The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. X-ray Structure Determination
2.3. Computational Details
3. Results and Discussion
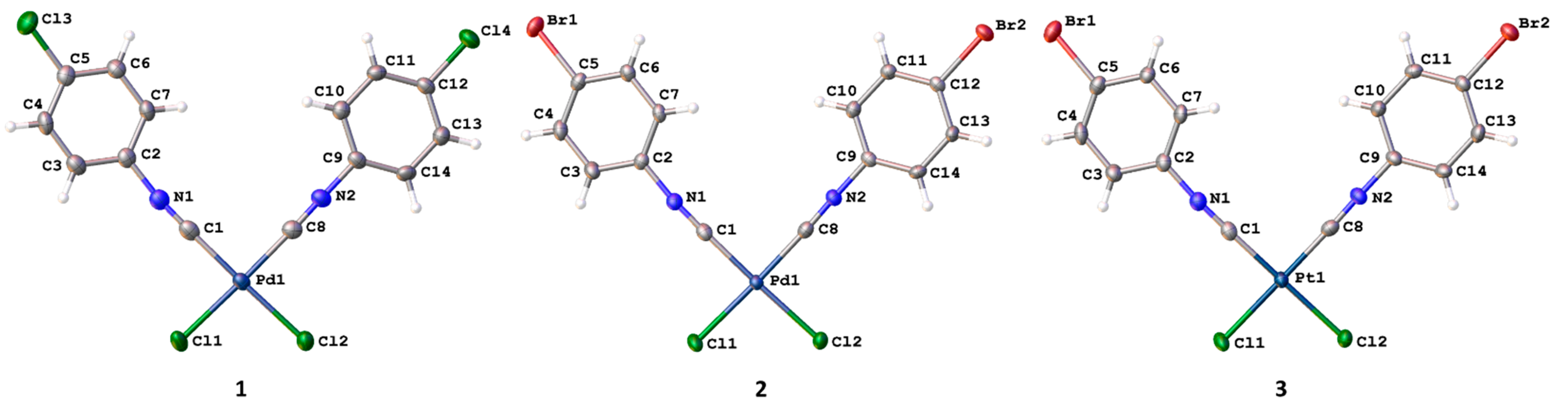
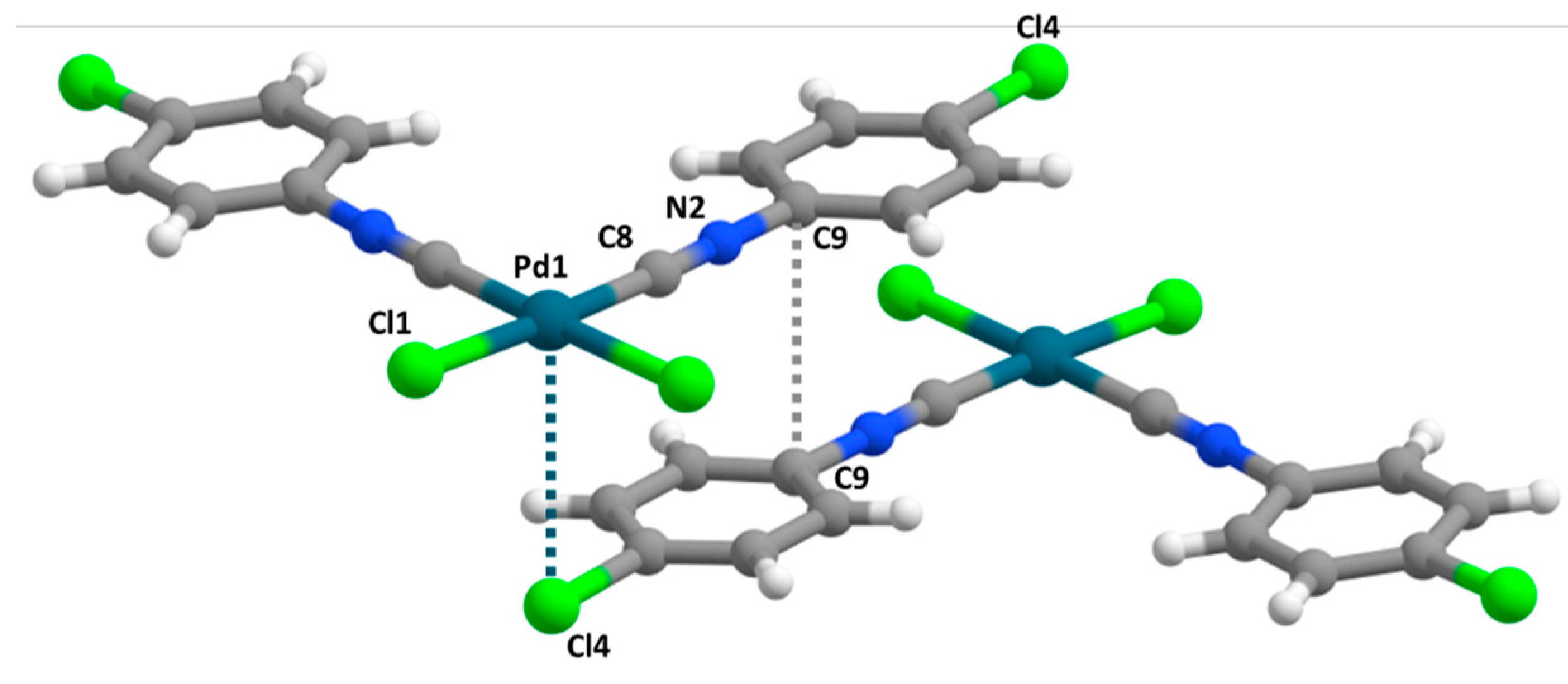
3.1. General Description of the X-ray Structures
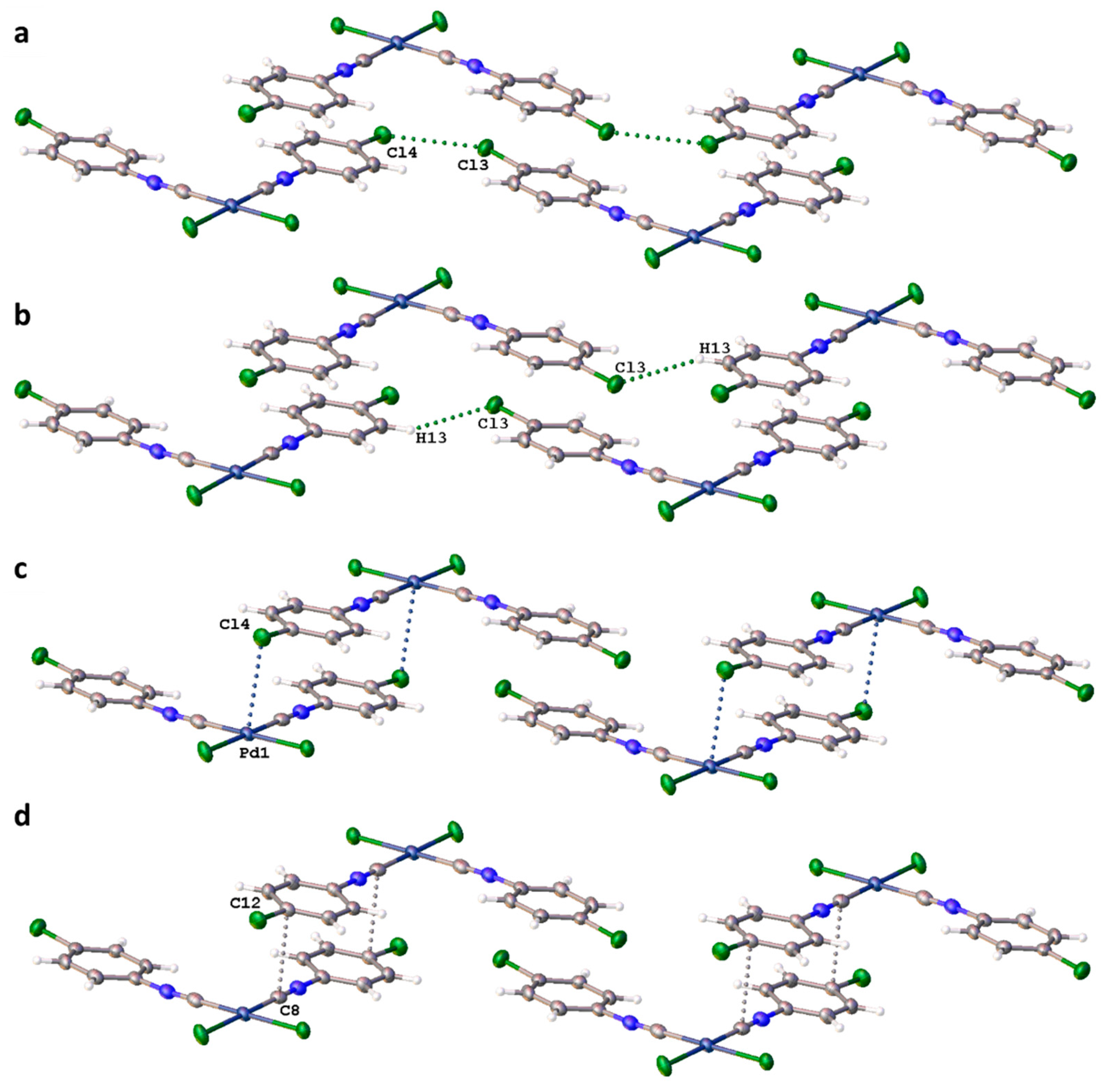
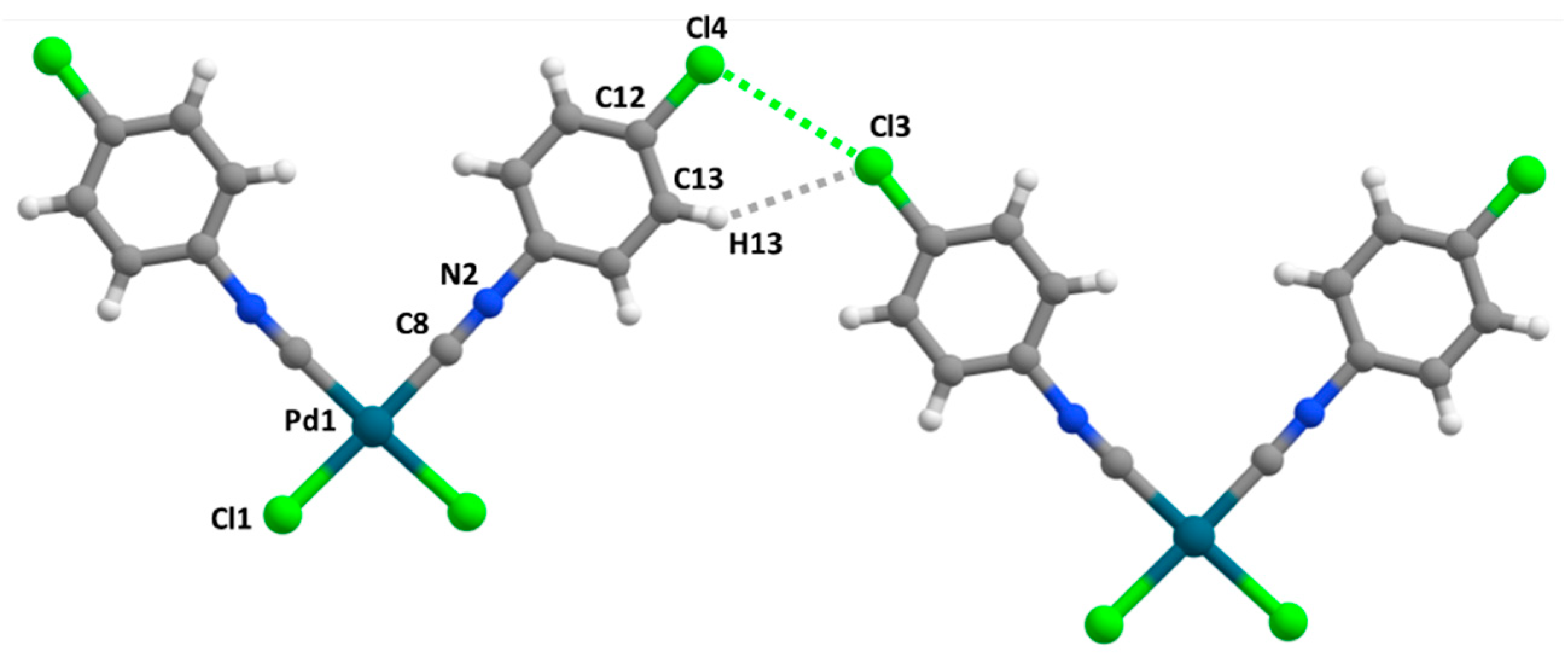
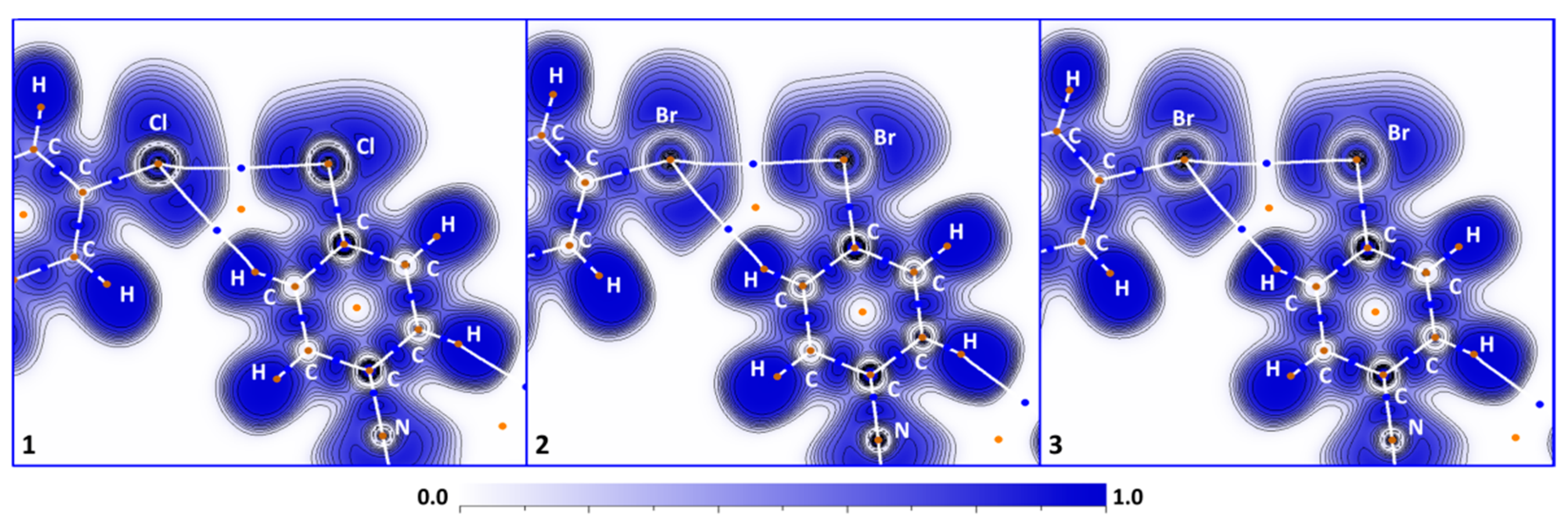
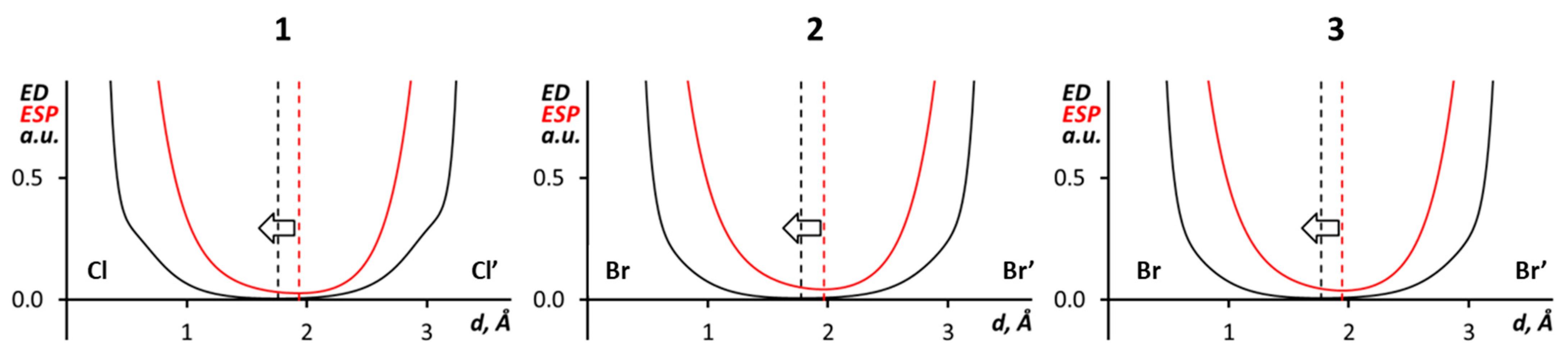
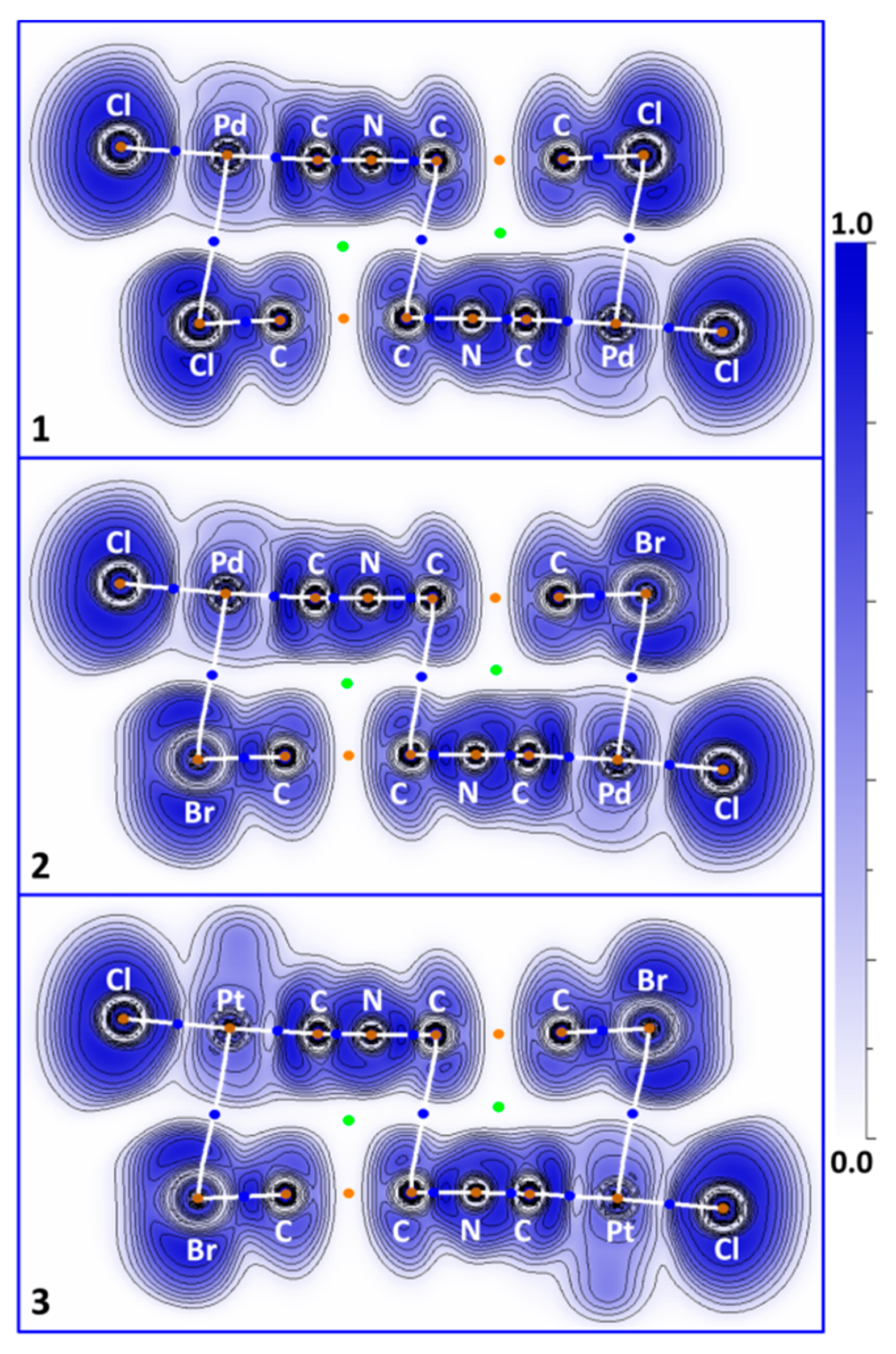
3.2. C–X···X’–C Halogen Bonding
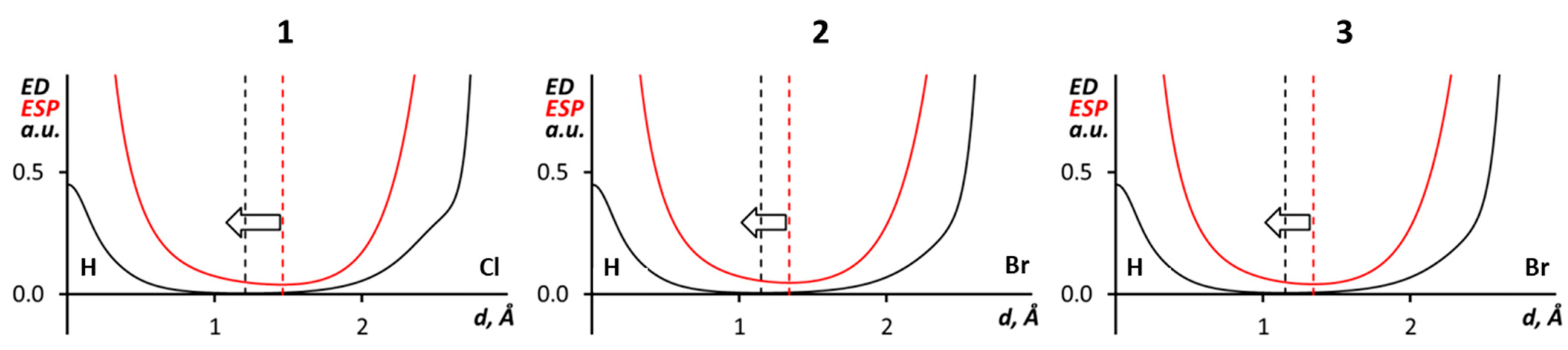
3.3. C–H···X–C Hydrogen Bonding
3.4. M···X Semicoordination
3.5. C···C’ Contacts Involving CNR Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusi, M. Engineering Crystal Properties through Solid Solutions. Cryst. Growth Des. 2018, 18, 3704–3712. [Google Scholar] [CrossRef] [Green Version]
- Lusi, M. A rough guide to molecular solid solutions: Design, synthesis and characterization of mixed crystals. CrystEngComm 2018, 20, 7042–7052. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef]
- Adolf, C.R.R.; Ferlay, S.; Hosseini, M.W. Molecular tectonics: Control of crystalline sequences. CrystEngComm 2018, 20, 2233–2236. [Google Scholar] [CrossRef]
- Nemec, V.; Lisac, K.; Bedeković, N.; Fotović, L.; Stilinović, V.; Cinčić, D. Crystal engineering strategies towards halogen-bonded metal–organic multi-component solids: Salts, cocrystals and salt cocrystals. CrystEngComm 2021, 23, 3063–3083. [Google Scholar] [CrossRef]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Turunen, L.; Hansen, J.H.; Erdelyi, M. Halogen Bonding: An Odd Chemistry? Chem. Rec. 2021, 21, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.J. The halogen bond: Nature and applications. Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.Q.; Wang, L.Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef] [PubMed]
- Shixaliyev, N.Q.; Gurbanov, A.V.; Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Martins, L.M.D.R.S.; Muzalevskiy, V.M.; Nenajdenko, V.G.; Pombeiro, A.J.L. Halogen-bonded tris(2,4-bis(trichloromethyl)-1,3,5-triazapentadienato)-M(III) [M = Mn, Fe, Co] complexes and their catalytic activity in the peroxidative oxidation of 1-phenylethanol to acetophenone. New J. Chem. 2014, 38, 4807–4815. [Google Scholar] [CrossRef]
- Zubkov, F.I.; Mertsalov, D.F.; Zaytsev, V.P.; Varlamov, A.V.; Gurbanov, A.V.; Dorovatovskii, P.V.; Timofeeva, T.V.; Khrustalev, V.N.; Mahmudov, K.T. Halogen bonding in Wagner-Meerwein rearrangement products. J. Mol. Liq. 2018, 249, 949–952. [Google Scholar] [CrossRef]
- Asgarova, A.R.; Khalilov, A.N.; Brito, I.; Maharramov, A.M.; Shikhaliyev, N.G.; Cisterna, J.; Cardenas, A.; Gurbanov, A.V.; Zubkov, F.I.; Mahmudov, K.T. Hydrogen and halogen bonding in the haloetherification products in chalcone. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kaasik, M.; Kanger, T. Supramolecular Halogen Bonds in Asymmetric Catalysis. Front. Chem. 2020, 8, 64. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically Enhanced Solubility of Halide-Containing Organometallic Species in Diiodomethane: The Role of Solvent⋅⋅⋅Complex Halogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef] [PubMed]
- von der Heiden, D.; Vanderkooy, A.; Erdélyi, M. Halogen bonding in solution: NMR spectroscopic approaches. Coord. Chem. Rev. 2020, 407, 213147. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Isostructural Materials Achieved by Using Structurally Equivalent Donors and Acceptors in Halogen-Bonded Cocrystals. Chem. Eur. J. 2008, 14, 747–753. [Google Scholar] [CrossRef]
- Hutchins, K.M.; Kummer, K.A.; Groeneman, R.H.; Reinheimer, E.W.; Sinnwell, M.A.; Swenson, D.C.; MacGillivray, L.R. Thermal expansion properties of three isostructural co-crystals composed of isosteric components: Interplay between halogen and hydrogen bonds. CrystEngComm 2016, 18, 8354–8357. [Google Scholar] [CrossRef]
- Jones, R.H.; Knight, K.S.; Marshall, W.G.; Clews, J.; Darton, R.J.; Pyatt, D.; Coles, S.J.; Horton, P.N. Colossal thermal expansion and negative thermal expansion in simple halogen bonded complexes. CrystEngComm 2014, 16, 237–243. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Saha, B.K. The effect of temperature on interhalogen interactions in a series of isostructural organic systems. New J. Chem. 2014, 38, 897–901. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Saha, B.K. A thermal expansion investigation of the melting point anomaly in trihalomesitylenes. Chem. Commun. 2015, 51, 9829–9832. [Google Scholar] [CrossRef] [Green Version]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen Contacts-Induced Unusual Coloring in BiIII Bromide Complex: Anion-to-Cation Charge Transfer via Br⋅⋅⋅Br Interactions. Chem. Eur. J. 2017, 23, 15612–15616. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Yushina, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with halopyridinium cations: Appearance or non-appearance of unusual colouring. CrystEngComm 2018, 20, 7766–7772. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Shikhaliyev, N.Q.; Suleymanova, G.T.; Gurbanov, A.V.; Babayeva, G.V.; Mammadova, G.Z.; Zubkov, F.I.; Nenajdenko, V.G.; Mahmudov, K.T.; Pombeiro, A.J.L. Pnicogen, halogen and hydrogen bonds in (E)-1-(2,2-dichloro-1-(2-nitrophenyl)vinyl)-2-(para-substituted phenyl)-diazenes. Dyes Pigm. 2018, 159, 135–141. [Google Scholar] [CrossRef]
- Shikhaliyev, N.Q.; Ahmadova, N.E.; Gurbanov, A.V.; Maharramov, A.M.; Mammadova, G.Z.; Nenajdenko, V.G.; Zubkov, F.I.; Mahmudov, K.T.; Pombeiro, A.J.L. Tetrel, halogen and hydrogen bonds in bis(4-((E)-(2,2-dichloro-1-(4-substitutedphenyl)vinyl)diazenyl)phenyl)methane dyes. Dyes Pigm. 2018, 150, 377–381. [Google Scholar] [CrossRef]
- Shikhaliyev, N.Q.; Kuznetsov, M.L.; Maharramov, A.M.; Gurbanov, A.V.; Ahmadova, N.E.; Nenajdenko, V.G.; Mahmudov, K.T.; Pombeiro, A.J.L. Noncovalent interactions in the design of bis-azo dyes. CrystEngComm 2019, 21, 5032–5038. [Google Scholar] [CrossRef]
- Sivchik, V.V.; Solomatina, A.I.; Chen, Y.T.; Karttunen, A.J.; Tunik, S.P.; Chou, P.T.; Koshevoy, I.O. Halogen Bonding to Amplify Luminescence: A Case Study Using a Platinum Cyclometalated Complex. Angew. Chem. Int. Ed. 2015, 54, 14057–14060. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Jin, W.J. Halogen bonding in room-temperature phosphorescent materials. Coord. Chem. Rev. 2020, 404, 213107. [Google Scholar] [CrossRef]
- Bhowal, R.; Biswas, S.; Adiyeri Saseendran, D.P.; Koner, A.L.; Chopra, D. Tuning the solid-state emission by co-crystallization through σ- and π-hole directed intermolecular interactions. CrystEngComm 2019, 21, 1940–1947. [Google Scholar] [CrossRef]
- Xiao, L.; Fu, H. Enhanced Room-Temperature Phosphorescence through Intermolecular Halogen/Hydrogen Bonding. Chem. Eur. J. 2019, 25, 714–723. [Google Scholar] [CrossRef]
- Maity, S.K.; Bera, S.; Paikar, A.; Pramanik, A.; Haldar, D. Halogen bond induced phosphorescence of capped γ-amino acid in the solid state. Chem. Commun. 2013, 49, 9051–9053. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wu, Y.; Yu, Z.; Xu, Z.; Li, J.; Liu, Y.; Yao, J.; Fu, H. Room-Temperature Phosphorescence in Pure Organic Materials: Halogen Bonding Switching Effects. Chem. Eur. J. 2018, 24, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Shi, H.; Tian, D.; Ma, H.; Cheng, Z.; Wu, Q.; Gu, M.; Huang, L.; An, Z.; Peng, Q.; et al. Enhancing Ultralong Organic Phosphorescence by Effective π-Type Halogen Bonding. Adv. Funct. Mater. 2018, 28, 1705045. [Google Scholar] [CrossRef]
- Sivchik, V.; Sarker, R.K.; Liu, Z.-Y.; Chung, K.-Y.; Grachova, E.V.; Karttunen, A.J.; Chou, P.-T.; Koshevoy, I.O. Improvement of the Photophysical Performance of Platinum-Cyclometalated Complexes in Halogen-Bonded Adducts. Chem. Eur. J. 2018, 24, 11475–11484. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Yan, D. Two-component molecular cocrystals of 9-acetylanthracene with highly tunable one-/two-photon fluorescence and aggregation induced emission. J. Mater. Chem. C 2016, 4, 2527–2534. [Google Scholar] [CrossRef]
- Fan, G.; Yan, D. Positional isomers of cyanostilbene: Two-component molecular assembly and multiple-stimuli responsive luminescence. Sci. Rep. 2014, 4, 4933. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Hu, R.X.; Pang, X.; Gao, H.Y.; Jin, W.J. The phosphorescent co-crystals of 1,4-diiodotetrafluorobenzene and bent 3-ring-N-heterocyclic hydrocarbons by C-I center dot center dot center dot N and C-I center dot center dot center dot pi halogen bonds. CrystEngComm 2014, 16, 7942–7948. [Google Scholar] [CrossRef]
- Yan, D.; Evans, D.G. Molecular crystalline materials with tunable luminescent properties: From polymorphs to multi-component solids. Mater. Horiz. 2014, 1, 46–57. [Google Scholar] [CrossRef]
- Varughese, S. Non-covalent routes to tune the optical properties of molecular materials. J. Mater. Chem. C 2014, 2, 3499–3516. [Google Scholar] [CrossRef]
- Katkova, S.A.; Luzyanin, K.V.; Novikov, A.S.; Kinzhalov, M.A. Modulation of luminescence properties for [cyclometalated]-PtII(isocyanide) complexes upon co-crystallisation with halosubstituted perfluorinated arenes. New J. Chem. 2021, 45, 2948–2952. [Google Scholar] [CrossRef]
- Brown, A.; Beer, P.D. Halogen bonding anion recognition. Chem. Commun. 2016, 52, 8645–8658. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Sansotera, M.; Terraneo, G. Halogen bonding: A general route in anion recognition and coordination. Chem. Soc. Rev. 2010, 39, 3772–3783. [Google Scholar] [CrossRef]
- Ho, P.S. Biomolecular Halogen Bonds. In Halogen Bonding I: Impact on Materials Chemistry and Life Sciences; Metrangolo, P., Resnati, G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–276. [Google Scholar]
- Amjad, A.; Clemente-Juan, J.M.; Coronado, E.; Luis, F.; Evangelisti, M.; Espallargas, G.M.; del Barco, E. Tunable crossover between one- and three-dimensional magnetic dynamics in Co(II) single-chain magnets organized by halogen bonding. Phys. Rev. B 2016, 93, 224418. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Su, D.; Wuest, J.D. Use of hydrogen bonds to control molecular aggregation. Self-assembly of three-dimensional networks with large chambers. J. Am. Chem. Soc. 1991, 113, 4696–4698. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Saccone, M.; Catalano, L. Halogen Bonding beyond Crystals in Materials Science. J. Phys. Chem. B 2019, 123, 9281–9290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen Bonding in Two-Dimensional Crystal Engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Kashina, M.V.; Kinzhalov, M.A.; Smirnov, A.S.; Ivanov, D.M.; Novikov, A.S.; Kukushkin, V.Y. Dihalomethanes as Bent Bifunctional XB/XB-Donating Building Blocks for Construction of Metal-involving Halogen Bonded Hexagons. Chem. Asian J. 2019, 14, 3915–3920. [Google Scholar] [CrossRef] [PubMed]
- Kryukova, M.A.; Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Smirnov, A.S.; Bokach, N.A.; Kukushkin, V.Y. Four-Center Nodes: Supramolecular Synthons Based on Cyclic Halogen Bonding. Chem. Eur. J. 2019, 25, 13671–13675. [Google Scholar] [CrossRef]
- Buldakov, A.V.; Kinzhalov, M.A.; Kryukova, M.A.; Ivanov, D.M.; Novikov, A.S.; Smirnov, A.S.; Starova, G.L.; Bokach, N.A.; Kukushkin, V.Y. Isomorphous Series of PdII-Containing Halogen Bond Donors Exhibiting Cl/Br/I Triple Halogen Isostructural Exchange. Cryst. Growth Des. 2020, 3, 1975–1984. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. JACS Au 2021. [Google Scholar] [CrossRef]
- Efimenko, Z.M.; Eliseeva, A.A.; Ivanov, D.M.; Galmés, B.; Frontera, A.; Bokach, N.A.; Kukushkin, V.Y. Bifurcated μ2-I···(N,O) Halogen Bonding: The Case of (Nitrosoguanidinate)NiII Cocrystals with Iodine(I)-Based σ-Hole Donors. Cryst. Growth Des. 2021, 21, 588–596. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Novikov, A.S.; Rozhkov, A.V.; Kornyakov, I.V.; Dubovtsev, A.Y.; Kukushkin, V.Y. Hexaiododiplatinate(II) as a useful supramolecular synthon for halogen bond involving crystal engineering. Dalton Trans. 2020, 49, 356–367. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2008, 42, 339–341. [Google Scholar] [CrossRef]
- Agilent, C. CrysAlis PRO.; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Barros, C.L.; de Oliveira, P.J.P.; Jorge, F.E.; Canal Neto, A.; Campos, M. Gaussian basis set of double zeta quality for atoms Rb through Xe: Application in non-relativistic and relativistic calculations of atomic and molecular properties. Mol. Phys. 2010, 108, 1965–1972. [Google Scholar] [CrossRef]
- de Berrêdo, R.C.; Jorge, F.E. All-electron double zeta basis sets for platinum: Estimating scalar relativistic effects on platinum(II) anticancer drugs. J. Mol. Struct. 2010, 961, 107–112. [Google Scholar] [CrossRef]
- Jorge, F.E.; Canal Neto, A.; Camiletti, G.G.; Machado, S.F. Contracted Gaussian basis sets for Douglas–Kroll–Hess calculations: Estimating scalar relativistic effects of some atomic and molecular properties. J. Chem. Phys. 2009, 130, 064108. [Google Scholar] [CrossRef]
- Canal Neto, A.; Jorge, F.E. All-electron double zeta basis sets for the most fifth-row atoms: Application in DFT spectroscopic constant calculations. Chem. Phys. Lett. 2013, 582, 158–162. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Mata, I.; Molins, E.; Alkorta, I.; Espinosa, E. Topological Properties of the Electrostatic Potential in Weak and Moderate N···H Hydrogen Bonds. J. Phys. Chem. A 2007, 111, 6425–6433. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zhurko, G.A. Chemcraft 1.8—Graphical Software for Visualization of Quantum Chemistry Computations; Ivanovo, Russia, 2005. Available online: https://scholar.google.com/scholar_lookup?hl=en&publication_year=2004&author=G.+A.+Zhurko&author=D.+A.+Zhurko&title=Chemcraft+-+Graphical+Program+for+Visualization+of+Quantum+Chemistry+Computations (accessed on 7 July 2021).
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Katkova, S.A.; Suslonov, V.V. Synthesis of Platinum(II) Phoshine Isocyanide Complexes and Study of Their Stability in Isomerization and Ligand Disproportionation Reactions. Russ. J. Gen. Chem. 2018, 88, 1180–1187. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Zolotarev, A.A.; Boyarskiy, V.P. Crystal structure of cis-[PdCl2(CNMes)2]. J. Struct. Chem. 2016, 57, 822–825. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Luzyanin, K.V.; Boyarskaya, I.A.; Starova, G.L.; Boyarskiy, V.P. Synthetic and structural investigation of [PdBr2(CNR)2] (R = Cy, Xyl). J. Mol. Struct. 2014, 1068, 222–227. [Google Scholar] [CrossRef]
- Vicente, J.; Arcas, A.; Fernández-Hernández, J.M.; Aullón, G.; Bautista, D. Acetonyl Platinum(II) Complexes. Organometallics 2007, 26, 6155–6169. [Google Scholar] [CrossRef]
- Sluch, I.M.; Miranda, A.J.; Elbjeirami, O.; Omary, M.A.; Slaughter, L.M. Interplay of Metallophilic Interactions, π–π Stacking, and Ligand Substituent Effects in the Structures and Luminescence Properties of Neutral PtII and PdII Aryl Isocyanide Complexes. Inorg. Chem. 2012, 51, 10728–10746. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Nguyen-Dang, T.T. Quantum Theory of Atoms in Molecules–Dalton Revisited. In Advances in Quantum Chemistry; Löwdin, P.-O., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 14, pp. 63–124. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Bartashevich, E.; Yushina, I.; Kropotina, K.; Muhitdinova, S.; Tsirelson, V. Testing the tools for revealing and characterizing the iodine-iodine halogen bond in crystals. Struct. Sci. Cryst. Eng. Mater. 2017, B73, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Matveychuk, Y.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Bifurcated Halogen Bonding C–Br···η2(Cl–Pt). Cryst. Growth Des. 2019, 19, 1364–1376. [Google Scholar] [CrossRef]
- Bulatova, M.; Ivanov, D.M.; Haukka, M. Classics Meet Classics: Theoretical and Experimental Studies of Halogen Bonding in Adducts of Platinum(II) 1,5-Cyclooctadiene Halide Complexes with Diiodine, Iodoform, and 1,4-Diiodotetrafluorobenzene. Cryst. Growth Des. 2021, 21, 974–987. [Google Scholar] [CrossRef]
- Lamberts, K.; Handels, P.; Englert, U.; Aubert, E.; Espinosa, E. Stabilization of polyiodide chains via anion⋯anion interactions: Experiment and theory. CrystEngComm 2016, 18, 3832–3841. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic–Nucleophilic Dualism of Nickel(II) toward Ni···I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
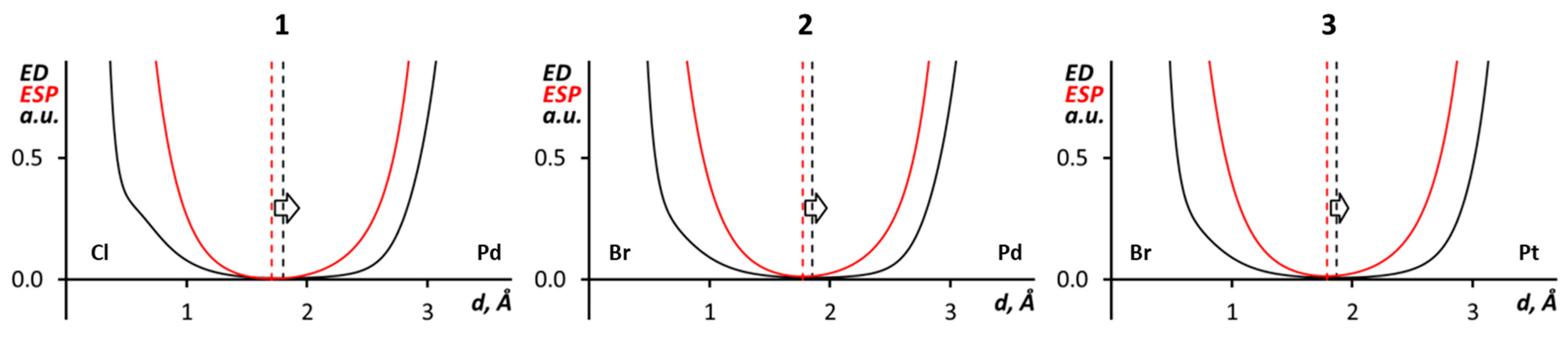
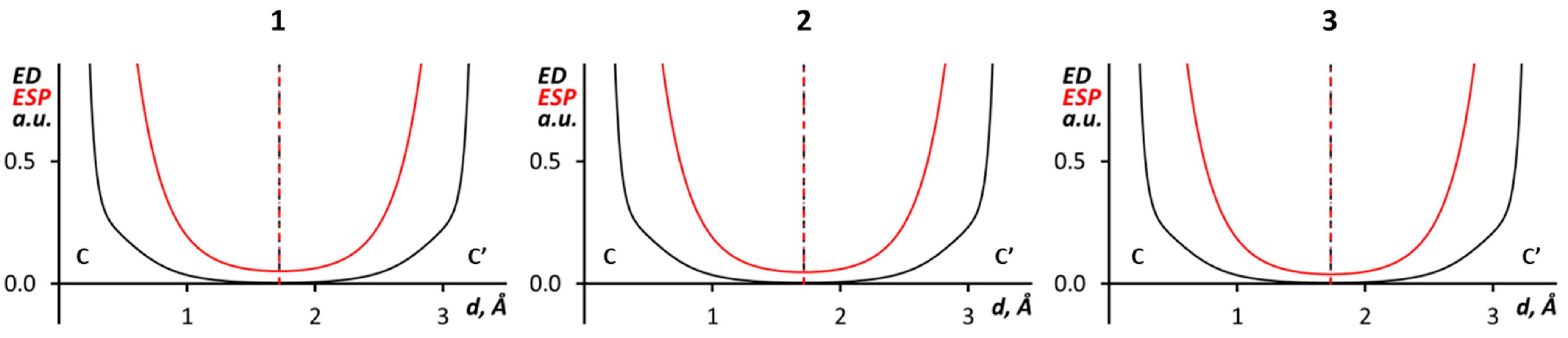
| Structure | d(X···X’), Å | ∠(C–X···X’), ° | ∠(X···X’–C), ° | R |
|---|---|---|---|---|
| 1 | 3.5933(13) | 155.78(11) | 101.05(11) | 1.03 1/1.01 2 |
| 2 | 3.6923(5) | 162.69(9) | 97.01(9) | 0.99 1 |
| 3 | 3.6759(9) | 161.87(17) | 96.87(16) | 0.99 1 |
| Cluster | Interaction | Sign(λ2)ρ | V(r) | G(r) |
|---|---|---|---|---|
| (1)2 | Cl···Cl’ | −0.006 | −0.003 | 0.004 |
| (2)2 | Br···Br’ | −0.007 | −0.004 | 0.004 |
| (3)2 | Br···Br’ | −0.007 | −0.004 | 0.004 |
| (1)2 | H···Cl | −0.004 | −0.002 | 0.003 |
| (2)2 | H···Br | −0.006 | −0.003 | 0.004 |
| (3)2 | H···Br | −0.006 | −0.003 | 0.004 |
| (1)2 | Pd···Cl | −0.007 | −0.004 | 0.004 |
| (2)2 | Pd···Br | −0.009 | −0.005 | 0.005 |
| (3)2 | Pd···Br | −0.008 | −0.004 | 0.004 |
| (1)2 | C···C’ | −0.005 | −0.002 | 0.003 |
| (2)2 | C···C’ | −0.005 | −0.002 | 0.003 |
| (3)2 | C···C’ | −0.005 | −0.002 | 0.003 |
| Structure | d(H···X), Å | d(C(H)···X), Å | ∠(C–H···X), ° | R 1 | R 2 |
|---|---|---|---|---|---|
| 1 | 3.0615(9) | 3.891(3) | 146.87(17) | 1.04 | 1.07 |
| 2 | 3.0447(3) | 3.906(3) | 159.13(18) | 1.03 | 1.06 |
| 3 | 3.04776(11) | 3.896(7) | 149.6(4) | 1.03 | 1.07 |
| Structure | d(X···M), Å | ∠(M···X and M Coordination Plane), ° | ∠(C–X···M), ° | R 1 | R 2 |
|---|---|---|---|---|---|
| 1 | 3.6687(9) | 79.12(2) | 77.93(11) | 1.09 | 0.92 |
| 2 | 3.6450(4) | 80.950(19) | 78.35(10) | 1.05 | 0.91 |
| 3 | 3.7445(6) | 79.76(4) | 76.68(16) | 1.07 | 0.90 |
| Structure | d(C···C’), Å | R |
|---|---|---|
| 1 | 3.430(6) | 0.97 |
| 2 | 3.497(5) | 0.99 |
| 3 | 3.454(11) | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashina, M.V.; Ivanov, D.M.; Kinzhalov, M.A. The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds. Crystals 2021, 11, 799. https://doi.org/10.3390/cryst11070799
Kashina MV, Ivanov DM, Kinzhalov MA. The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds. Crystals. 2021; 11(7):799. https://doi.org/10.3390/cryst11070799
Chicago/Turabian StyleKashina, Maria V., Daniil M. Ivanov, and Mikhail A. Kinzhalov. 2021. "The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds" Crystals 11, no. 7: 799. https://doi.org/10.3390/cryst11070799
APA StyleKashina, M. V., Ivanov, D. M., & Kinzhalov, M. A. (2021). The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds. Crystals, 11(7), 799. https://doi.org/10.3390/cryst11070799






