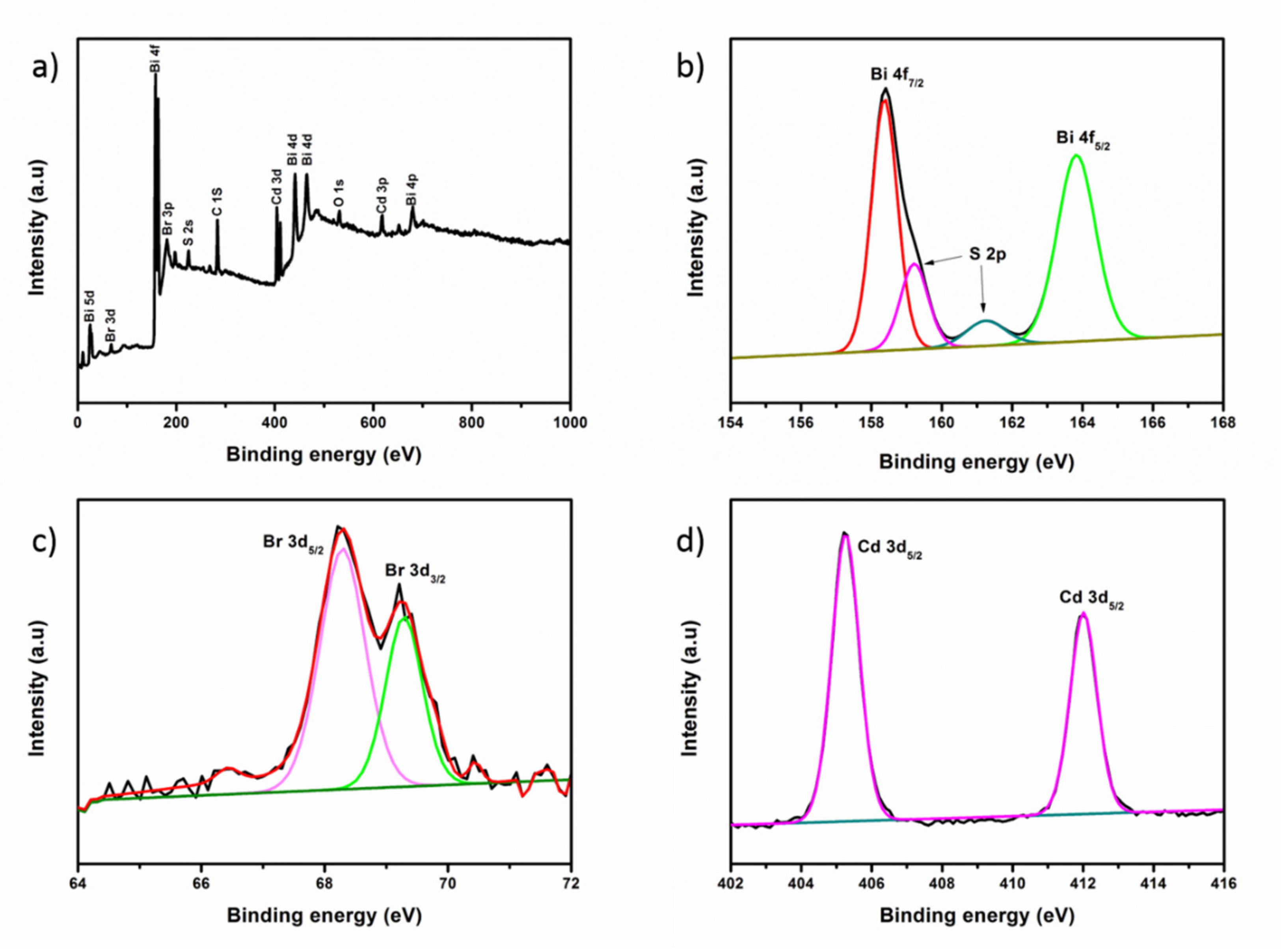
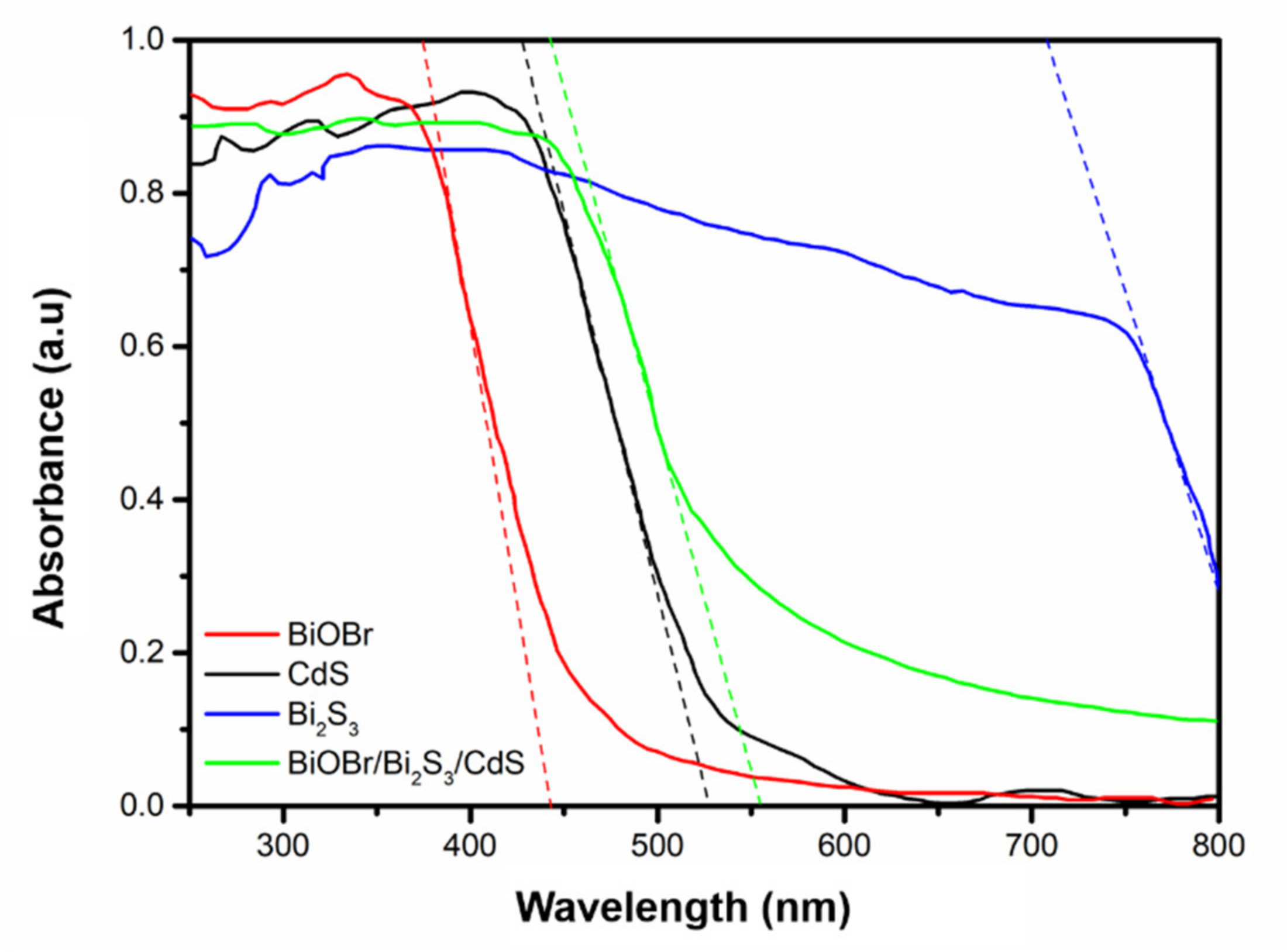
3.1. Characterization of BiOBr/Bi2S3/CdS Composite Material
3.2. Photocatalytic Performance
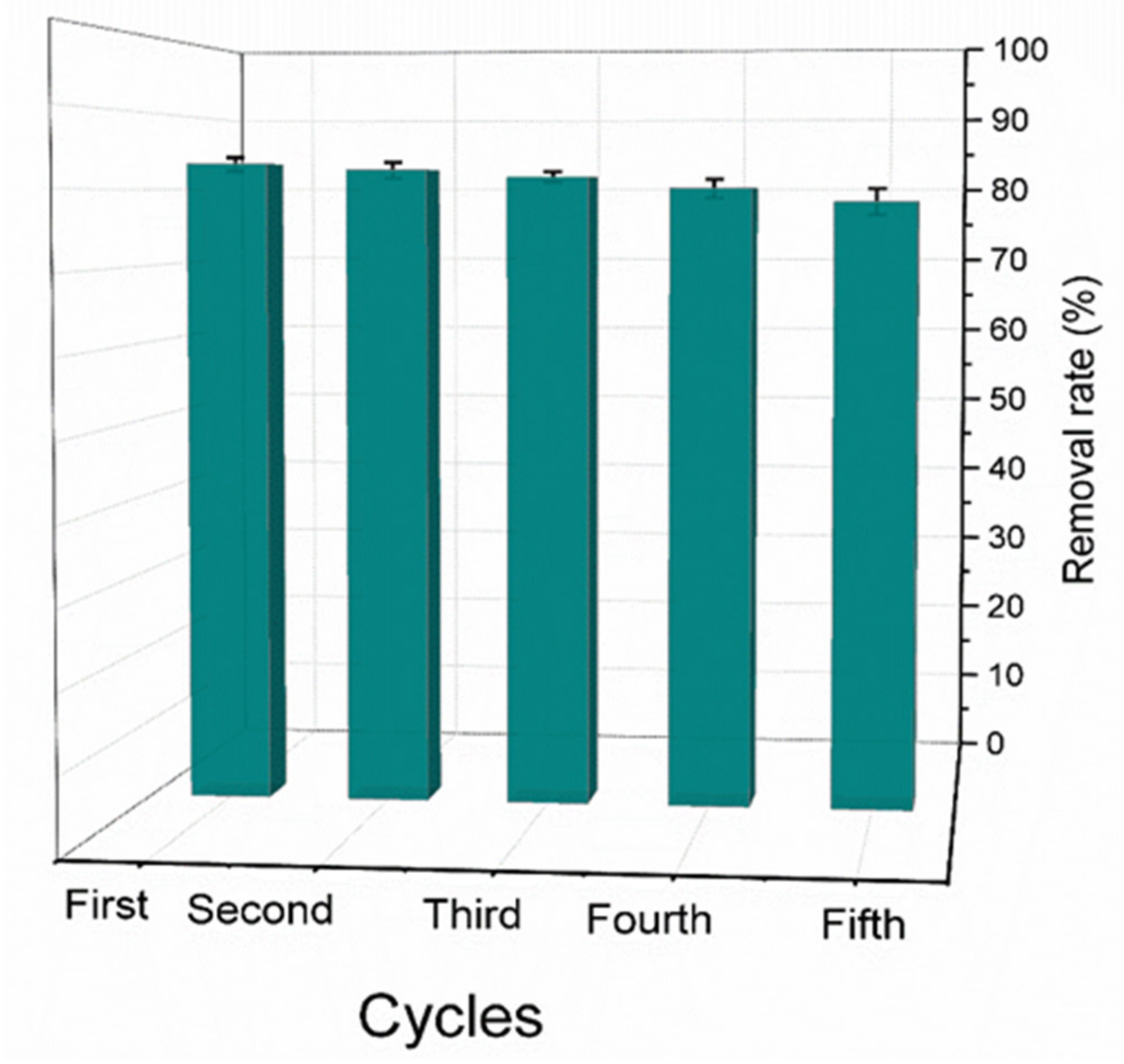
3.3. Cycling Experiments
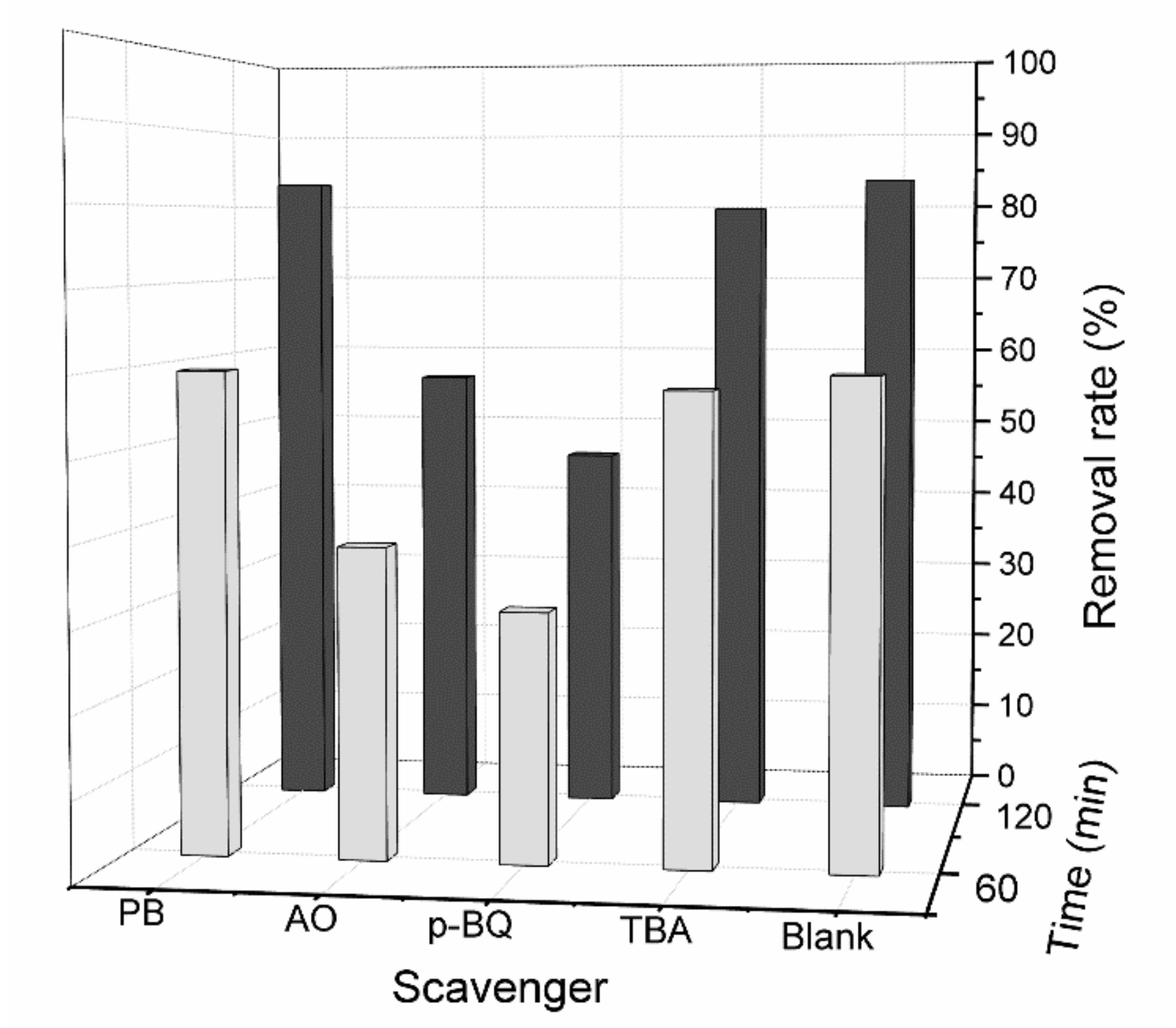
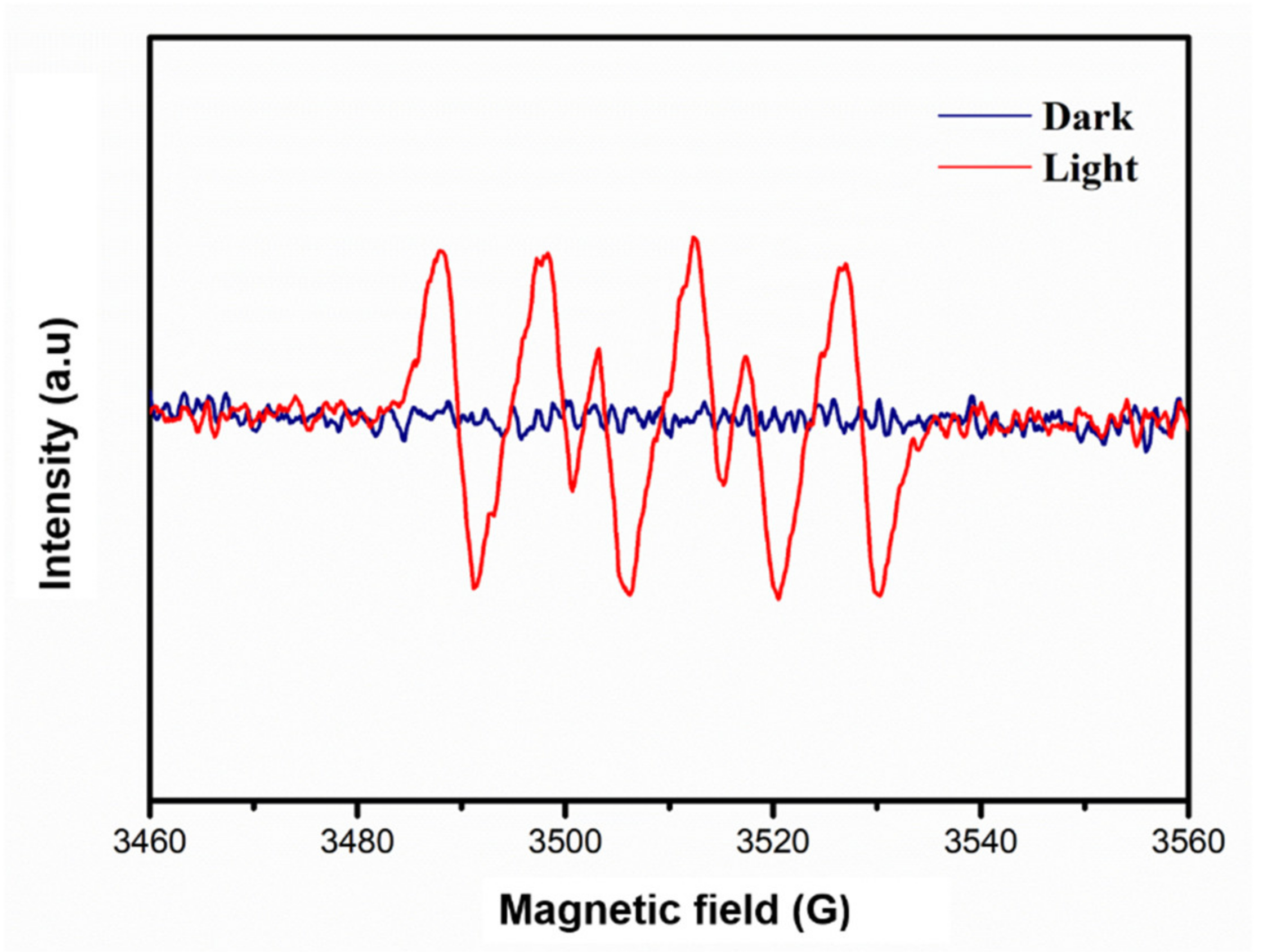
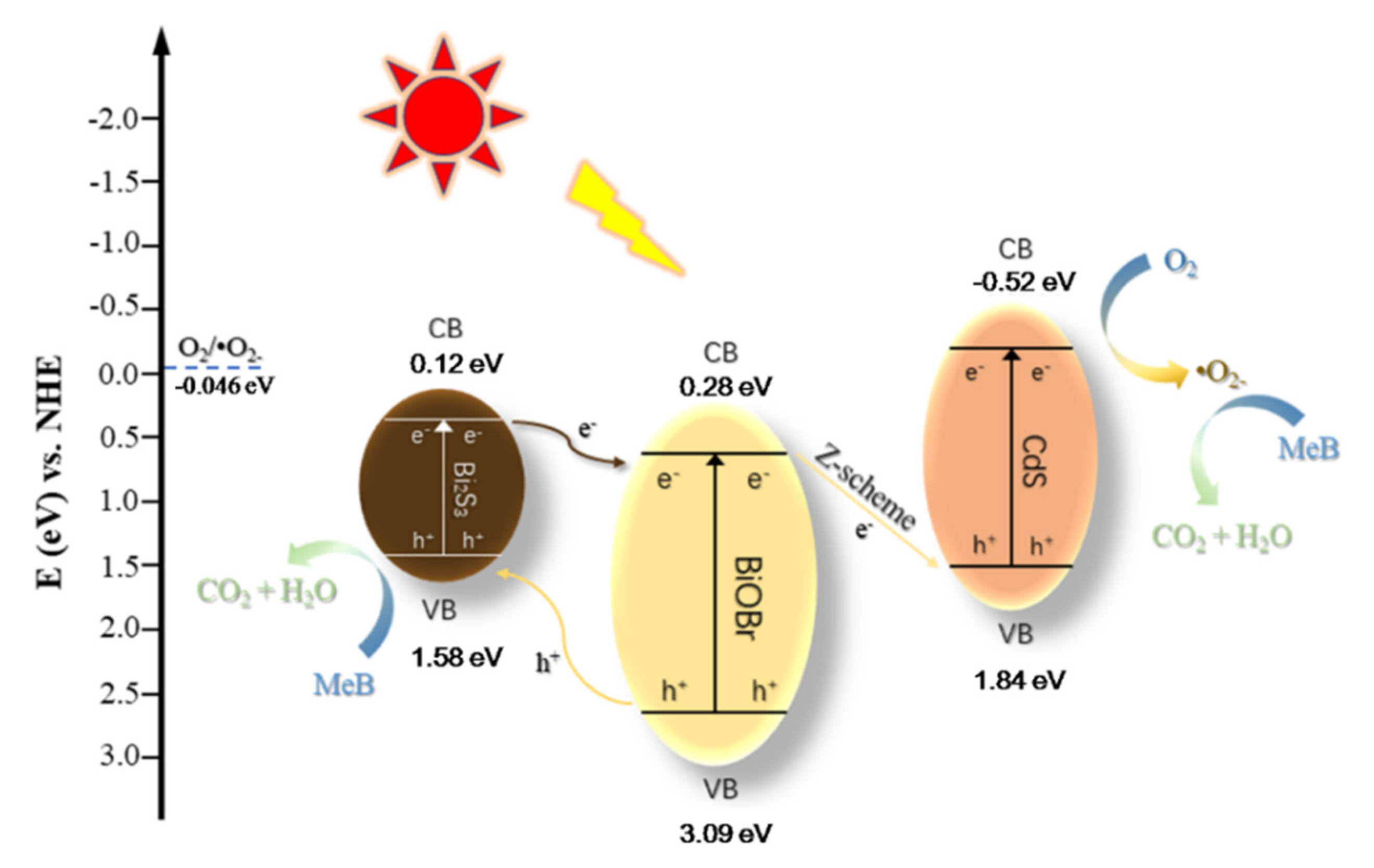
3.4. Exploration of Photocatalytic Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Symbols
| A | absorbance of the dye solution [-] |
| A0 | initial absorbance of the dye solution [-] |
| C | concentration of the dye solution [mol L−1] |
| C0 | initial concentration of the dye solution [mol L−1] |
| E0 | formation potential of O2− [eV] |
| Eg | band gap energy [eV] |
| ECB | edge potentials of conduction band [eV] |
| EVB | edge potentials of the valence band [eV] |
| Ee | energy of free electrons on the hydrogen scale [eV] |
| K | rate constant [min−1] |
| t | reaction time [min] |
| R2 | correlation coefficient [-] |
| X | electronegativity of BiOBr, Bi2S3 and CdS [eV] |
| Greek letters | |
| Λg | band edge wavelength [m] |
| Abbreviations | |
| AO | ammonium oxalate |
| CB | conduction band |
| PB | potassium bromate |
| p-BQ | p-benzoquinone |
| TAA | thioacetamide |
| TBA | tert-butanol |
| VB | valence band |
References
- Chiam, S.L.; Pung, S.Y.; Yeoh, F.Y. Recent developments in MnO2-based photocatalysts for organic dye removal: A review. Environ. Sci. Pollut. Res. 2020, 27, 5759–5778. [Google Scholar] [CrossRef]
- Lin, S.; Song, Z.L.; Che, G.B.; Ren, A.; Li, P.; Liu, C.B.; Zhang, J.H. Adsorption behavior of metal-organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mat. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, J.; Zhang, C.L.; Yue, Q.Y.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Wang, S.B.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Ali, I.; Karim, S.M.A.; Firoz, M.S.H.; Chowdhury, A.; Morton, D.W.; Angove, M.J. Removal of dye from polluted water using novel nano manganese oxide-based materials. J. Water Process. Eng. 2019, 32, 21. [Google Scholar] [CrossRef]
- Ranjith, R.; Renganathan, V.; Chen, S.-M.; Selvan, N.S.; Rajam, P.S. Green synthesis of reduced graphene oxide supported TiO2/Co3O4 nanocomposite for photocatalytic degradation of methylene blue and crystal violet. Ceram. Int. 2019, 45, 12926–12933. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Wang, M.; Kong, D.; Zhang, Y.; Chen, J.F.; Dai, L. Uniform Two-Dimensional Co3O4Porous Sheets: Facile Synthesis and Enhanced Photocatalytic Performance. Chem. Eng. Technol. 2016, 39, 891–898. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Baniamerian, H.; Teimoori, M.; Saberi, M. Fe2O3/TiO2/Activated Carbon Nanocomposite with Synergistic Effect of Adsorption and Photocatalysis. Chem. Eng. Technol. 2021, 44, 130–139. [Google Scholar] [CrossRef]
- Truong, D.H.; Vo, V.; Van Gerven, T.; Leblebici, M.E. A Facile Method for the Synthesis of a MoS2/g-C3N4. Photocatalyst. Chem. Eng. Technol. 2019, 42, 2691–2699. [Google Scholar] [CrossRef]
- Yan, P.; Jiang, D.; Tian, Y.; Xu, L.; Qian, J.; Li, H.; Xia, J.; Li, H. A sensitive signal-on photoelectrochemical sensor for tetracycline determination using visible-light-driven flower-like CN/BiOBr composites. Biosens. Bioelectron. 2018, 111, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.X.; Di, J.; Li, H.T.; Xu, H.; Li, H.M.; Guo, S.J. Ionic liquid-induced strategy for carbon quantum dots/BiOX (X = Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl. Catal. B-Environ. 2016, 181, 260–269. [Google Scholar] [CrossRef]
- Shenoy, S.; Sridharan, K. Bismuth oxybromide nanoplates embedded on activated charcoal as effective visible light driven photocatalyst. Chem. Phys. Lett. 2020, 749, 137435. [Google Scholar] [CrossRef]
- Sridharan, K.; Shenoy, S.; Kumar, S.G.; Terashima, C.; Fujishima, A.; Pitchaimuthu, S. Advanced Two-Dimensional Heterojunction Photocatalysts of Stoichiometric and Non-Stoichiometric Bismuth Oxyhalides with Graphitic Carbon Nitride for Sustainable Energy and Environmental Applications. Catalysts 2021, 11, 426. [Google Scholar] [CrossRef]
- Di, T.; Xu, Q.; Ho, W.; Tang, H.; Xiang, Q.; Yu, J. Review on Metal Sulphide-based Z-scheme Photocatalysts. ChemCatChem 2019, 11, 1394–1411. [Google Scholar] [CrossRef]
- Guan, S.; Yang, H.; Sun, X.; Xian, T. Preparation and promising application of novel LaFeO3/BiOBr heterojunction photocatalysts for photocatalytic and photo-Fenton removal of dyes. Opt. Mater. 2020, 100, 109644. [Google Scholar] [CrossRef]
- Yang, Z.; Tong, X.; Feng, J.; He, S.; Fu, M.; Niu, X.; Zhang, T.; Liang, H.; Ding, A.; Feng, X. Flower-like BiOBr/UiO-66-NH2 nanosphere with improved photocatalytic property for norfloxacin removal. Chemosphere 2019, 220, 98–106. [Google Scholar] [CrossRef]
- Wang, W.N.; Zhang, C.Y.; Zhang, M.F.; Pei, P.; Zhou, W.; Zha, Z.B.; Shao, M.; Qian, H.-S. Precisely photothermal controlled releasing of antibacterial agent from Bi2S3 hollow microspheres triggered by NIR light for water sterilization. Chem. Eng. J. 2020, 381, 122630. [Google Scholar] [CrossRef]
- Yang, M.; Shi, Y.; Li, Y.; Li, H.; Luo, N.; Li, J.; Fan, J.; Zhou, A. Construction of 2D Bi2S3/CdS Nanosheet Arrays for Enhanced Photoelectrochemical Hydrogen Evolution. J. Electron. Mater. 2019, 48, 6397–6405. [Google Scholar] [CrossRef]
- Liang, Q.; Ploychompoo, S.; Chen, J.; Zhou, T.; Luo, H. Simultaneous Cr(VI) reduction and bisphenol A degradation by a 3D Z-scheme Bi2S3-BiVO4 graphene aerogel under visible light. Chem. Eng. J. 2020, 384, 123256. [Google Scholar] [CrossRef]
- Shi, X.J.; Chen, X.; Chen, X.L.; Zhou, S.M.; Lou, S.Y.; Wang, Y.Q.; Yuan, L. PVP assisted hydrothermal synthesis of BiOBr hierarchical nanostructures and high photocatalytic capacity. Chem. Eng. J. 2013, 222, 120–127. [Google Scholar] [CrossRef]
- Deng, W.; Pan, F.P.; Batchelor, B.; Jung, B.M.; Zhang, P.; Abdel-Wahab, A.; Zhou, H.C.; Li, Y. Mesoporous TiO2-BiOBr microspheres with tailorable adsorption capacities for photodegradation of organic water pollutants: Probing adsorption-photocatalysis synergy by combining experiments and kinetic modeling. Environ. Sci. Wat. Res. Technol. 2019, 5, 769–781. [Google Scholar] [CrossRef]
- Cui, W.; An, W.; Liu, L.; Hu, J.; Liang, Y. Synthesis of CdS/BiOBr composite and its enhanced photocatalytic degradation for Rhodamine B. Appl. Surf. Sci. 2014, 319, 298–305. [Google Scholar] [CrossRef]
- Qiu, J.; Wu, M.; Yu, L.; Li, J.; Di, J.; Zhang, S.; Luo, Z.; Zhang, S.; Li, Z.; Wu, Z. Vanadate-Rich BiOBr/Bi Nanosheets for Effective Adsorption and Visible-Light-Driven Photodegradation of Rhodamine B. J. Nanosci. Nanotechnol. 2020, 20, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Kang, C.L.; Xiao, K.K.; Wang, X.Y. Fabrication of Bi2S3/MOFs composites without noble metals for enhanced photoreduction of Cr (VI). Sep. Purif. Technol. 2020, 241, 8. [Google Scholar] [CrossRef]
- Imam, S.S.; Adnan, R.; Mohd Kaus, N.H. Room-temperature synthesis of flower-like BiOBr/Bi2S3 composites for the catalytic degradation of fluoroquinolones using indoor fluorescent light illumination. Colloids Surf. A 2020, 585, 124069. [Google Scholar] [CrossRef]
- Wu, S.; Xie, Y.; Zhang, X.; Huang, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X. In situ synthesis of adsorptive β-Bi2O3/BiOBr photocatalyst with enhanced degradation efficiency. J. Mater. Res. 2019, 34, 3450–3461. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Khusnun, N.F.; Ali, M.W.; Haron, S. Role of reduced graphene oxide in improving interfacial charge transfer of hybridized rGO/silica/zirconia for enhanced Bisphenol A photodegradation. J. Alloys Compd. 2019, 789, 221–230. [Google Scholar] [CrossRef]
- Cui, Y.; Jia, Q.; Li, H.; Han, J.; Zhu, L.; Li, S.; Zou, Y.; Yang, J. Photocatalytic activities of Bi2S3/BiOBr nanocomposites synthesized by a facile hydrothermal process. Appl. Surf. Sci. 2014, 290, 233–239. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Fang, D.; Liang, J.; Zhou, L. Simulated solarlight catalytic reduction of Cr(VI) on microwave–ultrasonication synthesized flower-like CuO in the presence of tartaric acid. Mater. Chem. Phys. 2016, 171, 386–393. [Google Scholar] [CrossRef]
- Shenoy, S.; Ahmed, S.; Lo, I.M.C.; Singh, S.; Sridharan, K. Rapid sonochemical synthesis of copper doped ZnO grafted on graphene as a multi-component hierarchically structured visible-light-driven photocatalyst. Mater. Res. Bull. 2021, 140, 111290. [Google Scholar] [CrossRef]
- Khampuanbut, A.; Santalelat, S.; Pankiew, A.; Channei, D.; Pornsuwan, S.; Faungnawakij, K.; Phanichphant, S.; Inceesungvorn, B. Visible-light-driven WO3/BiOBr heterojunction photocatalysts for oxidative coupling of amines to imines: Energy band alignment and mechanistic insight. J. Colloid Interface Sci. 2020, 560, 213–224. [Google Scholar] [CrossRef]
- Shenoy, S.; Jang, E.; Park, T.J.; Gopinath, C.S.; Sridharan, K. Cadmium sulfide nanostructures: Influence of morphology on the photocatalytic degradation of erioglaucine and hydrogen generation. Appl. Surf. Sci. 2019, 483, 696–705. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Wide spectral degradation of Norfloxacin by Ag@BiPO4/BiOBr/BiFeO3 nano-assembly: Elucidating the photocatalytic mechanism under different light sources. J. Hazard. Mater. 2019, 364, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Liu, X.; Liu, Z.; Zeng, G.; Liang, Q.; Liang, C.; Cheng, Y.; Zhang, W.; Liu, Y.; Gong, S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019, 368, 730–745. [Google Scholar] [CrossRef]
- Dong, X.A.; Zhang, W.; Sun, Y.; Li, J.; Cen, W.; Cui, Z.; Huang, H.; Dong, F. Visible-light-induced charge transfer pathway and photocatalysis mechanism on Bi semimetal@defective BiOBr hierarchical microspheres. J. Catal. 2018, 357, 41–50. [Google Scholar] [CrossRef]
- Zhu, S.R.; Qi, Q.; Zhao, W.N.; Fang, Y.; Han, L. Enhanced photocatalytic activity in hybrid composite combined BiOBr nanosheets and Bi2S3 nanoparticles. J. Phys. Chem. Solids 2018, 121, 163–171. [Google Scholar] [CrossRef]

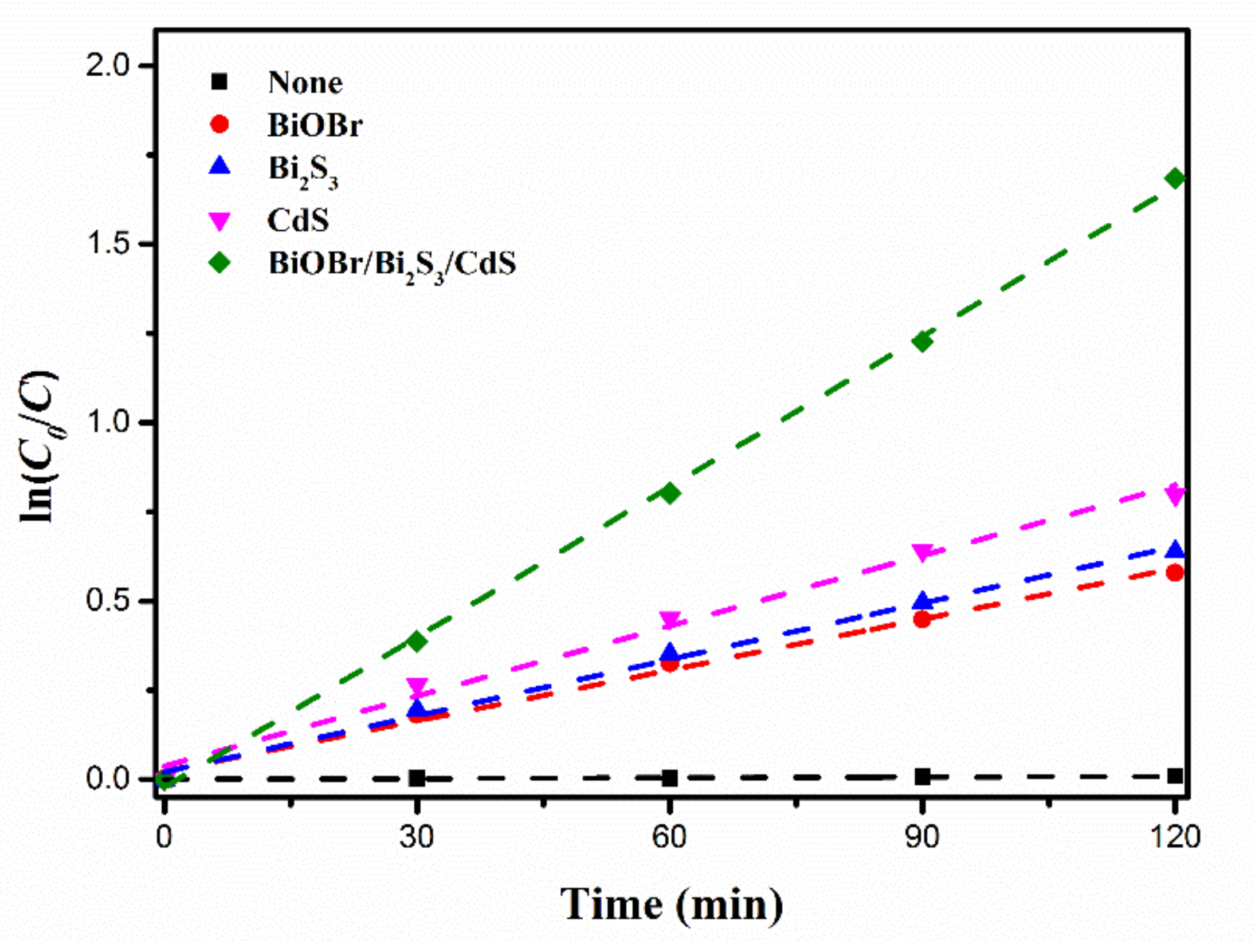
| Catalyst | k (min−1) | (R2) |
|---|---|---|
| BiOBr | 0.00475 | 0.9916 |
| Bi2S3 | 0.00526 | 0.9941 |
| CdS | 0.00656 | 0.9872 |
| BiOBr/Bi2S3/CdS | 0.01404 | 0.9987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Xing, Z.; Li, Y.; Han, J.; Lorenz, H.; Chen, J. Synthetic BiOBr/Bi2S3/CdS Crystalline Material and Its Degradation of Dye under Visible Light. Crystals 2021, 11, 899. https://doi.org/10.3390/cryst11080899
Jin Y, Xing Z, Li Y, Han J, Lorenz H, Chen J. Synthetic BiOBr/Bi2S3/CdS Crystalline Material and Its Degradation of Dye under Visible Light. Crystals. 2021; 11(8):899. https://doi.org/10.3390/cryst11080899
Chicago/Turabian StyleJin, Yunhan, Zhe Xing, Yinhui Li, Jian Han, Heike Lorenz, and Jianxin Chen. 2021. "Synthetic BiOBr/Bi2S3/CdS Crystalline Material and Its Degradation of Dye under Visible Light" Crystals 11, no. 8: 899. https://doi.org/10.3390/cryst11080899
APA StyleJin, Y., Xing, Z., Li, Y., Han, J., Lorenz, H., & Chen, J. (2021). Synthetic BiOBr/Bi2S3/CdS Crystalline Material and Its Degradation of Dye under Visible Light. Crystals, 11(8), 899. https://doi.org/10.3390/cryst11080899










