Abstract
Two cadmium(II) coordination polymers, with thiocyanate and pyridine-based ligands e.g., 3-acetamidopyridine (3-Acpy) and niazid (nicotinic acid hydrazide, nia), namely one-dimensional {[Cd(SCN)2(3-Acpy)]}n (1) and two-dimensional {[Cd(SCN)2(nia)]}n (2), are prepared in the mixture of water and ethanol. The adjacent cadmium(II) ions in 1 are bridged by two N,S-thiocyanate ions and an N,O-bridging 3-Acpy molecule, forming infinite one-dimensional polymeric chains, which are assembled by the intermolecular N–H∙∙∙S hydrogen bonds in one direction and by the intermolecular S∙∙∙S chalcogen bonds in another direction. Within the coordination network of 2, the adjacent cadmium(II) ions are bridged by N,S-thiocyanate ions in one direction and by N,O,N’-chelating and bridging nia molecules in another direction. The coordination networks of 2 are assembled by the intermolecular N–H∙∙∙S and N–H∙∙∙N hydrogen bonds and S∙∙∙S chalcogen bonds. Being the only supramolecular interactions responsible for assembling the polymer chains of 1 in the particular direction, the chalcogen S∙∙∙S bonds are more significant in the structure of 1, whilst the chalcogen S∙∙∙S bonds which act in cooperation with the N–H∙∙∙S and N–H∙∙∙N hydrogen bonds are of less significance in the structure of 2.
1. Introduction
Although not yet as established and studied as hydrogen and halogen bonding, chalcogen bonding (ChB) has recently gained more attention as an important supramolecular interaction with possible applications in biochemistry, crystal engineering, the design of new materials, and catalysis [1,2,3,4,5]. The chalcogen bond (D–Ch∙∙∙A) is a non-covalent interaction between a chalcogen bond donor D and a chalcogen bond acceptor A. Chalcogen bond donor D is a Lewis acid and acceptor A is a Lewis base, while Ch is a chalcogen atom (of group 16). The chalcogen bonds belong to the group of σ-hole interactions. The σ-hole interactions, particularly halogen bonds, have become more popular in the last two decades, particularly due to their strength and high directionality. Nowadays, however, chalcogen bonds attract more attention, though their nature and mechanism of formation still remain unclear [1,5,6]. In the case of the chalcogen bonds, the interactions are formed between a positive (electrophilic) region on the chalcogen atom and a negative electron density (nucleophilic region) on the chalcogen bond acceptor [1,4]. The anisotropy in the surface electrostatic potential on chalcogen atoms is crucial for the formation of chalcogen bonds, which become more evident when the radius of chalcogen atoms increases. The main characteristic of the chalcogen bonds, as opposed to hydrogen bonds, is their directional nature, which is a consequence of the aforementioned anisotropy [7]. The strength of the chalcogen bonds seems to be dependent on the electron-accepting capability of the respective chalcogen molecule. Although understood from the crystal structures of various chalcogenated compounds for some time, the chalcogen bonds did not gain desired importance and become relevant supramolecular interactions in crystal engineering, in the way that halogen bonds did [4,6].
Niazid (nia, nicotinic acid hydrazide), equipped with an N,O-donor set of atoms, could be a useful ligand for the design of coordination polymers of various dimensionalities due to its ability to chelately bind to metal ions via hydrazide N and carbonyl O atoms and, as well as bridge via pyridine N atom [8]. However, in spite of having such the potential to make coordination polymers, only a few coordination polymers with the described coordination mode of nia have been reported, e.g., coordination polymers of nickel(II) [9], lead(II) [10], and cadmium(II) [11]. Three different cadmium(II) coordination polymers of nia have been prepared and described, depending on the metal-to-ligand molar ratios and the solvents used [11]. Although 3-acetamidopyridine (3-Acpy) is structurally similar to nia, also containing the N,O-set of atoms as possible donors, it lacks bridging and chelating potential due to the spatial arrangement of N and O atoms. Indeed, only a few metal complexes of 3-Acpy are known, each of them containing 3-Acpy ligand coordinated in an N-monodentate fashion (via pyridine N atom) [12,13].
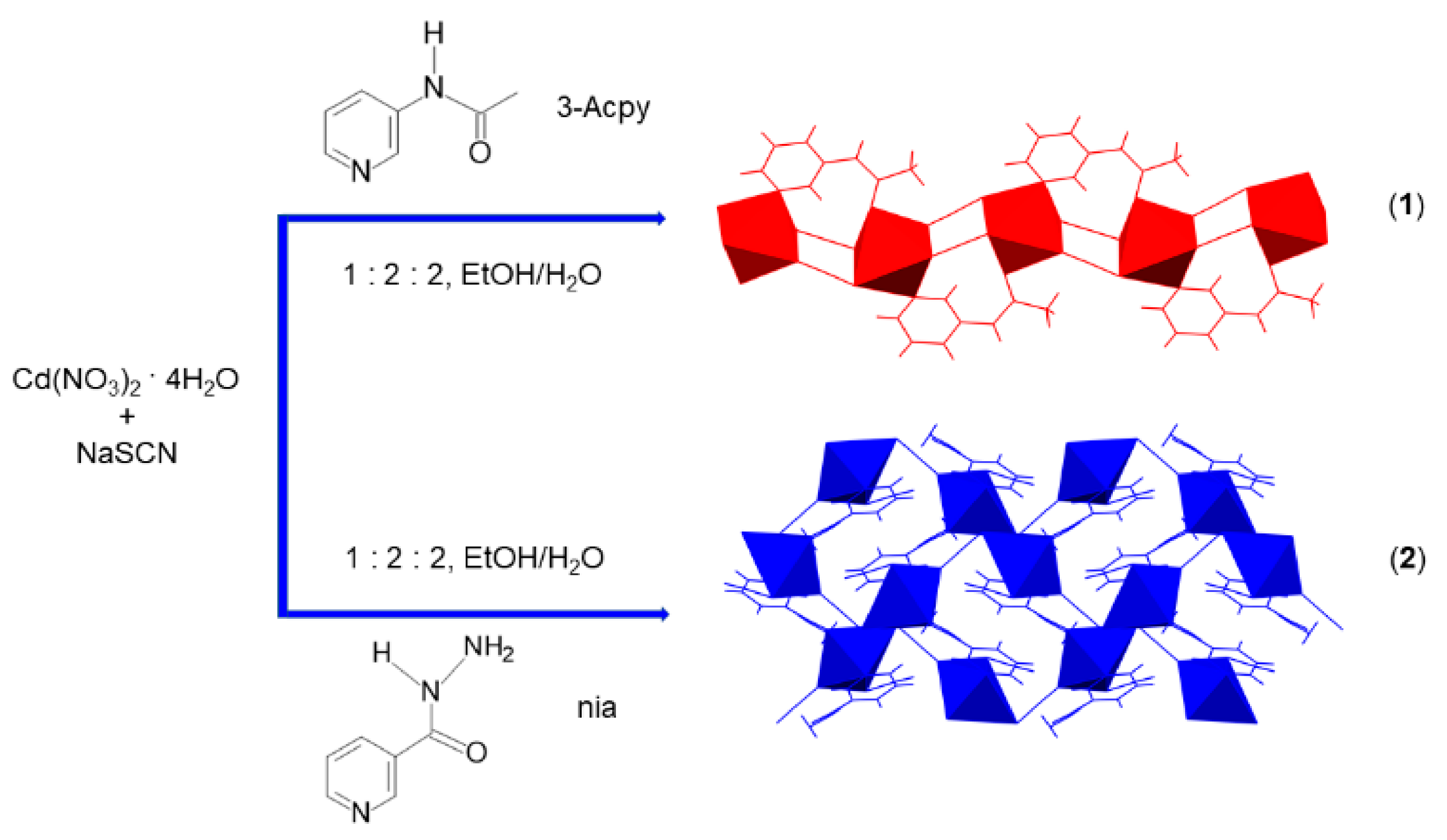
We wanted to explore the possibilities of delivering more diverse cadmium(II) coordination polymers by using mixed ligands—organic pyridine-based ligands (nia or 3-Acpy) and highly versatile thiocyanate ion as an inorganic linker. The thiocyanate ion is able to coordinate to the same metal ion via both N and S atoms, enabling its bridging capacity. Additionally, we wanted to examine if the introduction of the thiocyanate could increase the dimensionality of the cadmium(II) coordination polymer formed, especially when combined with chelating and bridging nia, as opposed to the combination with monodentate 3-Acpy. However our main purpose was to explore the potential for linking metal–organic building units via chalcogen bonds upon introduction of the sulfur-containing ligand that might enable the formation of the chalcogen S∙∙∙S bonding in the structures of the prepared cadmium(II) coordination polymers. In the construction of coordination polymers, we opted for a cadmium(II) ion due to its highly unpredictable and unreliable coordination environment, as this would increase our chances of obtaining more diverse coordination polymers. In line with these guidelines, we prepared a one-dimensional (1-D) cadmium(II) coordination polymer with 3-Acpy and thiocyanate and two-dimensional (2-D) cadmium(II) coordination polymer with nia and thiocyanate under the same experimental conditions (Scheme 1), namely {[Cd(SCN)2(3-Acpy)]}n (1) and {[Cd(SCN)2(nia)]}n (2). We then specifically checked if the chalcogen S∙∙∙S bonds appeared in the structures of 1 and 2. Furthermore, we determined their role and significance in the supramolecular assemblies of the discussed cadmium(II) coordination polymers.
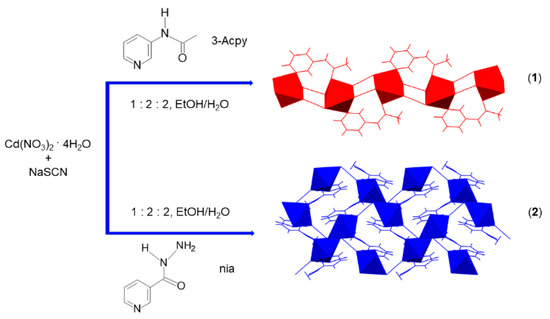
Scheme 1.
Preparation of one-dimensional {[Cd(SCN)2(3-Acpy)]}n (1) and two-dimensional {[Cd(SCN)2(nia)]}n (2) coordination polymers by the reaction of cadmium(II) nitrate, sodium thiocyanate and 3-acetamidopyridine (3-Acpy) or niazid (nia), respectively.
2. Results and Discussion
Cadmium(II) coordination polymers in this study were obtained by using 3-acetamidopyridine (3-Acpy) and niazide (nia) ligands. The reaction of the freshly prepared crystalline product 3-Acpy with cadmium(II) nitrate tetrahydrate and sodium thiocyanate (in a molar ratio 2:1:2) from the water–ethanol mixture yielded a 1-D coordination polymer {[Cd(SCN)2(3-Acpy)]}n (1) (Scheme 1). The 2-D coordination polymer {[Cd(SCN)2(nia)]}n (2) was also obtained by a reaction of nia with cadmium(II) nitrate tetrahydrate and sodium thiocyanate (in a molar ratio 2:1:2) from the water-ethanol mixture (Scheme 1).
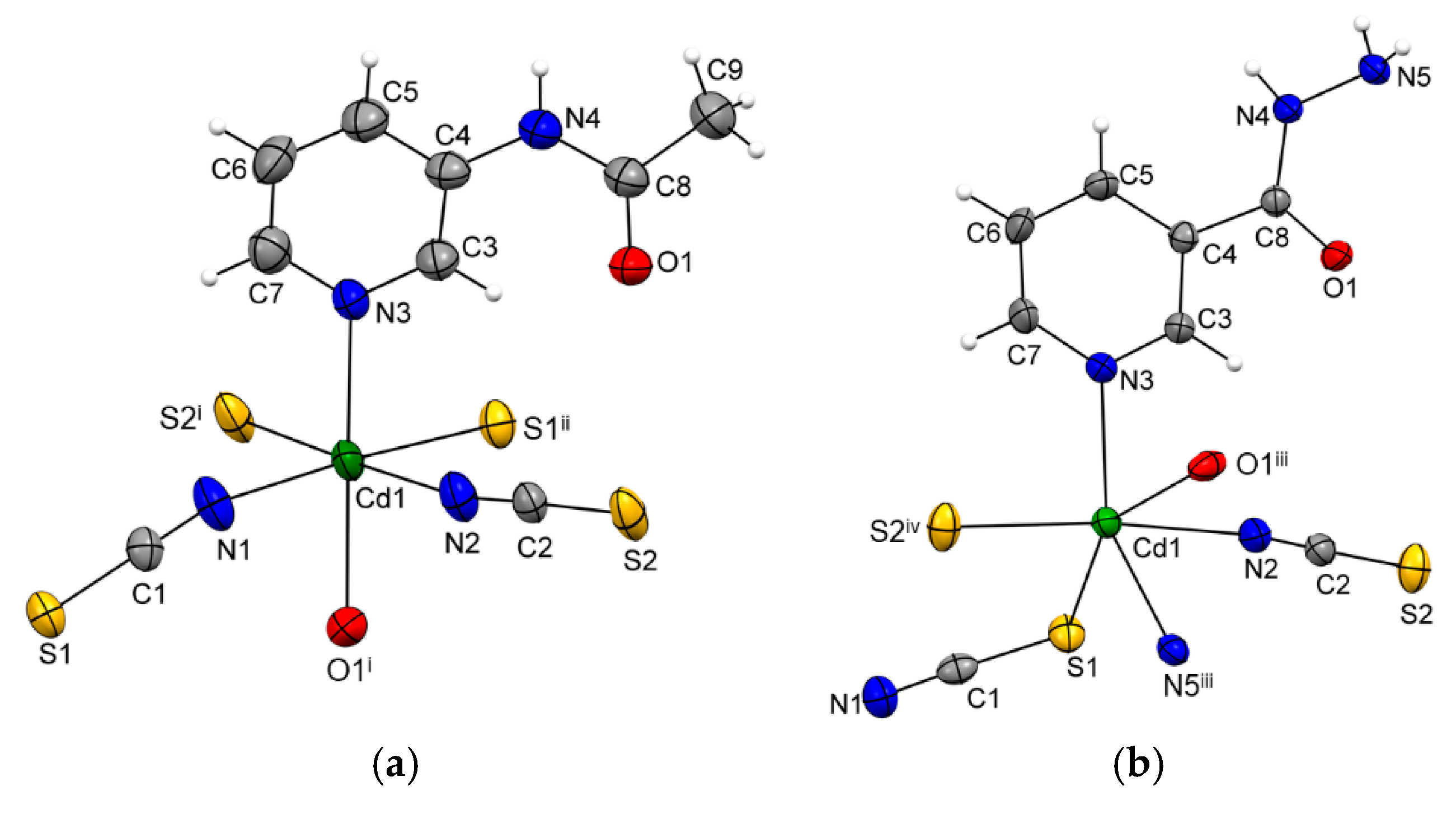
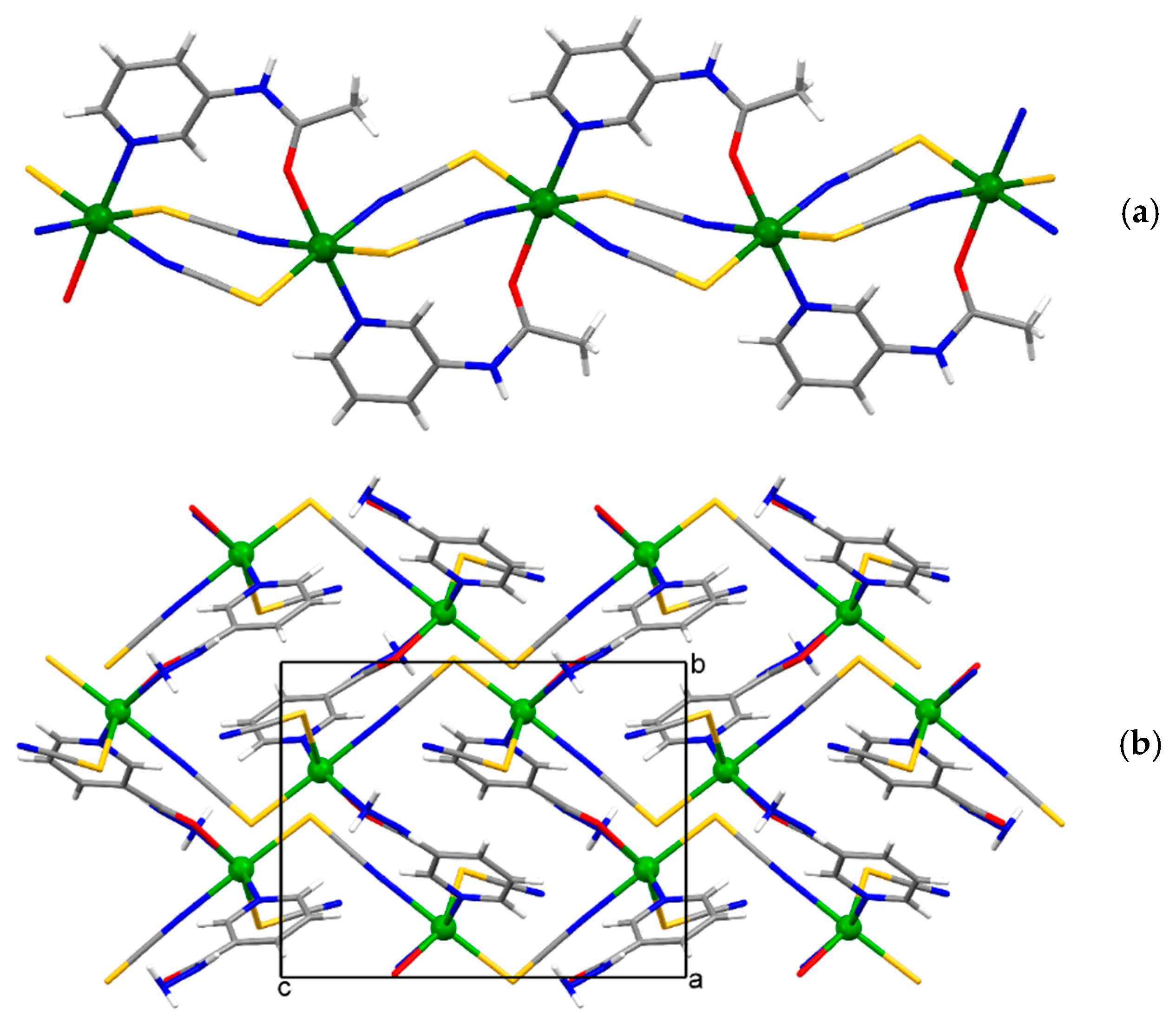
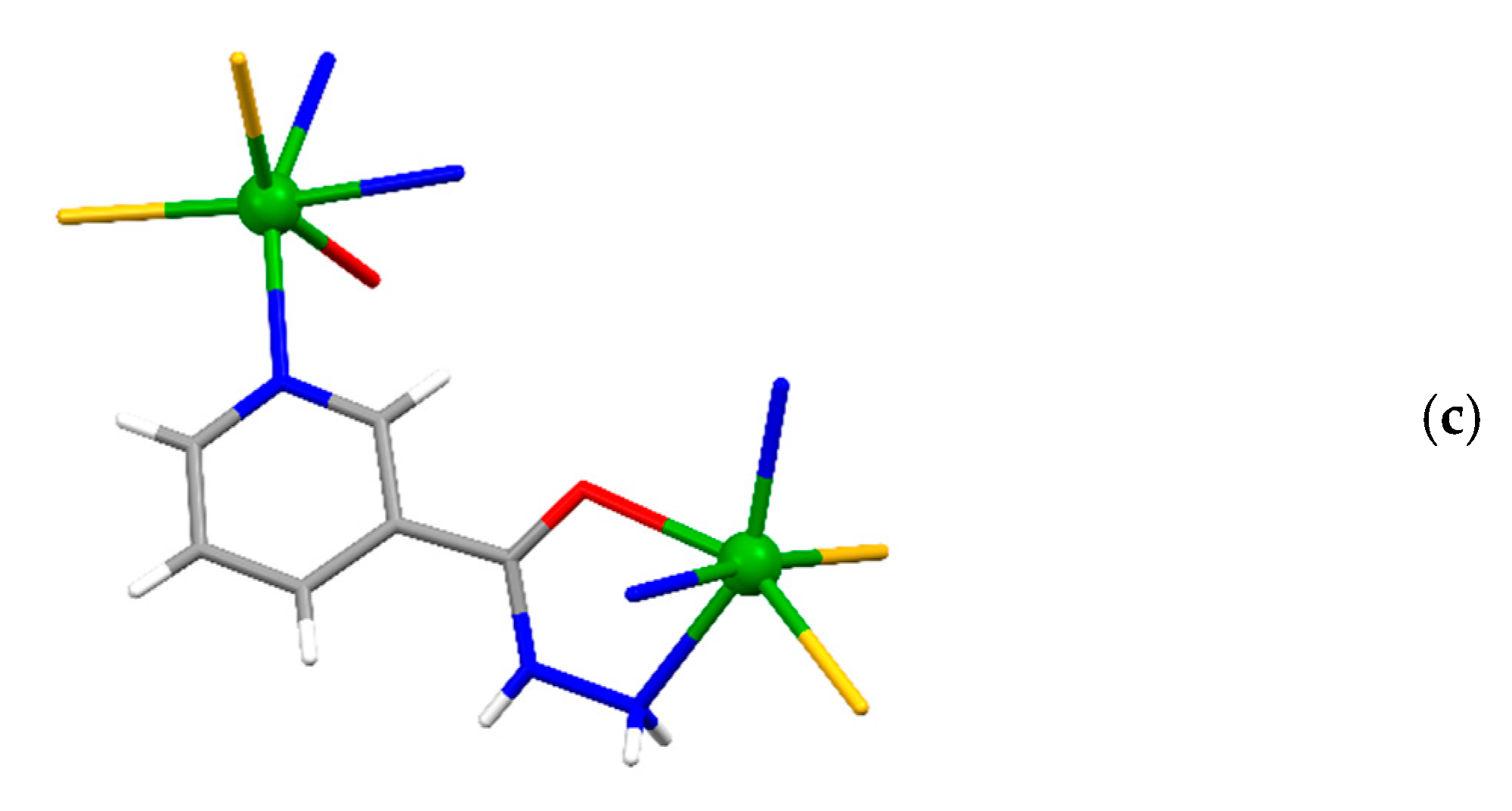
The asymmetric unit of {[Cd(SCN)2(3-Acpy)]}n (1) consists of a cadmium(II) ion, two thiocyanate ions, and one 3-Acpy molecule. The cadmium(II) ion is octahedrally coordinated with two thiocyanate N1 and N2 atoms and two thiocyanate S1ii and S2i atoms in the equatorial plane, whilst 3-Acpy pyridine N3 atom and carbonyl O1 atom are bound in the axial positions (N3−Cd1−O1i = 175.2(1)°; symmetry codes (i) −x + 1, y + 1/2, −z + 1/2; (ii) −x + 1, y − 1/2, −z + 1/2). The thiocyanate N1 and N2 atoms are bound in cis position (N2−Cd1−N1 = 94.8(1)°), as are the thiocyanate S1ii and S2i atoms (S2i −Cd1−S1ii = 83.81(3)°; Figure 1a and Table 1). The adjacent cadmium(II) ions in 1 are bridged by two N,S-thiocyanate ions in an antiparallel fashion and by an N,O-bridging 3-Acpy molecule, forming an infinite 1-D polymeric chain extending in the [0 1 0] direction (Figure 2a). The asymmetric unit of {[Cd(SCN)2(nia)]}n (2) also consists of a cadmium(II) ion, two thiocyanate ions and one nia molecule. The cadmium(II) ion is octahedrally coordinated with the discrete thiocyanate S1 atom and nia pyridine N3, hydrazide N5iii and carbonyl O1iii atoms in the equatorial plane, whilst the bridging thiocyanate N2 and S2iv atoms are bound in the axial positions (N2−Cd1−S2iv = 174.22(7)°; symmetry codes (iii) −x + 1, y + 1/2, −z + 1/2; (iv) x, −y + 3/2, z + 1/2; Figure 1b and Table 1). The cadmium(II) ions in 2 are bridged with N,S-thiocyanate ions and N,O,N’-nia molecules into an infinite 2-D network extending parallel to the (1 0 0) plane (Figure 2b). Within the coordination network, the adjacent cadmium(II) ions are bridged in two different ways: by N,S-thiocyanate ions (a single thiocyanate ion per two neighboring cadmium(II) ions) in the [0 0 1] direction and by N,O,N’-nia molecules in the [0 1 0] direction (Figure 2b). Each N,O,N’-nia molecule acts as a chelating and bridging ligand (via hydrazide N5 and carbonyl O1 atoms, forming a five-membered chelate ring Cd1/O1/C8/N4/N5, and via pyridine N3 atom) between two neighboring cadmium(II) ions (Figure 2c). The conformation of the mentioned chelate ring can best be described as an envelope on the Cd1 atom. The octahedral coordination environment around the cadmium(II) ion in 1 is only slightly distorted, as is evident from the angles for the trans (172.40(9)°–175.2(1)°) and cis (83.81(3)°–94.8(1)°) pairs of the ligating atoms (Table 1). However, the octahedral coordination environment around the cadmium(II) ion in 2 is highly distorted, as the angles for the trans (152.83(5)°–174.22(7)°) and cis (70.14(7)°–108.79(6)°) pairs of the ligating atoms indicate (Table 1). The reason for such a distortion is the N,O-bidentate binding of the nia ligand, leading to the formation of the five-membered chelate ring (Figure 2c) with the very small N5iii–Cd1–O1iii angle (70.14(7)°).
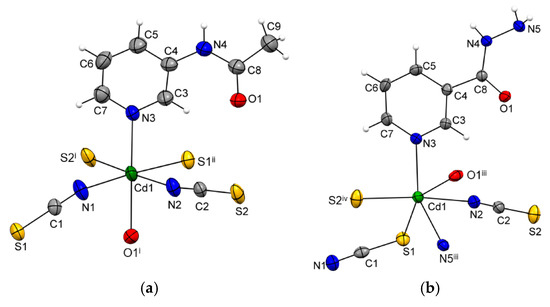
Figure 1.
ORTEP-style plots of {[Cd(SCN)2(3-Acpy)]}n (1) (a) and {[Cd(SCN)2(nia)]}n (2) (b), with the atomic numbering schemes. The thermal ellipsoids are drawn at the 40% probability level at 296(2) K and hydrogen atoms are shown as spheres of arbitrary radii (symmetry codes (i) −x + 1, y + 1/2, −z + 1/2; (ii) −x + 1, y − 1/2, -z + 1/2; (iii) −x + 1, y + 1/2, −z + 1/2; (iv) x, −y + 3/2, z + 1/2).

Table 1.
Selected bond lengths (Å) and angles (°) for {[Cd(SCN)2(3-Acpy)]}n (1) and {[Cd(SCN)2(nia)]}n (2).
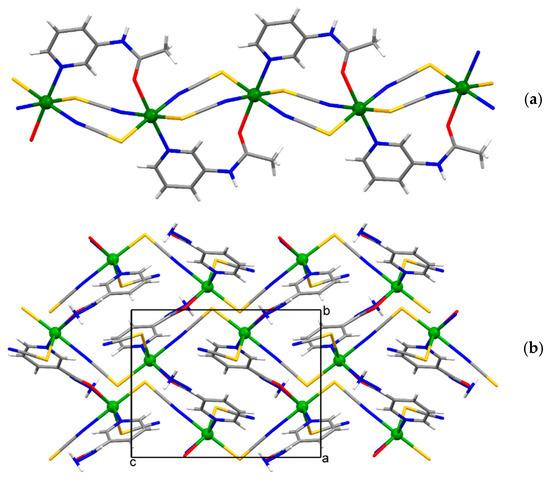
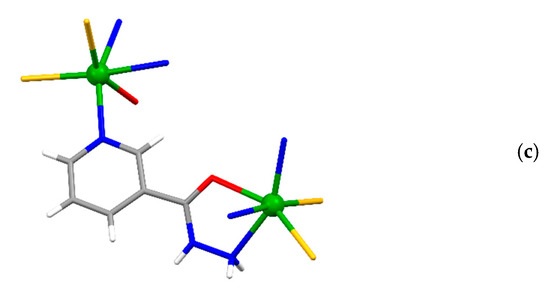
Figure 2.
The infinite 1-D polymeric chain of {[Cd(SCN)2(3-Acpy)]}n (1) extending along the [0 1 0] direction (a), the 2-D network of {[Cd(SCN)2(nia)]}n (2) extending parallel to the (1 0 0) plane (b) and N,O,N’-nia molecule as a chelating and bridging ligand between two neighboring cadmium(II) ions in the 2-D network of {[Cd(SCN)2(nia)]}n (2), forming a five-membered chelate ring (c).
There are many cadmium(II) coordination polymers structurally similar to 1 and 2, containing bridging thiocyanate ions and either bridging [14,15,16,17,18,19] or terminal [17,19,20] pyridine-based ligands, substituted at position -3, in the Cambridge Structural Database. We focused solely on the structures containing bridging or terminal pyridine-based ligands with simple substituents, excluding the ring substituents, e.g., N,N-diethylnicotinamide [14], pyridine-3-carboxylate [15], 3-(3-pyridyl)acrylate [16], 3-acetylpyridine [17], 3-aminomethylpyridine [18], 3-hydroxymethylpyridine [19] and 3-aminopyridine [20]. The Cd−N and Cd−S (thiocyanate N and S atoms), Cd−N (pyridine N) and Cd−O bond lengths in 1 and 2 are comparable to those seen in the related cadmium(II) coordination polymers containing thiocyanate ions and mentioned bridging or terminal pyridine-based ligands [11,14,15,16,17,18,19,20].
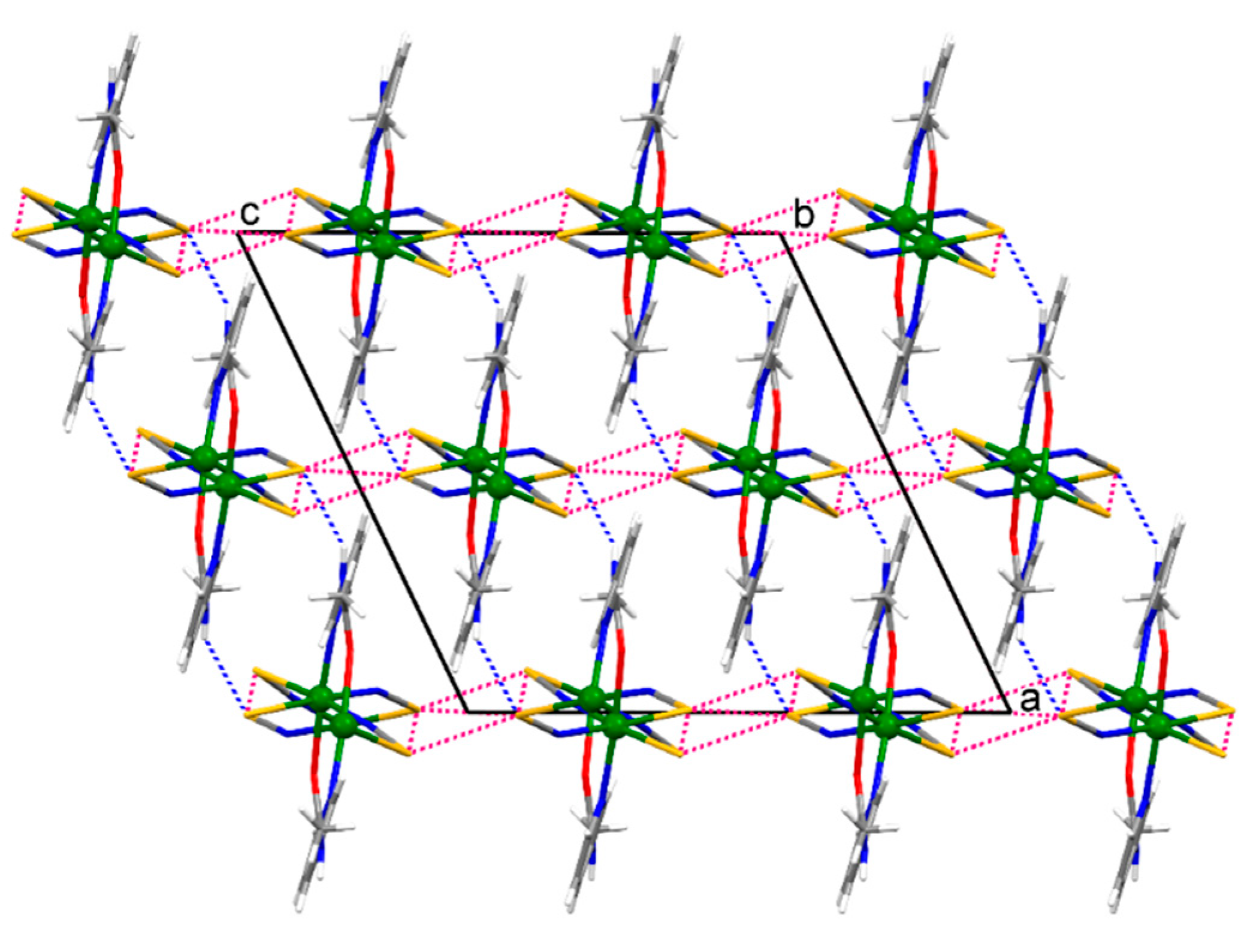
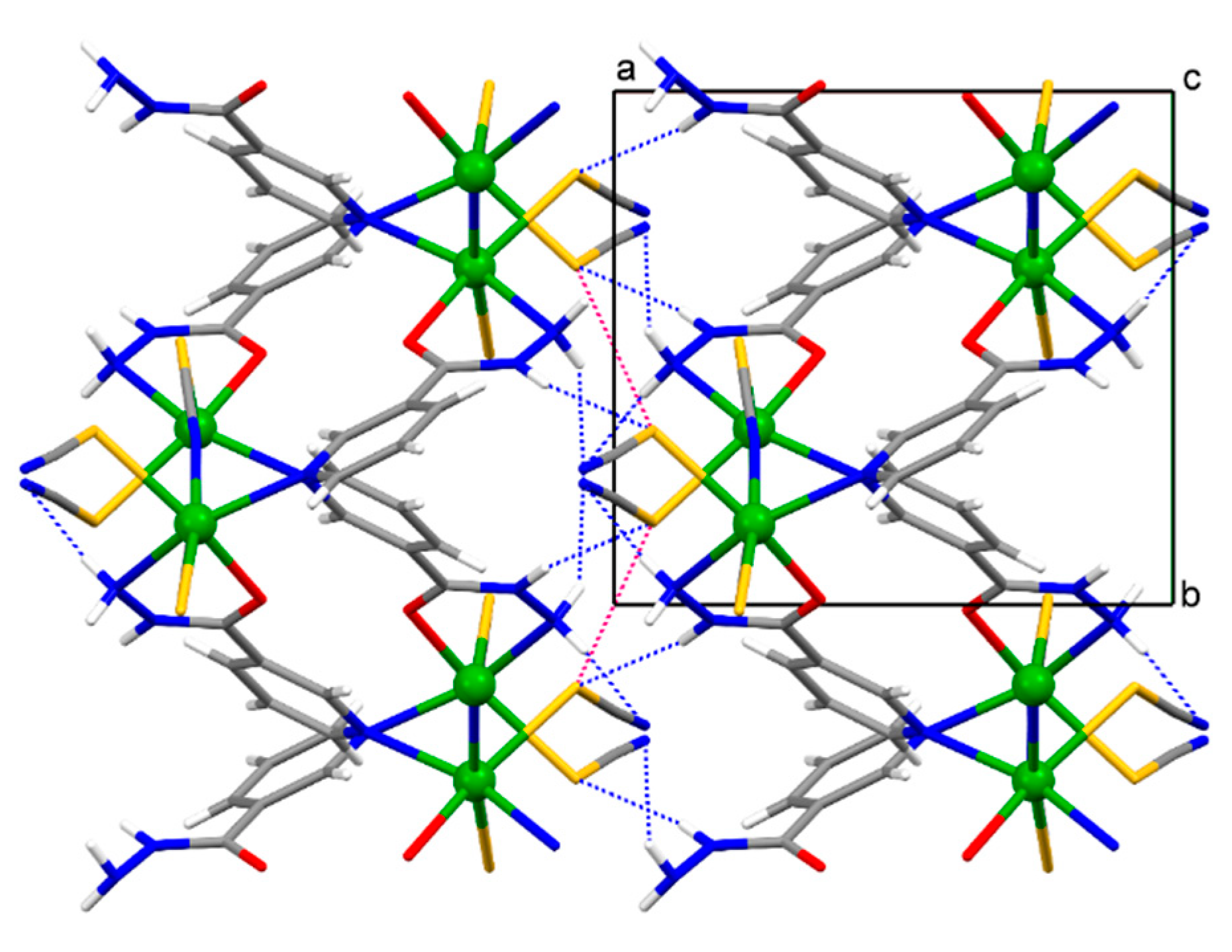
The crystal structure of 1 features strong intermolecular N–H∙∙∙S and weak intramolecular C–H∙∙∙O hydrogen bonds, while there are strong intermolecular N–H∙∙∙S and N–H∙∙∙N and weak C–H∙∙∙N hydrogen bonds in the crystal structure of 2 (Table 2). If the structure of 1 is viewed down the [0 1 0] direction (the direction along which the polymeric chain is running), the polymeric chains are connected by the intermolecular N–H∙∙∙S hydrogen bonds along the [1 0 0] direction and by the intermolecular S∙∙∙S chalcogen bonds (S1∙∙∙S1v distance = 3.427(1) Å, angles Cd1–S1∙∙∙S1v = 110.08(3)° and S1v∙∙∙S1–C1 = 146.2(1)°; S2∙∙∙S1v distance = 3.733(2) Å, angles Cd1–S2∙∙∙S1v = 103.53(4)°, Cd1–S1∙∙∙S2v = 166.62(4)° and S1v∙∙∙S2–C2 = 129.1(1)°, symmetry code (v) −x, 1 − y, −z) along the [0 0 1] direction within the framework (Figure 3). The intramolecular S∙∙∙S chalcogen bonds are also formed within the polymeric chain of 1 (S1∙∙∙S2 distance = 3.615(2) Å, angles C1–S1∙∙∙S2 = 149.6(1)° and S1∙∙∙S2–C2 = 136.2(2)°). In turn, the 2-D coordination networks of 2 are assembled along the [1 0 0] direction by the intermolecular N–H∙∙∙S and N–H∙∙∙N hydrogen bonds and by the intermolecular S∙∙∙S chalcogen bonds (S1∙∙∙S1vi distance = 3.616(1) Å, angle Cd1–S1∙∙∙S1vi = 156.79(3)°, symmetry code (vi) −x, −y, −z; Figure 4). The chalcogen S∙∙∙S bonds found in the supramolecular assemblies of 1 and 2 are of comparable distances to those seen in the crystal structures of monomeric zinc(II), cadmium(II) and mercury(II) complexes with thiocyanate and picolinamide [21] or nicotinamide [22]. These complexes were reported some time ago, but have currently been revisited.

Table 2.
The hydrogen bond geometry for {[Cd(SCN)2(3-Acpy)]}n (1) and {[Cd(SCN)2(nia)]}n (2).
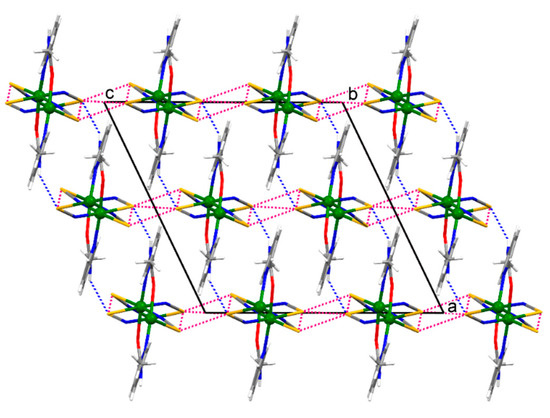
Figure 3.
A fragment of the infinite framework of {[Cd(SCN)2(3-Acpy)]}n (1) viewed down the [0 1 0] direction. The polymeric chains, represented as monomeric molecules in this projection, are connected by the intermolecular N–H∙∙∙S hydrogen bonds (represented by the blue dotted lines) along the [1 0 0] direction and are connected by the intermolecular S∙∙∙S chalcogen bonds (represented by the red dotted lines) along the [0 0 1] direction within the framework. The intramolecular S∙∙∙S chalcogen bonds are also shown by the red dotted lines.
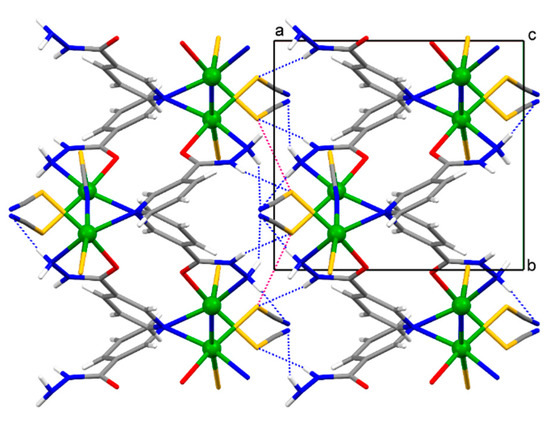
Figure 4.
A fragment of the infinite framework of {[Cd(SCN)2(nia)]}n (2) viewed down the [0 0 1] direction. The 2-D networks are connected by the intermolecular N–H∙∙∙S and N–H∙∙∙N hydrogen bonds (represented by the blue dotted lines) and by the S∙∙∙S chalcogen bonds (represented by the red dotted lines) along the [1 0 0] direction within the framework.
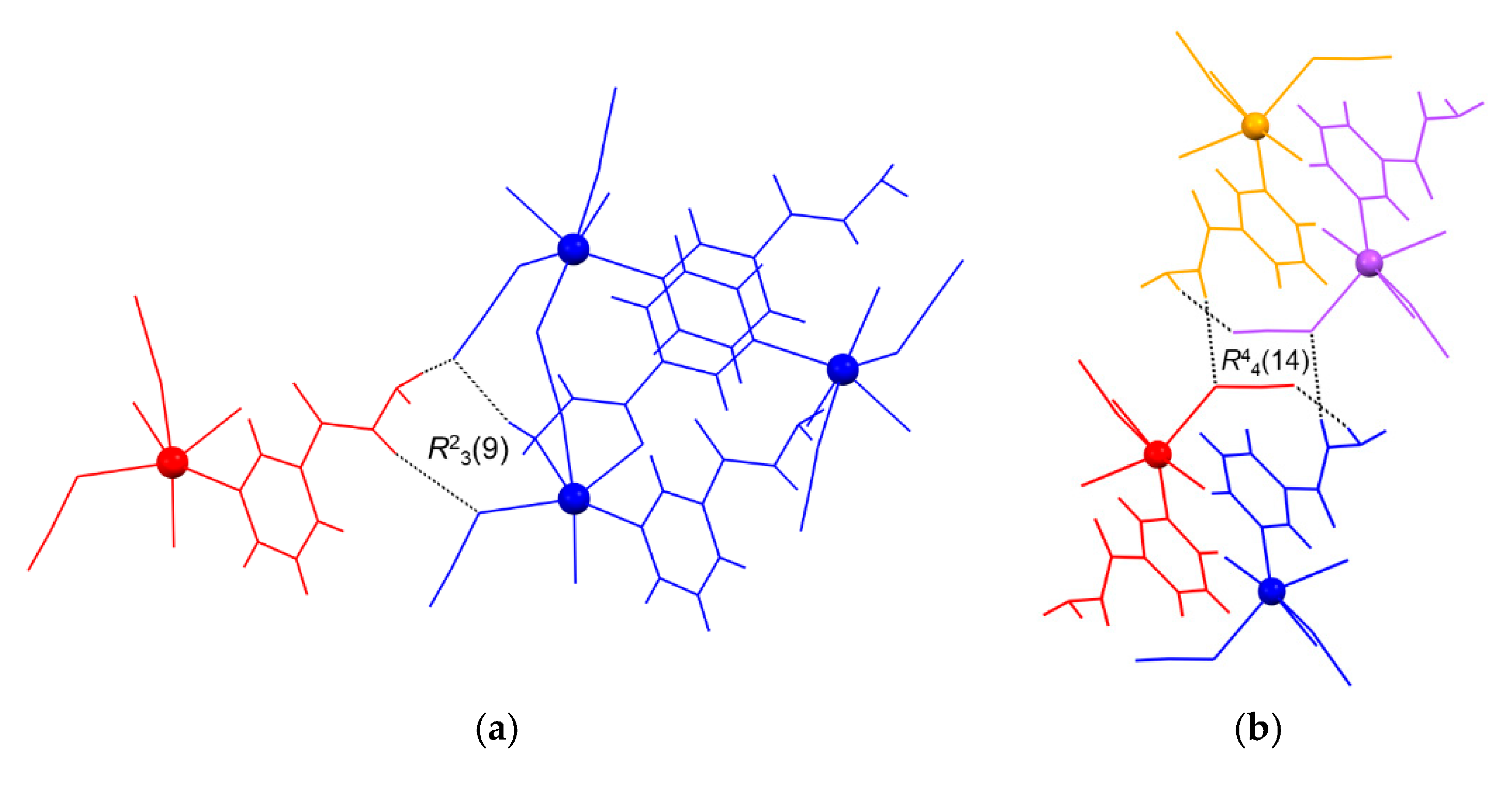
The most distinguished hydrogen-bonded ring motifs within the framework of 2 are the dimeric R23(9) and the centrosymmetric tetrameric R44(14) motifs (Figure 5). The R23(9) motif is formed between two symmetry-related coordination networks (represented as molecules in blue and red) via two nia hydrazide N atoms of the red molecule, one nia hydrazide N and two thiocyanate N atoms of the blue molecule (Figure 5a). The centrosymmetric R44(14) motif, however, is formed between four symmetry-related coordination networks (represented as molecules in blue, red, orange and violet) via two nia hydrazide N atoms of both blue and orange molecules, one thiocyanate N, and one thiocyanate S atom of both red and violet molecules (Figure 5b).
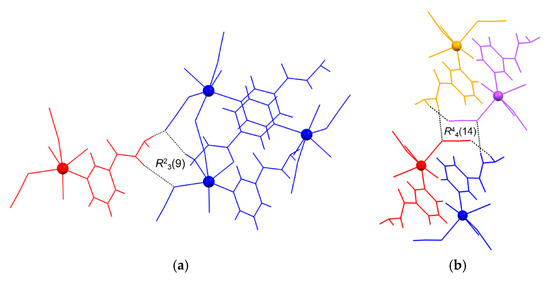
Figure 5.
The most distinguished hydrogen-bonded ring motifs (shown by dotted lines) found within the hydrogen-bonded framework of {[Cd(SCN)2(nia)]}n (2), e.g., the dimeric R23(9) (a) and the centrosymmetric tetrameric R44(14) (b) motifs. The various symmetry-related coordination networks are represented as molecules and shown in red and blue (a) and in orange, violet, red and blue (b) (see text).
3. Conclusions
We have prepared a 1-D cadmium(II) coordination polymer {[Cd(SCN)2(3-Acpy)]}n (1) and a 2-D polymer {[Cd(SCN)2(nia)]}n (2) under the same experimental conditions. The neighboring cadmium(II) ions are bridged by two thiocyanate ions and one 3-Acpy ligand (via its pyridine N and carbonyl O atoms), into a polymeric chain of 1. The adjacent cadmium(II) ions in 2 are bridged by thiocyanate ions in one direction, but also by chelating and bridging nia in another direction, leading to the formation of a coordination network. The coordination mode of 3-Acpy found in 1 (bridging via its pyridine N and carbonyl O atoms) is unprecedented, as until now, 3-Acpy acted exclusively as an N-monodentate ligand in the reported metal-containing compounds. On the other hand, the coordination mode of nia found in 2 was the expected one, previously established in the known coordination polymers. We did confirm that the dimensionality of a coordination polymer can be increased (from 1D in the case of 1 to 2D in the case of 2) if two bridging ligands (thiocyanate and nia) are used in the case of nia in 2. However, it was revealed that 3-Acpy also acts as a bridging ligand in 1, yielding a lower-dimensionality polymer than in the case of 2. As 1 and 2 both contain bridging thiocyanates and bridging 3-Acpy or nia, respectively, the difference in their dimensionality can solely be ascribed to the chelating ability of nia. Nia can, therefore, more efficiently bridge the cadmium(II) ions in a different direction (as compared to the direction the thiocyanate bridge along), thus leading to the higher dimensionality of 2, as opposed to 1. The introduction of thiocyanate ions indeed enabled the expected formation of the chalcogen S∙∙∙S bonds in the crystal packings of 1 and 2, though this was not of the same role or significance. It could be noted that the intermolecular chalcogen S∙∙∙S bonds are more significant in the structure of 1, as they are the only supramolecular interactions responsible for assembling the polymeric chains of 1 in this particular direction. In the structure of 2, the intermolecular chalcogen S∙∙∙S bonds also assemble the coordination networks of 2 in a particular direction, but this time in cooperation with the intermolecular N–H∙∙∙S and N–H∙∙∙N hydrogen bonds, acting along the same direction. In both structures, the employment of the strongest acceptor atoms (O atoms), in the coordination with the neighboring cadmium(II) ions, reduces the potential for establishing stronger supramolecular links, thus facilitating the supramolecular connectivity of metal-containing building units via desired S∙∙∙S links.
4. Materials and Methods
All commercially available chemicals were of reagent grade and were used as received, without further purification. 3-Acetamidopyridine (3-Acpy) was prepared according to the previously reported method [23,24]. CHN elemental analyses were carried out with a Perkin-Elmer 2400 Series II CHNS analyzer in Analytical Services Laboratories of the Ruđer Bošković Institute, Zagreb, Croatia. The IR spectra were obtained from KBr pellets in the range 4000–400 cm–1 on a Perkin-Elmer Spectrum Two FT-IR spectrometer.
4.1. Synthesis of {[Cd(SCN)2(3-Acpy)]}n (1) in Bulk
Cadmium(II) nitrate tetrahydrate (0.74 g; 2.40 mmol) was dissolved in water (2 mL); sodium thiocyanate (0.389 g; 4.80 mmol) was also dissolved in water (3 mL). The two solutions were mixed and added to 3-acetamidopyridine (0.66 g; 4.85 mmol), dissolved in ethanol (5 mL). A white powder of 1 was obtained in a period of 1–2 days, filtered off, washed with small amount of cold water–ethanol mixture, (1:1, v/v) and dried in vacuo. Yield: 97% (0.84 g). Anal. Calcd. (Mr = 364.71): C, 29.64%; H, 2.22%; N, 15.37%. Found: C, 29.02%; H, 1.93%; N, 14.96%. IR (KBr, cm−1): 3294(m, ν(NH)), 3117(w, ν(NH)), 3068(w, ν(CH)pyridine), 2119(vs, ν(CN)thiocyanate), 2104(vs, ν(CN)thiocyanate), 1663(s, ν(C=O), 1584(m, ν(CN)pyridine), 1539(s, ν(−CN)amide, δ(−NH)amide), 1475 (w, ν(CC)pyridine), 1427(s, ν(CC)pyridine), 1373(w, ω(CH)), 1334(m, ν(CN)pyridine), 1291(m, δ(CCH)pyridine), 809(m, δ(CNC)pyridine), 696(m, ν(Cd−S)), 640(w, δ(SCN)). The IR spectrum can be found in the Supplementary Materials.
Preparation of Single Crystals of 1
3-Acetamidopyridine (0.011 g; 0.081 mmol) was dissolved in ethanol (5 mL), cadmium(II) nitrate tetrahydrate (0.013 g; 0.42 mmol) was dissolved in water (2 mL), and sodium thiocyanate (0.007 g; 0.086 mmol) was dissolved in water (3 mL). All the solutions were mixed through moderate stirring, and the final solution was left at room temperature to evaporate slowly. Colorless crystals suitable for single-crystal X-ray structure determination were formed in a period of two weeks.
4.2. Synthesis of {[Cd(SCN)2(nia)]}n (2) in Bulk
Cadmium(II) nitrate tetrahydrate (0.07 g; 0.23 mmol) was dissolved in ethanol (2 mL) and niazid (0.07 g; 0.51 mmol) was dissolved in 3 mL of water–ethanol mixture (1:2, v/v). The two solutions were mixed together and added to the solution of sodium thiocyanate (0.04 g; 0.49 mmol) in ethanol (3 mL). A white powder of 2 was formed in a period of 3–4 days, filtered off, washed with small amounts of cold water and dried in vacuo. Yield: 50% (0.04 g). Anal. Calcd. (Mr = 365.71): C, 26.27%; H, 1.93%; N, 19.15%. Found: C, 24.98%; H, 1.44%; N, 18.77%. IR (KBr, cm−1): 3442(w, ν(NH)), 3223(m, ν(NH)), 3144(m, ν(NH)), 3091(w, ν(CH)pyridine), 3040(w, ν(CH)pyridine), 3006(w, ν(CH)pyridine), 2095(vs, ν(CN)thiocyanate), 2073(vs, ν(CN)thiocyanate), 1652(s, ν(C=O)), 1592(m, ν(CN)pyridine), 1536(m, ν(−CN)amide, δ(−NH)amide), 1417(m, ν(CC)pyridine), 1337(m, ω(CH)), 1199(m, δ(CCH)pyridine), 1116(m, ω(CH)pyridine), 1068(m, ω(CH)pyridine), 1017(m, ωoop(CH)pyridine), 699(m, ν(Cd−S)), 648(m, δ(SCN)). The IR spectrum can be found in the Supplementary Materials.
Preparation of Single Crystals of 2
Cadmium(II) nitrate tetrahydrate (0.07 g; 0.23 mmol) was dissolved in ethanol (2.5 mL) and niazid (0.07 g; 0.51 mmol) was dissolved in 3 mL of water–ethanol mixture (1:2, v/v). The solutions were mixed and transferred to a test tube and carefully layered with 1 mL of ethanol, followed by the careful addition of 2.5 mL of ethanol solution of sodium thiocyanate (0.04 g; 0.49 mmol). Colorless crystals of 2, suitable for single-crystal X-ray structure determination, were formed in a couple of weeks.
4.3. X-ray Crystallographic Analysis
The suitable single crystals of 1 and 2 were selected and mounted in Paratone-N oil onto thin glass fibers. The data collection was carried out on an Oxford Diffraction Xcalibur four-circle kappa geometry diffractometer with Xcalibur Sapphire 3 CCD detector, using graphite monochromated MoKα (λ = 0.71073 Å) radiation at room temperature (296(2) K) and by applying the CrysAlis PRO Software system [25]. The data reduction and cell refinement were performed by the CrysAlis PRO Software system [25]. The structures were solved by SHELXT [26] and refined by SHELXL-2018/3 [27]. The refinement procedure was done by full-matrix least-squares methods based on F2 values against all reflections. The figures were made with MERCURY (Version 2020.2.0) [28]. The crystallographic data for 1 and 2 are summarized in Table 3.

Table 3.
The crystallographic data for {[Cd(SCN)2(3-Acpy)]}n (1) and {[Cd(SCN)2(nia)]}n (2).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11060697/s1, IR spectra of 1 and 2. Deposition numbers 2086781 (for 1) and 2086782 (for 2) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Author Contributions
Conceptualization, B.-M.K. and M.Đ.; methodology, N.M.M., N.P., B.-M.K. and M.Đ.; validation, N.M.M., N.P., B.-M.K. and M.Đ.; formal analysis, N.M.M., N.P., B.-M.K. and M.Đ.; investigation, N.M.M., N.P., B.-M.K. and M.Đ.; resources, M.Đ.; data curation, M.Đ.; writing—original draft preparation, B.-M.K.; writing—review and editing, B.-M.K. and M.Đ.; visualization, B.-M.K.; supervision, B.-M.K. and M.Đ.; project administration, M.Đ.; funding acquisition, M.Đ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation, grant number IP-2019-04-1242. The APC was funded by the Foundation of the Croatian Academy of Sciences and Arts and by the institutional funding of the Faculty of Science, University of Zagreb.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- De Azevedo Santos, L.; Ramalho, T.C.; Hamlin, T.A.; Bickelhaupt, F.M. Chalcogen bonds: Hierarchical ab initio benchmark and density functional theory performance study. J. Comput. Chem. 2021, 42, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Asai, K.; Jacquot de Rouville, H.-P.; Shimajiri, T.; Heitz, V.; Fujii-Shinomiya, H.; Suzuki, T. Molecular recognition by chalcogen bond: Selective charge-transfer crystal formation of dimethylnaphthalene with selenadiazolotetracyanonaphthoquinodimethane. Eur. J. Org. Chem. 2021, 990–997. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, P.-P.; Wang, Y. Chalcogen···π bonding catalysis. Angew. Chem. Int. Ed. 2021, 60, 9395–9400. [Google Scholar] [CrossRef]
- Haakansson, C.T.; Corkish, T.R.; Watson, P.D.; Robinson, H.T.; Tsui, T.; McKinley, A.J.; Wild, D.A. Spectroscopic investigation of chalcogen bonding: Halide–carbon disulfide complexes. ChemPhysChem 2021, 22, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef]
- Dhaka, A.; Jeannin, O.; Jeon, I.-R.; Aubert, E.; Espinosa, E.; Fourmigué, M. Activating chalcogen bonding (ChB) in alkylseleno/alkyltelluroacetylenes toward chalcogen bonding directionality control. Angew. Chem. Int. Ed. 2020, 59, 23583–23587. [Google Scholar] [CrossRef]
- Peloquin, A.J.; McMillen, C.D.; Iacono, S.T.; Pennington, W.T. Halogen and chalcogen bonding between the triphenylphosphine chalcogenides (Ph3P=E.; E=O, S, Se) and iodofluorobenzenes. ChemPlusChem 2021, 86, 549–557. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.-H.; Qian, H.-F.; Peng, Y.-X.; Huang, W. Tetranuclear Zn(II) and mononuclear Ni(II) based coordination polymers derived from a pair of isomeric 1,2,4-triazole ligands 3,5-disubstituted by pyridine and acetate ethyl ester groups. Polyhedron 2016, 106, 138–143. [Google Scholar] [CrossRef]
- Mirtamizdoust, B. Sonochemical synthesis of nano lead(II) metal-organic coordination polymer; New precursor for the preparation of nano-materials. Ultrason. Sonochem. 2017, 35, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Notash, B. 1D helical cadmium coordination polymers containing hydrazide ligand: The role of solvent and molar ratio. J. Mol. Struct. 2018, 1156, 534–543. [Google Scholar] [CrossRef]
- Wang, X.-L.; Rong, X.; Liu, D.-N.; Lin, H.-Y.; Liu, G.-C.; Wang, X.; Song, G. Diverse polyoxometalate-based metal–organic complexes constructed by a tetrazole- and pyridyl-containing asymmetric amide ligand or its in situ transformed ligand. CrystEngComm 2016, 18, 5101–5109. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Hussain, I.; Forbes, S.; Desper, J. Versatile ligands for the construction of layered metal-containing networks. Aust. J. Chem. 2009, 62, 899–908. [Google Scholar] [CrossRef]
- Bigoli, F.; Braibanti, A.; Pellinghelli, M.A.; Tiripicchio, A. The crystal and molecular structure of mono-(N,N-diethylnicotinamide)cadmium dithiocyanate. Acta Crystallogr. 1972, B28, 962–966. [Google Scholar] [CrossRef]
- Shahverdizadeh, G.H.; Morsali, A. Sonochemical synthesis of one-dimensional nano-structure of three-dimensional cadmium(II) coordination polymer. J. Inorg. Organomet. Polym. 2011, 21, 694–699. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Zhou, Q.-X.; Yue, L.; Zhao, X.-Q. Synthesis and structural characterization of a novel coordination polymer [Cd2(SCN)2(C8H6NO2)2(H2O)4]n. J. Coord. Chem. 2004, 57, 741–746. [Google Scholar] [CrossRef]
- Werner, J.; Boeckmann, J.; Näther, C. Investigations on the structure diversity and thermal degradation behavior of CdII and ZnII thiocyanato coordination compounds based on 3-acetylpyridine as neutral co-ligand. Z. Anorg. Allg. Chem. 2012, 638, 2257–2264. [Google Scholar] [CrossRef]
- Neumann, T.; Germann, L.S.; Moudrakovski, I.; Dinnebier, R.E.; dos Santos Cunha, C.; Terraschke, H.; Näther, C. Synthesis, crystal structures, and properties of M(NCS)2-3-aminomethylpyridine coordination compounds (M = Cd, Zn). Z. Anorg. Allg. Chem. 2017, 643, 1904–1912. [Google Scholar] [CrossRef]
- Neumann, T.; dos Santos Cunha, C.; Terraschke, H.; Germann, L.S.; Dinnebier, R.E.; Jess, I.; Näther, C. Synthesis, structures, and physical properties of thiocyanate coordination compounds with 3-hydroxymethylpyridine. Z. Anorg. Allg. Chem. 2017, 643, 1497–1507. [Google Scholar] [CrossRef]
- Saber, M.R.; Abu-Youssef, M.A.M.; Goher, M.A.S.; Sabra, B.A.; Hafez, A.K.; Badr, A.M.-A.; Mautner, F.A. 1D cadmium(II) thiocyanate systems: Synthesis and characterization of three new polymeric 1D cadmium(II) thiocyanato complexes. J. Mol. Struct. 2012, 1008, 17–23. [Google Scholar] [CrossRef]
- Đaković, M.; Popović, Z.; Giester, G.; Rajić-Linarić, M. Thiocyanate complexes of the group 12 metals with pyridine-2-carboxamide: Synthesis and structural characterization. Polyhedron 2008, 27, 210–222. [Google Scholar] [CrossRef]
- Đaković, M.; Popović, Z.; Giester, G.; Rajić-Linarić, M. Synthesis, spectroscopic and structural investigation of Zn(NCS)2(nicotinamide)2 and [Hg(SCN)2(nicotinamide)]n. Polyhedron 2008, 27, 465–472. [Google Scholar] [CrossRef]
- Li, C.-d.; Rittmann, L.S.; Tsiftsoglou, A.S.; Bhargava, K.K.; Sartorelli, A.C. Pyridine derivatives as potent inducers of erythroid differentiation in friend leukemia cells. J. Med. Chem. 1978, 21, 874–877. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Hussain, I.; Forbes, S.; Desper, J. Exploring the hydrogen-bond preference of N–H moieties in co-crystals assembled via O–H(acid)⋅⋅⋅N(py) intermolecular interactions. CrystEngComm 2007, 9, 46–54. [Google Scholar] [CrossRef]
- CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, Oxfordshire, UK, 2014.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).