Effects of Expansive Additives on the Shrinkage Behavior of Coal Gangue Based Alkali Activated Materials
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Mixture Proportions
2.3. Testing Methods
3. Results and Discussion
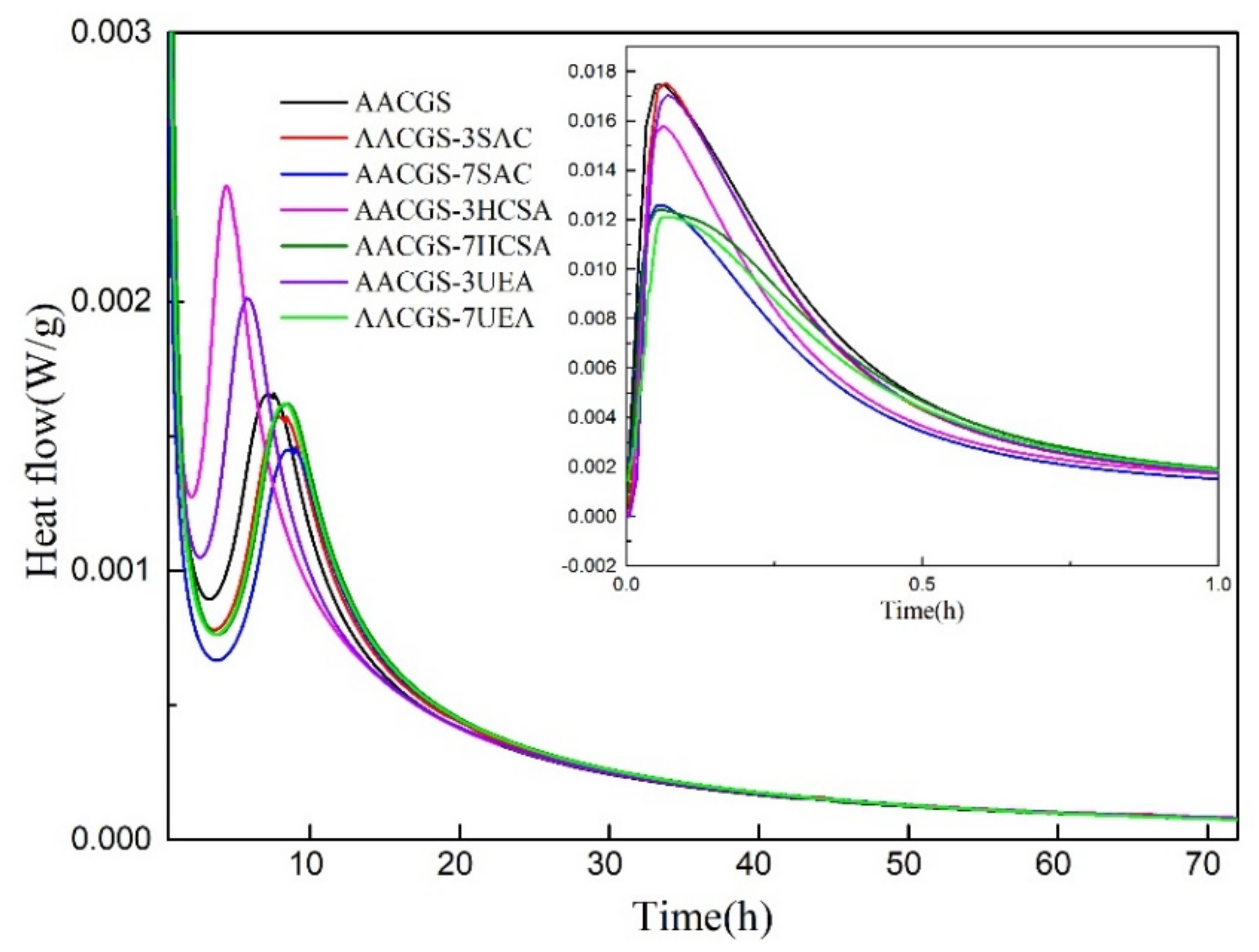
3.1. Reaction Heat Release
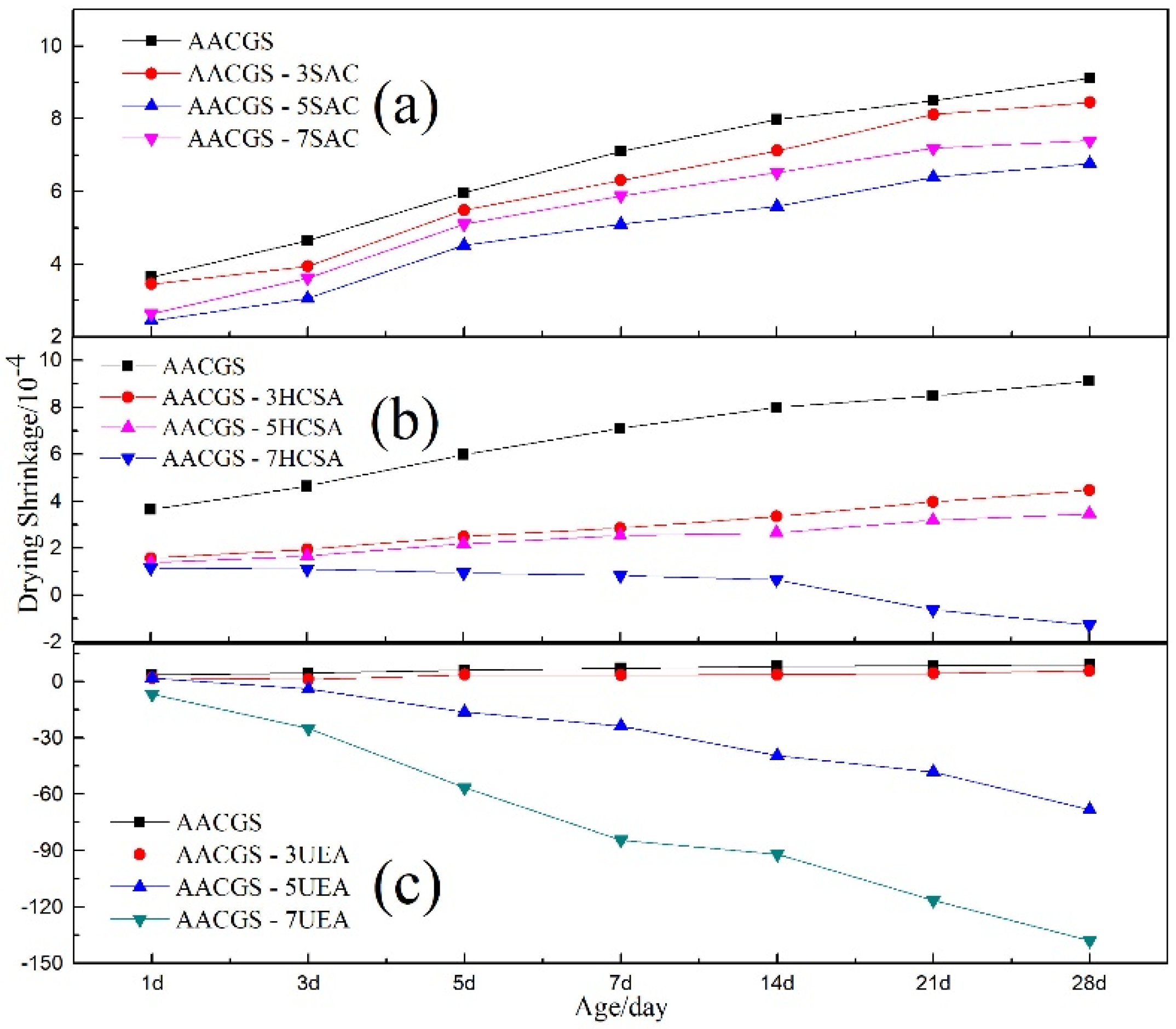
3.2. Drying Shrinkage
3.3. Thermogravimetry Analysis
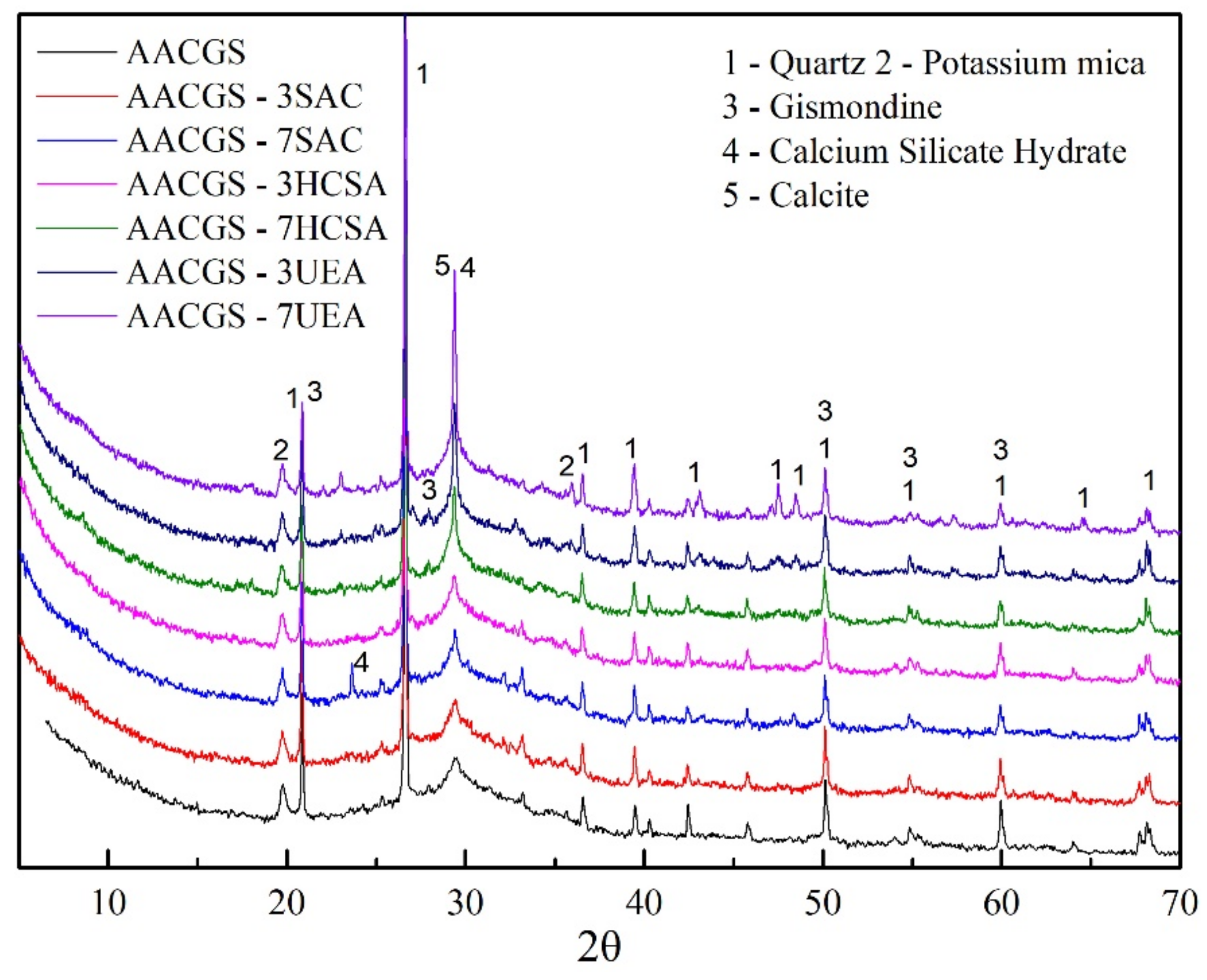
3.4. X-ray Diffraction Analysis
3.5. Brunner−Emmet−Teller (BET) Analysis
3.6. Compressive Strength
4. Conclusions
- In alkali activated binding system, the addition of SAC inhibits the early stage reaction to some extent. Incorporating HCSA and UEA promotes the reaction with a relatively low dosage of 3%, while an opposite effect is shown with a higher replacement of 7%.
- All types of applied additives are able to mitigate the drying shrinkage of alkali activated coal gangue-slag composite, but excessive dosage of HCSA and UEA would result in expansion. The pore characteristics of the hardened matrix are well related with their shrinkage behavior.
- Addition of SAC brings hydrotalcite phases within the reaction products, while HCSA and UEA addition mainly introduces CaCO3 and Ca(OH)2. Additionally formed phases are not observed from the XRD analysis due to their relatively low dosage and poor crystalline nature under the condition of alkali activation.
- Applying SAC benefits the compressive strength in general, and an optimum dosage around 5% is suggested. The compressive strength is very sensitive to HCSA and UEA dosage; higher replacements would result in dramatic strength reduction due to expansion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.J.; Yan, X.P. Experimental study on the durability of the concrete with coal gangue aggregate. J. China Coal Soc. 2013, 38, 1215–1219. [Google Scholar]
- Wang, X.; Zhong, N.; Han, X. Impacts of coal gangue stockpiling on polycyclic aromatic hydrocarbons pollution in soil environment. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2013, 33, 3092–3100. [Google Scholar]
- Lü, Q.; Dong, X.; Zhu, Z.; Dong, Y. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite. J. Hazard. Mater. 2014, 273, 136–145. [Google Scholar] [CrossRef]
- Chuncai, Z.; Guijian, L.; Dun, W.; Fang, T.; Wang, R.; Xiang, F. Mobility behavior and environmental implications of trace elements associated with coal gangue: A case study at the Huainan Coalfield in China. Chemosphere 2014, 95, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Fabiańska, M.J.; Ciesielczuk, J.; Kruszewski, Ł.; Misz-Kennan, M.; Blake, D.R.; Stracher, G.; Moszumańska, I. Gaseous compounds and efflorescences generated in self-heating coal-waste dumps—A case study from the Upper and Lower Silesian Coal Basins (Poland). Int. J. Coal Geol. 2013, 116, 247–261. [Google Scholar] [CrossRef]
- Ribeiro, J.; da Silva, E.F.; Flores, D. Burning of coal waste piles from Douro Coalfield (Portugal): Petrological, geochemical and mineralogical characterization. Int. J. Coal Geol. 2010, 81, 359–372. [Google Scholar] [CrossRef]
- Aiqin, L.; Shaoping, K.; Hua, D. The discussion of comprehensive utilization of coal gangue. China Resour. Compr. Util. 2004, 2, 11–14. [Google Scholar]
- Haibin, L.; Zhenling, L. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar] [CrossRef]
- Li, D.; Song, X.; Gong, C.; Pan, Z. Research on cementitious behavior and mechanism of pozzolanic cement with coal gangue. Cem. Concr. Res. 2006, 36, 1752–1759. [Google Scholar] [CrossRef]
- Song, Q.; Yu, R.; Shui, Z.; Wang, Y. Physical and chemical coupling effect of metakaolin induced chloride trapping capacity variation for Ultra High Performance Fibre Reinforced Concrete (UHPFRC). Constr. Build. Mater. 2019, 223, 765–774. [Google Scholar] [CrossRef]
- Wang, Y.; Shui, Z.; Gao, X.; Huang, Y.; Yu, R.; Ling, G. Chloride binding behaviors of metakaolin-lime hydrated blends: Influence of gypsum and atmospheric carbonation. Constr. Build. Mater. 2019, 201, 380–390. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, J.; Yu, L.; Xu, J.; Jiao, K.; Wang, Y. Effect of Activated Coal Gangue in North China on the Compressive Strength and Hydration Process of Cement. J. Mater. Civ. Eng. 2019, 31, 04019022. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, C.; Palomo, A.; Deventer, S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline activation of metakaolin: Effect of calcium hydroxide in the products of reaction. J. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+ Ca) and metakaolin (Si+ Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Allahverdi, A.; Skvara, F. Sulfuric acid attack on hardened paste of geopolymer cements-Part 1. Mechanism of corrosion at relatively high concentrations. Ceram. Silik. 2005, 49, 225. [Google Scholar]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Firouz, M.; Illikainen, M. Drying shrinkage in alkali-activated binders–a critical review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Cartwright, C.; Rajabipour, F.; Radlińska, A. Shrinkage characteristics of alkali-activated slag cements. J. Mater. Civ. Eng. 2014, 27, B4014007. [Google Scholar] [CrossRef]
- Thomas, R.J.; Lezama, D.; Peethamparan, S. On drying shrinkage in alkali-activated concrete: Improving dimensional stability by aging or heat-curing. Cem. Concr. Res. 2017, 91, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Kheradmand, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Shrinkage performance of fly ash alkali-activated cement based binder mortars. KSCE J. Civ. Eng. 2018, 22, 1854–1864. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.Q.; Chen, H.Y.; Zhu, H.G.; Shi, Y.Y.; Ni, Y.D.; Huo, Q.J.; Hang, Z.T. Study on the drying shrinkage of alkali-activated coal gangue-slag mortar and its mechanisms. Constr. Build. Mater. 2019, 225, 204–213. [Google Scholar]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Effect of admixtures on properties of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1367–1374. [Google Scholar] [CrossRef]
- Yip, C.K.; Provis, J.L.; Lukey, G.C.; Deventer, J. Carbonate mineral addition to metakaolin-based geopolymers. Cem. Concr. Compos. 2008, 30, 979–985. [Google Scholar] [CrossRef]
- Güneyisi, E.; Gesoğlu, M.; Mermerdaş, K. Improving strength, drying shrinkage, and pore structure of concrete using metakaolin. Mater. Struct. 2008, 41, 937–949. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Strength and drying shrinkage of slag paste activated by sodium carbonate and reactive MgO. Constr. Build. Mater. 2015, 81, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.L.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Yang, L.Y.; Jia, Z.J.; Zhang, Y.M.; Dai, J.G. Effects of nano-TiO2 on strength, shrinkage and microstructure of alkali activated slag pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Injorhor, B.; Phoo-ngernkham, T.; Damrongwiriyanupap, N.; Li, L.Y.; Sukontasukkul, P.; Chindaprasirt, P. Drying shrinkage, strength and microstructure of alkali-activated high-calcium fly ash using FGD-gypsum and dolomite as expansive additive. Cem. Concr. Compos. 2020, 114, 103760. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Alonso, S.; Palomo, A. Calorimetric study of alkaline activation of calcium hydroxide–metakaolin solid mixtures. Cem. Concr. Res. 2001, 31, 25–30. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Mohammed, M.S.; Alomayri, T. Single and dual effects of magnesia and alumina nano-particles on strength and drying shrinkage of alkali activated slag. Constr. Build. Mater. 2019, 228, 116827. [Google Scholar] [CrossRef]
- Liska, M. Properties and Applications of Reactive Magnesia Cements in Porous Blocks; University of Cambridge: Cambridge, UK, 2010. [Google Scholar]
- Abdel-Gawwad, H.A.; Heikal, M.; Mohammed, M.S.; El-Aleem, S.; Hassan, H.S.; Garcia, S.R.V.; Rashad, A.M. Evaluating the impact of nano-magnesium calcite waste on the performance of cement mortar in normal and sulfate-rich media. Constr. Build. Mater. 2019, 203, 392–400. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Khalil, K.A. Application of thermal treatment on cement kiln dust and feldspar to create one-part geopolymer cement. Constr. Build. Mater. 2018, 187, 231–237. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; El-Enein, S.A.A.; Heikal, M.; El-Aleem, S.; Amer, A.A.; El-Kattan, I.M. Synergistic effects of curing conditions and magnesium oxide addition on the physico-mechanical properties and firing resistivity of Portland cement mortar. Constr. Build. Mater. 2018, 176, 676–689. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different additives on the properties of alkali-activated slag–A guide for Civil Engineer. Constr. Build. Mater. 2013, 47, 29–55. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Nie, L.L.; Sun, S.B.; Yao, X.D.; Tian, Y.L. Study on the properties of ordinary portland cement and fast hardening sulphoaluminate cement mortar. Concr. Cem. Prod. 2014, 3, 10–13. [Google Scholar]
- Zhao, S.; Li, C.; Jia, F.; Wu, X.N. Study on self healing of shrinkage cracks in HCSA shrinkage compensating concrete. Expans. Agent Expans. Concrete 2017, 3, 1–4. [Google Scholar]


| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | TiO2 | SrO | L.O.I | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C G | 53.11 | 26.43 | 2.88 | 0.77 | 0.44 | 0.52 | 0.34 | 0.34 | 0.79 | 0 | 13.14 |
| GBFS | 32.4 | 15.1 | 0.46 | 41.23 | 6.61 | 2.18 | 0.48 | 0.27 | 0.84 | 0 | 0.22 |
| SAC | 6.19 | 19.35 | 7.72 | 43.31 | 1.24 | 14.58 | 0.14 | 0.12 | 1.11 | 0.20 | 6.04 |
| HCSA | 2.89 | 3.40 | 0.76 | 68.89 | 2.11 | 18.76 | 0.26 | 0.05 | 0.15 | 0.26 | 2.47 |
| UEA | 5.33 | 2.35 | 0.74 | 71.59 | 4.18 | 10.38 | 0.31 | 0.05 | 0.14 | 0.13 | 4.80 |
| Sample ID | CG | GFBS | SAC | HCSA | UEA | Na2O | Activator Modulus | W/B Ratio | B/S Ratio |
|---|---|---|---|---|---|---|---|---|---|
| AACGS | 40 | 60 | \ | \ | \ | 8 | 1.6 | 0.4 | 0.5 |
| AACGS-3SAC | 38.5 | 58.5 | 3 | \ | \ | ||||
| AACGS-5SAC | 37.5 | 57.5 | 5 | \ | \ | ||||
| AACGS-7SAC | 36.5 | 56.5 | 7 | \ | \ | ||||
| AACGS-3HCSA | 38.5 | 58.5 | \ | 3 | \ | ||||
| AACGS-5HCSA | 37.5 | 57.5 | \ | 5 | \ | ||||
| AACGS-7HCSA | 36.5 | 56.5 | \ | 7 | \ | ||||
| AACGS-3UEA | 38.5 | 58.5 | \ | \ | 3 | ||||
| AACGS-5UEA | 37.5 | 57.5 | \ | \ | 5 | ||||
| AACGS-7UEA | 36.5 | 56.5 | \ | \ | 7 |
| Mix | Mass Loss (%) | |||
|---|---|---|---|---|
| H2O (40–200 °C) | Hydrotalcite (200–400 °C) | Ca(OH)2 (400–600 °C) | CaCO3 (600–800 °C) | |
| AACGS | 12.01% | 3.08% | 3.29% | 1.02% |
| AACGS-3SAC | 14.09% | 3.19% | 3.11% | 0.79% |
| AACGS-7SAC | 14.48% | 3.13% | 2.80% | 0.65% |
| AACGS-3HCSA | 14.65% | 3.35% | 3.17% | 0.90% |
| AACGS-7HCSA | 18.59% | 3.83% | 3.97% | 3.19% |
| AACGS-3UEA | 13.66% | 3.26% | 5.19% | 4.18% |
| AACGS-7UEA | 17.66% | 3.66% | 4.32% | 6.78% |
| Group | Specific Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|
| AACGS | 33.40 | 7.54 |
| AACGS-3SAC | 36.39 | 8.04 |
| AACGS-7SAC | 36.59 | 8.08 |
| AACGS-3HCSA | 35.35 | 8.11 |
| AACGS-7HCSA | 35.38 | 8.16 |
| AACGS-3UEA | 48.06 | 8.15 |
| AACGS-7UEA | 61.67 | 8.85 |
| Group | Compressive Strength (MPa) | ||
|---|---|---|---|
| 3 d/MPa | 7 d/MPa | 28 d/MPa | |
| AACGS | 39.4 | 42.3 | 51.7 |
| AACGS-3SAC | 44.4 | 48.2 | 56.3 |
| AACGS-5SAC | 43.9 | 49.4 | 63.1 |
| AACGS-7SAC | 42.8 | 46.5 | 60.6 |
| AACGS-3HCSA | 36.2 | 37.3 | 45.9 |
| AACGS-5HCSA | 39.8 | 21.8 | 16.6 |
| AACGS-7HCSA | 24.7 | 15.6 | 11.6 |
| AACGS-3UEA | 32.3 | 43.6 | 20.6 |
| AACGS-5UEA | 29.1 | 36.5 | 9.3 |
| AACGS-7UEA | 25.2 | 21.8 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Liu, C.; Shui, Z.; Yu, R. Effects of Expansive Additives on the Shrinkage Behavior of Coal Gangue Based Alkali Activated Materials. Crystals 2021, 11, 816. https://doi.org/10.3390/cryst11070816
Gao X, Liu C, Shui Z, Yu R. Effects of Expansive Additives on the Shrinkage Behavior of Coal Gangue Based Alkali Activated Materials. Crystals. 2021; 11(7):816. https://doi.org/10.3390/cryst11070816
Chicago/Turabian StyleGao, Xu, Chao Liu, Zhonghe Shui, and Rui Yu. 2021. "Effects of Expansive Additives on the Shrinkage Behavior of Coal Gangue Based Alkali Activated Materials" Crystals 11, no. 7: 816. https://doi.org/10.3390/cryst11070816
APA StyleGao, X., Liu, C., Shui, Z., & Yu, R. (2021). Effects of Expansive Additives on the Shrinkage Behavior of Coal Gangue Based Alkali Activated Materials. Crystals, 11(7), 816. https://doi.org/10.3390/cryst11070816







