Abstract
Lime plaster mixes are becoming more and more popular in the world’s building materials market every year. Therefore, the issue of increasing the efficiency of lime finishing coatings is relevant. The paper aim is the modification of lime binders with specially synthesized calcium silicate hydrates (CSHs). To obtain the CSH filler, liquid sodium glass was used with a silicate module of 1.53–2.9 and a density of 1130–1663 kg/m3. Using differential thermal analysis (DTA), X-ray diffraction (XRD) patterns, synthesized calcium silicate hydrates, as well as dry plaster mixes, and finishing coatings based on using them were studied. The regularities of the filler synthesis were established depending on the temperature, density, and silicate modulus of liquid glass, the amount of the precipitant additive, the rate of its introduction, and the drying mode. As a result of processing the obtained experimental data, a mathematical model was obtained for the composition “lime + CSH”. The phase composition of the filler was revealed, which is characterized by the presence of calcium silicate hydrates of the tobermorite group, a solid solution CSH (B) in the form of a weakly crystallized gel, a solid solution of C–S–H (II), hydrohalites, and calcites. It was found that the use of the fillers into the lime compositions, obtained with the rapid introduction of CaCl2 additive into water glass during the synthesis of the filler, promotes the acceleration of the plastic strength gain of lime compositions. It was revealed that the lime composites with the CSH filler are characterized by reduced shrinkage deformations up to 45%. The introduction of the CSH filler into the lime compositions increases the water resistance of the lime finishing layer by 36%. A technological scheme for the production of the lime dry plaster mixes has been developed; it can be introduced at existing factories of building materials without significant re-equipment of production.
1. Introduction
Every year, dry plaster mixes (DPMs) are becoming more and more popular in the world’s building materials market [1]. The modifiers that are used to create them give them new properties [2]. Modern compositions have high strength, density, crack resistance, frost resistance, etc. [3].
Currently, the most popular among plaster mixes are cement and gypsum mixes [4]. The demand for lime mixes is slightly lower due to low strength and water resistance [5]. However, lime plaster mixes have a number of advantages: They have good thermal insulation [6] and fireproof [7] properties; environmentally friendly [8]; have good adhesion to wood [9,10], brick [11] and cinder-concrete [12] surfaces; resistant to biological damage due to the high alkalinity of lime [13]; flexible [14] and easy to work with [15]. In addition, they have high vapor permeability [16,17,18], which allows the wall to “breathe” without accumulating condensate [19], thereby contributing to the improvement of the microclimate of the rooms finished with them by regulating the humidity of the environment [20]. Lime coatings are easily pierced with nails [21]. Lime-based plaster mixes can be applied to the surface to be finished at low positive and moderately negative temperatures [22,23]. Therefore, the issue of increasing the efficiency of lime finishing coatings is relevant.
To improve the performance of lime coatings, various additives are introduced into them, for example, water repellents or pozzolanic materials [24,25]. The use of wollastonite (calcium silicates) in plaster mixes, although it leads to a decrease in shrinkage and an increase in water-holding capacity, increases the strength of composites insignificantly and does not provide sufficient water resistance of lime mixes [26]. The increase in the efficiency of lime compositions due to the use of additives based on calcium silicate hydrates (CSHs) has hardly been studied.
The currently available methods for producing calcium silicate hydrates by ion exchange have a number of disadvantages associated with the multistage synthesis process [27].
Thus, the technology for the synthesis of calcium silicate hydrates used subsequently for the production of lime-based dry mixes requires improvement. This is due to the fact that the use of such filler in compositions with mineral binders dictates the need for a more complete use of its potential, namely, the reactivity of the filler [28].
The aim of the work is to modify lime binders with specially synthesized calcium silicate hydrates.
To achieve this aim, the following tasks were solved:
- Study of the regularities of the synthesis of finely dispersed fillers based on calcium silicate hydrates (relationship between a density of liquid glass, its concentration, an amount of precipitant and the properties of the resulting filler);
- Study of the structure formation of lime systems in the presence of CSH fillers;
- Development of compositions of lime-based dry mixes and determination of technological and operational properties of plaster mortar based on the developed compositions.
2. Materials and Methods
2.1. Characteristics of the Raw Materials Used
The initial binder was a lime (Atmis-sugar, Russia) with an activity of 75–84%, a true density of 2205 kg/m3, a bulk density of 500 kg/m3, and a specific surface area of 10,000 cm2/g. To obtain a filler based on CSH, we used liquid sodium glass with a silicate module M = 1.53–2.9 and a density = 1130–1663 kg/m3. Calcium chloride, iron (III) chloride, and aluminum sulfate were used as precipitants. The amount of addition of the precipitant was 30–50% of the mass of water glass in the form of a 7.5–15% solution, depending on the density and silicate module of the liquid glass.
A quartz sand (Ukhta, Russia) with a real density of 2611 kg/m3, a size module equal to 1.7, and a bulk density of 1490 kg/m3 was used as a filler.
The granulometric composition of the sand is given in Table 1.

Table 1.
Granulometric composition of the sand.
The binders were mixed with drinking water with a pH of 7.12.
Due to the high water demand of lime compositions in order to regulate the rheological, technological and functional properties of the finishing compositions, the following plasticizing additives were introduced into the mix recipe:
Kratasol that are salts of naphthalenesulfonic acids with a high content of high molecular weight fractions. S-3 (sulfonated polycondensates up to 82–84%; sodium sulfate is 8–10%; moisture no more than 10%). SP-3 is a mix of sodium salts of polymethylene naphthalenesulfonic acids of various molecular weights, industrial lignosulfonates, as well as industrial mix of sodium thiosulfate and sodium thiocyanate;
Hidetal P-4 (SKT-Standart, Novozybkov, Russia), Melment F15G and Melflux 1641F (BASF, Berlin, Germany)polycarboxylate-based superplasticizers. The amount of the additive was 0.7–2% by weight of the binder.
To obtain colored compositions, an additive-chromophore FeCl3 was introduced, as well as pigments (blue and yellow) and ocher (chromium oxide) in an amount from 1% to 5%. The blue and yellow pigments have a phthalocyanine organic chemical base.
To increase the water retention capacity, the dobpack Mecellose FMC 2094 (Lotte Fine Chemicals, Seoul, Korea) was also introduced into the formulation of the lime-sand composition in an amount of 0.1% by weight of the dry mix. To increase the strength characteristics, redispersible powders were also introduced into the formulation of the lime-sand composition: Pulver DM 1142P (Pulver, Kocaeli, Turkey) Neolith 4400, Neolith 7200 (Neolith, Gozzano, Italy) in the amount of 1%, 0.8%, and 1.8% of the dry mix mass, respectively. Neolith redispersible powders are produced by spray drying aqueous dispersions based on copolymers of vinyl acetate, acrylates, and versatates. They contain anticoagulants and anti-caking agents. Pulver DM1142P is a redispersible powder in the form of vinyl acetate, ethylene, and polyvinyl alcohol monomers.
2.2. Mix Design
When hardening in air, the lime mortars made with slaked lime give significant shrinkage. In this regard, the quartz sand of fraction 0.63–0.315 and 0.315–0.14 mm, in a ratio of 80:20 and bulk density of 1527 kg/m3, was introduced into the lime mortar as a fine aggregate. In this case, the ratio of binder:sand (B:S) were 1:3 and 1:4.
The mix design was carried out based on the different ratio of the addition of the synthesized silicate hydrates to lime, as well as from the different water:lime ratios (Table 2).

Table 2.
Mix design.
For each series of tests, six specimens of each composition were made. Moreover, the standard deviation of the results did not exceed 5% for all results.
2.3. Laboratory Equipment and Research Methods
The granulometric composition of the obtained fillers was estimated using an automatic laser diffractometer Fritsch Particle Sizer Analysette 22 (Fritsch, Berlin, Germany). The determination of the specific surface area of powder materials was carried out by the method of air permeability on a PSH-11 device (Pribory Khodakova, Moscow, Russia). The XRD patterns of the samples under study were obtained on a Thermo Scientific ARL X’TRA diffractometer (Thermo Fisher Scientific, Waltham, MA, USA). Photographs of CSH crystals were also obtained using a Nikon Eclipse E optical microscope (Nikon, Tokyo, Japan). IR spectrometry was carried out using a Varian Scimitar 1000 FT-IR (Varian Scimitar, Waltham, MA, USA) instrument. Differential thermal analysis of the samples was carried out using a thermogravimetric analyzer Shimadzu DTG-60H (Shimadzu, Tokyo, Japan).
The true density of the starting materials was determined using a Le Chatelier (Pribory Khodakova, Moscow, Russia) instrument. The bulk density was determined by filling a vessel with a capacity of 1000 cm3. The average density was determined as the ratio of the sample mass to its volume. The sorption capacity of the materials was studied by keeping them in desiccators at different humidity levels and for different times. The activity of the synthesized mineral additive was determined by its solubility in a 20% KOH solution. Determination of the amount of cohesive lime was determined in accordance with the Russian standard GOST 22688 to determine the total content of active CaO + MgO in calcium lime using a titration method based on the neutralization reaction of hydrated lime HCI:
CaO + 2HCI + H2O = CaCI2 + 2H2O
The rheological properties of the compositions were evaluated in terms of plastic strength, which was determined using a CP-3 conical plastometer. During the experiment, the influence of the mix formulation on the change in the rheological properties of the formulations was investigated. The variable factors were the ratio of the components “lime:CSH filler”, the conditions for the synthesis of the filler, the type and concentration of additives-plasticizers. Lime with an activity of 71% was used as a binder.
To assess the fracture toughness of the lime composites, the measurements of shrinkage deformations were carried out using an IZA-2 optical comparator.
Investigation of finishing layers for strength in compression and flexion was investigated using a setup developed by the authors on specimens 10 × 10 × 10 mm and 10 × 10 × 50 mm in size, respectively (Figure 1).
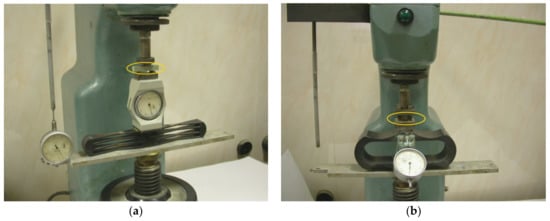
Figure 1.
Setup for determining the strength in flexion (a) and compression (b).
In addition, the crack resistance of the coatings was evaluated using the crack resistance coefficient, which was determined as the ratio of the flexural strength to the compressive strength of specimens aged 28 days.
Water resistance was evaluated by the softening coefficient, which is equal to the ratio of the compressive strength of a material saturated with water to the one of a dry material.
The water-holding capacity of the finishing compounds was determined in accordance with the Russian standard GOST 5802.
To assess the operational resistance of coatings based on lime-based dry mixes, frost resistance tests were carried out by alternating thawing and freezing of the finishing layer applied to the cement-sand substrate after 28 days of air-dry hardening. The assessment of the appearance of the coatings was carried out according to the Russian standard GOST 6992-68. In the absence of defects, the condition is considered excellent, if there are defects, but there are very few of them, then the condition is considered good. The condition of the coating, which is characterized by a loss of gloss up to 50% with a significant change in color, whitishness, bronzing and dirt retention, cracking up to 25% of the surface, the presence of a rash up to 25% and bubbles up to 5% of the surface.
3. Results and Discussion
3.1. Synthesis of Calcium Silicate Hydrates
When developing the technology for the production of the fillers, the following factors were taken into account: The density of the liquid glass, the amount of addition of the precipitant and the rate of its introduction, the mode of drying the precipitate and the time of its storage. From Table 3 it can be seen that, with an increase in the content of the precipitant additive during the synthesis of the filler, the compressive strength of lime compositions with the fillers increases.

Table 3.
Compressive strength of the modified lime composites (specimens 03A).
It was found that the output of the filler synthesized from the liquid glass in the presence of CaCl2 in the form of a 15% solution in an amount of 30% and 50% of the weight of the liquid glass was 85%, and the output of the filler synthesized in the presence of CaCl2 in the form of 7.5% solution in the amounts of 30% and 50% was 100%.
After drying at a temperature of 105 °C, the true density of the fillers was 2200 kg/m3; the bulk density was 2935 kg/m3. It was found that the particle size distribution of the silicate-containing filler is of a polyfraction nature, and the size distribution of the filler particles is two-modal (Figure 2).
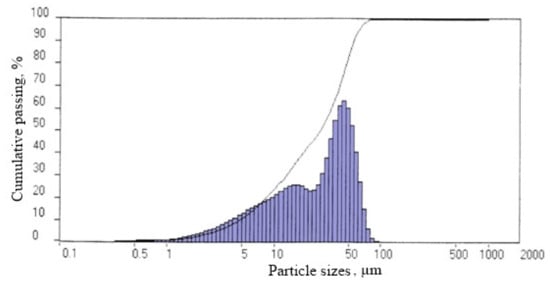
Figure 2.
Granulometric composition of the filler obtained by synthesis in the presence of CaCl2 in the form of a 15% solution.
Analysis of the experimental data presented in Figure 2 indicates that the average particle diameter is 34.19 μm, the particle size prevails in the range of 20–45 (35%) and 45–100 μm (23–32%), while more than 99% are particles with a size less than 73.23 μm. The content of particles in the range of 0.05–1 μm is 1%. The specific surface area is 5876 cm2/cm3.
With the slow introduction of the CaCl2 precipitating additive, a small number of large particles are formed in the sediment. Granulometric data indicate that particles with a size of 20–45 μm prevail, the content of which is 38.52%. The arithmetic mean of the size of the filler particles synthesized with the slow introduction of the CaCl2 precipitating additive is 29.35 μm.
The use of more dilute solutions (7.5%) leads to a slowdown in precipitation and the appearance of larger crystals. The content of particles with a size of 45–100 μm is 32.62%, crystals with a particle size of 100–200 μm appear, and their content is 0.04%.
With an increase in the maturation time of the sediment, crystal growth is observed (Figure 3). An increase in temperature to 50 °C during deposition accelerates the formation of a crystal lattice, resulting in the formation of larger crystals. The content of particles with a size of 45–100 µm, which is 33.21%, and the content of particles with a size of 100–200 µm, which is 1.02%, increases.
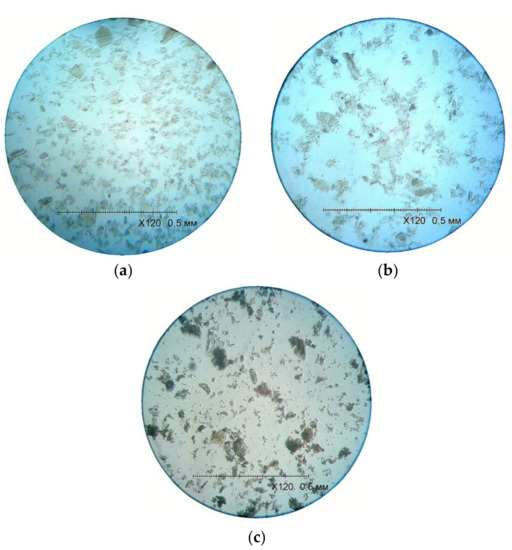
Figure 3.
Image of crystals that matured for two (a) and four (b) days in the filtrate under normal conditions, as well as for two days at a temperature of 50 °C (c).
It was found that for 7.5% and 15% CaCl2 solution, pH are 7.12 and 7.3, respectively. With an increase in the concentration of the solution (50% solution of the liquid glass with a density of ρ = 1335 kg/m3 and a silicate modulus of 2.9), the pH also increases to 11.8, and for a 50% solution of the liquid glass with a density of 1663 kg/m3 and a silicate modulus of 1.5, pH is 13.6 (Table 4).

Table 4.
Effect of precipitation conditions on the filler activity (specimens 015A).
The introduction of aluminum sulfate during the synthesis of the filler leads to an increase in the compressive strength of lime paste with a filler. This filler synthesized from the liquid glass in the presence of precipitating additives CaCl2 in an amount of 45% by weight of the liquid glass and Al2(SO4)3 in an amount of 11% by weight of the liquid glass. Thus, the compressive strength of lime paste with the filler synthesized in the presence of CaCl2 and Al2(SO4)3 precipitating additives is 5.0 MPa, the strength of the reference specimen of lime paste with the filler synthesized in the presence of CaCl2 precipitating additive is 4.6 MPa. Figure 4 shows an X-ray diffraction pattern of the filler synthesized in the presence of CaCl2 and Al2(SO4)3 precipitating additives. In the specimens of the filler synthesized with the joint introduction of CaCl2 and Al2(SO4)3, there are diffraction lines (Å) of the following compounds: calcium silicate hydrates C-S-H (I) and (II): 10.1337; 7.6742; 3.5796; 2.8290; 2.4662; 2.2261; 2.2061; 1.2625; gypsum: 4.3105; 4.3114; 2.2261; zeolites: 3.2658; 1.9981; 1.7130; calcite: 3.8583; 3.0395; 1.4115 aragonite: 1.8782; 1.2976; vaterite: 1.2625. The presence of additional compounds (zeolites, gypsum) and an additional amount of C-S-H type silicate hydrates interacting with lime helps to strengthen the structure of the lime paste.
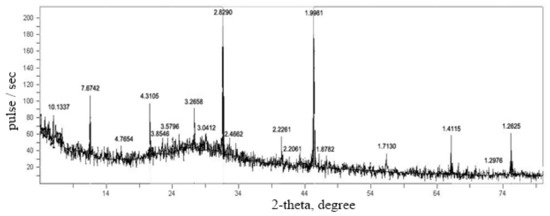
Figure 4.
X-ray diffraction pattern of the filler samples synthesized with the addition of CaCl2 and Al2(SO4)3.
To obtain colored fillers, an additive-chromophore FeCl3 was introduced, both together with the addition of CaCl2, and separately. An analysis of the experimental data showed that the compressive strength of the lime compositions with the filler synthesized from the liquid glass in the presence of the combined introduction of CaCl2 and FeCl3 additives is 28% higher compared to compositions based on the fillers synthesized in the presence of CaCl2 alone (Table 5).

Table 5.
Compressive strength of the lime composites (specimens 005A).
To determine the optimum density and the silicate module of the liquid glass, a full factorial experiment was designed. The main factor levels and variation intervals are shown in Table 6. The compressive strength of the lime composite was chosen as the optimization parameter. The homogeneity of the variances was assessed by the Cochran criterion, the adequacy of the models was checked by the Fisher criterion, the significance of the coefficients by the Student criterion at a significance level of 0.05.

Table 6.
Conditions for changing variables.
As a result of processing the obtained experimental data, a linear model was obtained for the composition “lime + CSH”:
The resulting model adequately describes the influence of the investigated factors on the compressive strength of the lime composite. The significance of the coefficients of the regression equation indicates a significant effect of a density and a silicate module on the optimization parameter. The interpretation of the absolute values of the coefficients of the regression equation and their signs indicates the predominant influence of the modulus of the liquid glass on the formation of compressive strength. The graphic interpretation of the resulting model is shown in Figure 5.
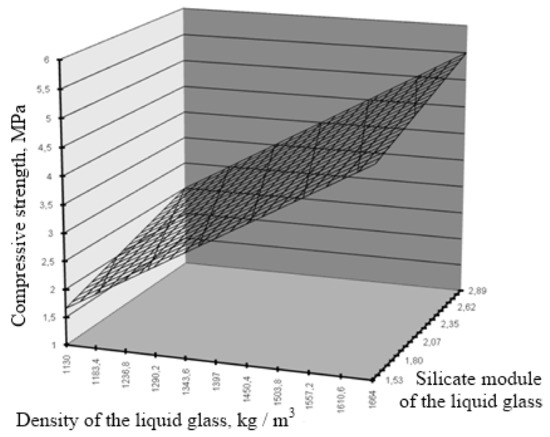
Figure 5.
Dependence of compressive strength on the characteristics of liquid glass in the “lime + CSH” system.
Table 7 shows that the activity of the filler varies depending on the synthesis mode in the range of 178–289 mg/g.

Table 7.
Dependence of the filler activity on the synthesis conditions.
The activity of the fillers depends on the drying temperature (Table 8). The most active is the filler dried after filtration at a temperature of 300 °C. Compressive strength Rcom of specimens at the age of 28 days of hardening in air-dry conditions, of the composition 10.3 (lime:CSH filler) by weight at a water:lime ratio equal to 0.7, when using the filler dried at temperature of 300 °C, is Rcom = 6.79 MPa. In addition, the composition with the use of the filler dried at a temperature of 105 °C is 4.56 MPa (i.e., the increase in compressive strength is 50%).

Table 8.
Strength of the lime compositions.
In order to study the influence of the terms and conditions of storage of the filler on its activity, part of the filler was stored in conditions excluding the access of moisture, and remaining part was stored in the open air at a relative humidity of 70–75% and a temperature of 18–20 °C. After storage of the filler for 10–40 days, specimens of the composition lime:CSH filler = 1:0.3 were molded with water:lime ratios of 0.7 and 0.9. The specimens hardened at a relative air humidity of 70% and a temperature of 18–20 °C (Table 9). Specimens molded immediately after drying of the fillers were taken as control ones.

Table 9.
Effect of storage conditions on the filler activity.
The research results shown in Table 9 indicate that during storage of the filler under conditions excluding moisture access, the activity of the filler practically does not change. Therefore, the value of the compressive strength of the samples molded at w/l = 0.65 on the filler immediately after drying is Rcom = 4.56 MPa, and molded on the filler after storage for 10–20 days is 4.56–4.50 MPa. Some fluctuations in compressive strength values are associated with statistical variability. After 20 days of storage in conditions that exclude moisture access, its activity decreases slightly. The decrease in the compressive strength of specimens molded at w/l = 0.7 on the filler after storage for 30–40 days is 8.3–19.7%, and at w/l = 0.9 is 10.5–24.6%.
Storage of fillers in air-dry conditions dramatically changes its activity. Thus, the decrease in the compressive strength of the lime compositions molded at w/l = 0.9 on the filler after storage for 30–40 days is 57.9–68.6%.
The generalized analysis of the results of experimental data made it possible to establish the optimal ratio of components, select the type, amount, and concentration of the solution of the precipitant additive involved in the synthesis of the filler, and also establish the optimal density and modulus of the liquid glass. The greatest advantages in terms of strength characteristics are possessed by compositions with the filler obtained by synthesis from the liquid glass, with a density of 1335–1663 kg/m3 and a silicate module of 1.53–2.9, in the presence of a precipitating additive CaCl2 in an amount of 30–50% of the weight of the liquid glass in the form of a 7.5–15% solution, and dried at a temperature of 105 °C.
3.2. Development of the Modified Lime Compositions
The hardening of solutions on slaked lime is due to the occurrence of two processes: Crystallization of Ca(OH)2 during drying of solutions and carbonization of hydroxide. This process takes place primarily in the surface layers. Carbonization of deep layers is long-lasting, because, firstly, the amount of CO2 in the atmosphere is only 0.04%, and, secondly, the resulting CaCO3 film has low permeability. Therefore, in the central part of well-compacted solutions, a significant amount of Ca(OH)2 remains for a long time. Evaporation of water from the solution also helps to increase the strength. The formation of CaCO3 leads to an increase in the strength and water resistance of products. If active additives are used as a filler, along with the formation of carbonates, calcium silicate hydrates may also appear, which increase the strength of solutions. The formation of a significant amount of silicate hydrates that improve the adhesion of the binder to the aggregate, and the high plastic strength of lime mortars is explained (Figure 6).
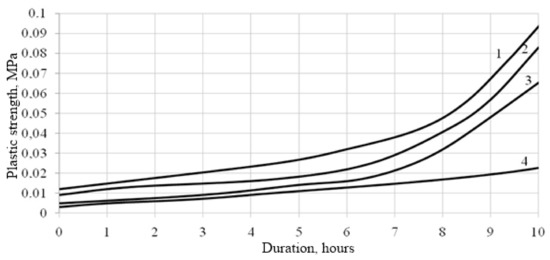
Figure 6.
Change in the plastic strength of the specimen 03E depending on the filler synthesis conditions. 1—the filler was synthesized with the rapid introduction of CaCl2 additive in an amount of 90% of the water glass mass, the specific surface of the filler is 5876 cm2/g; 2—the filler was synthesized with the slow introduction of CaCl2 additive in an amount of 90% of the water glass mass, the specific surface of the filler is 4669 cm2/g; 3—the filler was synthesized with the rapid introduction of CaCl2 additive in an amount of 90% of the weight of the liquid glass, and kept for three days, the specific surface of the filler is 4439 cm2/g; 4—lime paste (specimen Ref.).
It was found that the use of the CSH fillers in the lime compositions, obtained with the rapid introduction of CaCl2 additives into the liquid glass during the filler synthesis, accelerates the plastic strength gain of the lime compositions with this filler (Figure 6, curve 1). Thus, the plastic strength of the lime composition when using a filler obtained with the rapid introduction of CaCl2 additive, in the form of a solution of 15% concentration at the age of 4.5 h, is τ = 0.025 MPa, and the filler synthesized with the slow introduction of a precipitating additive is τ = 0.017 MPa (Figure 6, curve 2). The filler, obtained by holding the filtrate for three days, slows down the development of plastic strength (Figure 6, curve 3). At the age of 4.5 h, the plastic strength is τ = 0.012 MPa. Shrinkage reduction of the lime mortars is achieved by introducing quartz sand (Figure 7).
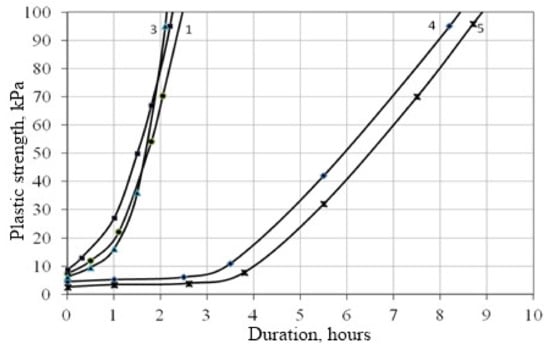
Figure 7.
Change in the plastic strength of the lime specimens: 1—03A+S-3 superplasticizer; 2—03B+SP-3 superplasticizer; 3—03A+Kratasol superplasticizer; 4—03C; 5—03D+S-3 superplasticizer.
It was found that the introduction of the S-3 superplasticizer leads to a slowdown in the gain of plastic strength (Figure 7, curve 5). A decrease in the amount of mixing water (w/b = 1.1), taking into account the water-reducing coefficient, naturally contributes to a faster gain of plastic strength in compositions with superplasticizers (Figure 7, curves 1, 2, 3). Thus, the plastic strength of the composition with the addition of S-3 at the time of 1 h of hardening is τ = 21.7 kPa, for the 03C composition, τ = 5.2 kPa. When the Kratasol additive is introduced into the formulation at the first stage of hardening (up to 1 h 20 min), there is a slight slowdown in the set of plastic strength compared to compositions containing the additives S-3 and SP-3; however, subsequently the plastic strength of the composition with the addition of Kratasol is higher.
The values of adhesion and compressive strength of the lime composites are shown in Table 10.

Table 10.
Adhesion and compressive strength of the lime composites (28 days).
The CSH filler addition to the DPM results in increased compressive strength and adhesion. Compressive strength and adhesion of the specimens with the following ratios “lime:CSH = 1:0.3; binder:sand = 1:3 and water/binder = 1.8”, tested at the age of 28 days of hardening in air-dry conditions at temperature of 18–20 °C and a relative humidity of 60–70%, are equal to 1.76 and 0.25 MPa, respectively, and specimens of the composition “binder:sand = 1:3 and water/binder = 1.8” are 0.96 and 0.16 MPa, respectively. A decrease in the amount of mixing water due to the introduction of the S-3 superplasticizer additive into the formulation causes a natural increase in the strength of the lime composite.
The results of assessing the shrinkage deformations of the finishing mixes are shown in Figure 8.
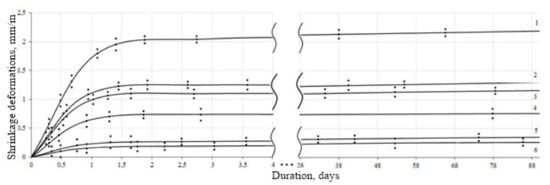
Figure 8.
Shrinkage kinetics of finishing mixes: 1—Ref-2; 2—Ref-1; 3—03B; 4—03A; 5—03D+S-3+Neolith 7200; 6—03C+S-3+Pulver DM 1142P.
Shrinkage deformations of specimens with ratios “Iime:sand = 1:3 and water/binder = 1.8” after three months were 2.12 mm/m (Figure 8, curve 1). When the CSH filler is introduced into the lime composition, a decrease in shrinkage is observed, which is for the composition “lime:CSH = 1:0.3, binder:sand = 1:3 and water:binder = 1.8” after three months was 1.16 mm/m (Figure 8, curve 2). An increase in the amount of sand in the mix helps to reduce shrinkage. Shrinkage deformations of specimens with ratios “lime:CSH = 1:0.3, binder:sand = 1: 4 and water/binder = 1.8” were 0.76 mm/m (Figure 8, curve 3). The introduction of superplasticizer S-3 and redispersible powders such as Neolith 7200 and Pulver DM 1142P into the composition of the DPM also leads to a decrease in shrinkage deformations. Shrinkage value after three months of hardening for compositions “lime:CSH = 1:0.3, binder:sand = 1:3 and water/binder = 1.0+S-3+Neolith 7200” and “lime:CSH = 1:0.3, binder:sand = 1: 4 and water/binder = 1.0+S-3+Pulver DM 1142P” was 0.34 and 0.26 mm/m, respectively (Figure 8, curves 4 and 5).
Table 11 shows the results of evaluating crack resistance through the ratio of the flexural strength Rflex to the compressive strength Rcom of specimens aged 28 days.

Table 11.
Crack resistance coefficient of the lime coatings.
The crack resistance coefficient of lime compositions is 0.26–0.28, depending on the lime:sand ratio. The introduction of the CSH filler into the dry mix increases the Rflex:Rcom ratio by 32–35%. Thus, crack resistance coefficient of the specimens with the composition “lime:CSH = 1:0.3, binder:sand = 1:3 and water/binder = 1.8” is 0.35, and for the specimens with the composition “lime:CSH = 1: 0,3, binder:sand = 1: 4 and water/binder = 1.8” it is equal to 0.37. With the introduction of redispersible powders, such as Neolith 7200 and Pulver DM 1142P in the presence of S-3 superplasticizer, taking into account the water-reducing coefficient in the finishing composition, the Rflex:Rcom ratio increases by 75–96%. The crack resistance coefficient of specimens with the composition “lime:CSH = 1:0.3, binder:sand = 1:3, water/binder = 1.0+S-3+Neolith 7200” it is 0.51, and specimens with the composition “lime:CSH = 1:0.3, binder:sand = 1: 4; water/binder = 1+S-3+Pulver DM 1142P” it is 0.49.
To increase the decorative properties, the pigments were introduced into the mix in an amount of 1% to 5%, while, in particular, the introduction of blue pigment into the DPM leads to a decrease in the strength characteristics by 10–13% (Table 12).

Table 12.
Compressive strength of the painted lime composites.
Table 13 shows that painted specimens with a yellow pigment, along with uncolored samples, withstood 50 cycles of frost resistance tests, while the condition of the coating after 50 test cycles was assessed satisfactorily, which is characterized by a loss of gloss up to 5%, a barely noticeable color change and the absence of whitishness, bronzing, dirt retention, peeling, cracking, rashes, and blistering of the surface.

Table 13.
Quality of the appearance of painted coatings.
The appearance of the specimens after 50 test cycles is shown in Figure 9.

Figure 9.
The appearance of the specimens after 50 test cycles: (a) 03D+S-3+Neolith 7200; (b) 03D+S-3+Neolith 7200 + 5% yellow pigment.
Finishing coatings prepared on the modified lime binder are characterized by increased water resistance (Table 14).

Table 14.
Water resistance of the finishing coatings.
Thus, the softening coefficients of the reference specimens are 0.42–0.45, and of the modified specimens are 0.53 and 0.56, depending on the ratio of binder:sand. With the introduction of the superplasticizer into the composition, the specimens with the Kratasol addition showed a higher value of the softening coefficient (0.68), which, apparently, is explained by the presence of a hydrophobic component in the additive, as well as the creation of a denser structure due to a decrease in the amount of mixing water. This is evidenced by the data on the kinetics of water absorption of the lime composites (Figure 10).
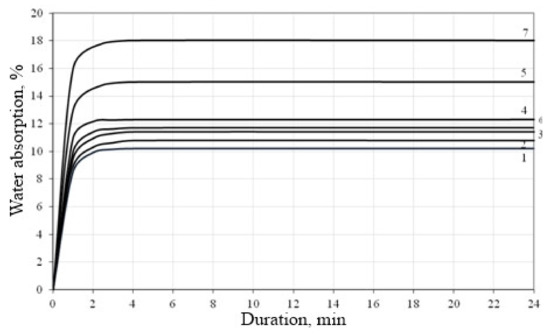
Figure 10.
Water absorption of the lime composites: 1—03C+Kratasol; 2—03C+S-3; 3—03C+Hidetal P-4; 4—03C+SP-3; 5—03A; 6—03D+S-3; 7—03B.
It was found that, during the initial 1–4 min, all samples showed intense water absorption, and subsequently the water absorption indicators stabilized. Water absorption of the composition “water/binder = 1.8, binder:sand = 1: 4” after 24 h was 15% by weight (Figure 10, curve 5), and the composition “water/binder = 1.8, binder:sand = 1:3” was 18%. When a superplasticizer is introduced into the mix, a decrease in water absorption is observed. A lower value of the water absorption index, which is 10.2% at 24 h water absorption (Figure 10, curve 1), is observed in the samples prepared with the use of the Kratasol additive.
Traditional limestone mixes form coatings that are characterized by high porosity and a significant volume of open pores [29,30,31,32]. The addition of the CSH to the mix leads to a decrease in porosity. Thus, the porosity of the specimen based on the reference composition is 37.5%, and with the CSH addition is 31.6%. The introduction of the additive S-3, as well as the Pulver DM 1142P, taking into account the water-reducing effect, allows the porosity to be reduced to 24.8–25.2%. Table 15 shows the results of determining the water absorption coefficient during capillary suction of the lime composites.

Table 15.
Water absorption during capillary suction.
The numerical values of the water absorption coefficient, which for compositions with Kratasol and S-3+Pulver DM 1142P additives, respectively, were 0.49 and 0.39 kg/(m2·h0.5), indicate that coatings in accordance with DIN 52617 are water-repellent and hydrophobic (less than 0.5 kg/(m2·h0.5).
Figure 11 shows the developed technological scheme for the production of the lime dry plaster mix. This technological scheme can be implemented in existing factories of building materials without significant re-equipment of production.
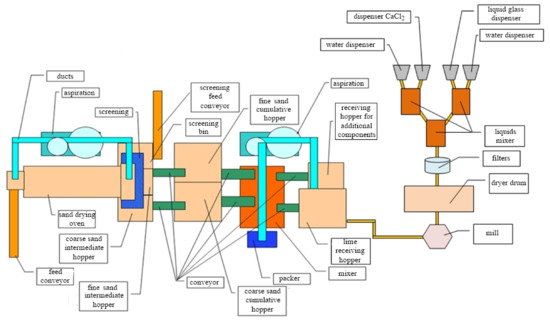
Figure 11.
Technological scheme for the production of lime dry plaster mix.
4. Conclusions
- A composition for exterior and interior decoration of walls of buildings has been developed in the form of a dry mix, including slaked lime, CSH filler, sand, superplasticizer S-3, and redispersible powder Neolith P7200. This composition makes it possible to obtain mortar mixes with a water-holding capacity of 98–99%. Coatings based on the proposed DPM are characterized by a vapor permeability coefficient of 0.05 mg/m·h·Pa, an adhesion strength of 0.6–0.9 MPa, and a compressive strength of 3–4 MPa.
- The regularities of the filler synthesis were established depending on the temperature, density and silicate modulus of liquid glass, the amount of the precipitant additive, the rate of its introduction, and the drying mode. The optimal content of the precipitant additive was found to be 30–50% of the mass of water glass in the form of a 7.5–15% solution. The optimal density of liquid glass is determined, which is kg/m3, depending on the modulus of liquid glass. The filler output is 85–100%, depending on the synthesis conditions.
- The phase composition of the filler was revealed, which is characterized by the presence of calcium silicate hydrates of the tobermorite group, solid solution CSH(B) in the form of weakly crystallized gel, solid solution C–S–H(II), hydrohalites, and calcites. It is shown that the curves of particle size distribution of the silicate-containing filler are of a polyfraction nature. The average particle diameter is 28–34 µm, depending on the filler synthesis mode. It was found that the filler based on calcium silicate hydrates has a high sorption capacity, which is 95% at 100% air humidity.
- It is shown that the filler based on calcium silicate hydrates has a hydraulic activity of 178–289 mg/g depending on the synthesis mode. It was found that the amount of free lime in the mixes was reduced by more than two times in the presence of CSH additive.
- Regularities of changes in rheological and technological properties of the lime finishing compositions are established depending on the conditions of the synthesis of the filler and its content, as well as the type of plasticizing additive.
- It was revealed that the lime compositions with the CSH filler are characterized by reduced shrinkage deformations up to 45%. The introduction of the CSH filler into the lime compositions increases the water resistance of the lime finishing layer by 36%.
- A technological scheme for the production of the lime dry plaster mixes was developed, it can be introduced at existing factories of building materials without significant re-equipment of production.
Author Contributions
Conceptualization, V.L.; methodology, K.S., Y.V., N.V. and R.F.; software, R.F.; validation, Y.V., V.L., K.S. and R.F.; resources, Y.V., N.V., K.S. and R.F.; writing—original draft preparation, V.L., R.F., K.S., N.V., V.U., M.A., M.S. and V.L.; writing—review and editing, Y.V., K.S., N.V. and Y.V.; visualization, V.L., R.F., N.V. and Y.V.; supervision, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research is partially funded by the Ministry of Science and Higher Education of the Russian Federation as part of World-class Research Center program: Advanced Digital Technologies (contract No. 075-15-2020-934 dated 17 November 2020).
Data Availability Statement
All data, models, and code generated or used during the study appear in the submitted article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zimich, V.V. Magnesium dry mixes for outer wall decoration. In Materials Science Forum; Trans Tech Publications Ltd.: Hong Kong, China, 2016. [Google Scholar]
- Elistratkin, M.Y.; Minakova, A.V.; Jamil, A.N.; Kukovitsky, V.V.; Eleyan, I.J.I. Composite Binders For Finishing Compositions. Constr. Mater. Prod. 2020. [Google Scholar] [CrossRef]
- Pavlík, Z.; Fořt, J.; Pavlíková, M.; Pokorný, J.; Trník, A.; Černý, R. Modified lime-cement plasters with enhanced thermal and hygric storage capacity for moderation of interior climate. Energy Build. 2016, 126, 113–127. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Vejmelková, E.; Keppert, M.; Keršner, Z.; Rovnaníková, P.; Černý, R. Mechanical, fracture-mechanical, hydric, thermal, and durability properties of lime-metakaolin plasters for renovation of historical buildings. Constr. Build. Mater. 2012. [Google Scholar] [CrossRef]
- Walker, R.; Pavía, S. Thermal performance of a selection of insulation materials suitable for historic buildings. Build. Environ. 2015, 94, 155–165. [Google Scholar] [CrossRef]
- Kingery, W.D.; Vandiver, P.B.; Prickett, M. The beginnings of pyrotechnology, part ii: Production and use of lime and gypsum plaster in the pre-pottery neolithic near east. J. Field Archaeol. 1988, 15, 219–243. [Google Scholar] [CrossRef]
- Fassina, V.; Favaro, M.; Naccari, A.; Pigo, M. Evaluation of compatibility and durability of a hydraulic line-based plaster applied on brick wall masonry of historical buildings affected by rising damp phenomena. J. Cult. Herit. 2002, 3, 45–51. [Google Scholar] [CrossRef]
- Horgnies, M.; Bayle, M.; Gueit, E.; Darque-Ceretti, E.; Aucouturier, M. Microstructure and Surface Properties of Frescoes Based on Lime and Cement: The Influence of the Artist’s Technique. Archaeometry 2015, 57, 344–361. [Google Scholar] [CrossRef]
- Ayzenshtadt, A.; Lesovik, V.; Frolova, M.; Tutygin, A.; Danilov, V. Nanostructured wood mineral composite. Procedia Eng. 2015, 117, 45–51. [Google Scholar] [CrossRef]
- Kahrovic, E.; Jakovljevic, V.; Zahirovic, A. Ftir investigation of pigments and binder of painted walls in heritage monuments. J. Sci. Arts 2020. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Alves, C.; Velosa, A.; Rocha, F. Bentonite as a natural additive for lime and lime-metakaolin mortars used for restoration of adobe buildings. Cem. Concr. Compos. 2015, 60, 99–110. [Google Scholar] [CrossRef]
- Jerman, M.; Scheinherrová, L.; Medveď, I.; Krejsová, J.; Doleželová, M.; Bezdička, P.; Černý, R. Effect of cyclic wetting and drying on microstructure, composition and length changes of lime-based plasters. Cem. Concr. Compos. 2019, 104, 103411. [Google Scholar] [CrossRef]
- Bu, J.; Teresa, R.G.; Brown, K.G.; Sanchez, F. Adsorption mechanisms of cesium at calcium-silicate-hydrate surfaces using molecular dynamics simulations. J. Nucl. Mater. 2019, 515, 35–51. [Google Scholar] [CrossRef]
- Kellenberger, D.; Althaus, H.-J.; Jungbluth, N.; Künninger, T.; Lehmann, M.; Thalmann, P. Life Cycle Inventories of Building Products; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007. [Google Scholar]
- Escadeillas, G.; Magniont, C.; Amziane, S.; Nozahic, V. Binders. In Bio-Aggregate-Based Building Materials: Applications to Hemp Concretes; Wiley-ISTE: Paris, France, 2013; ISBN 9781848214040. [Google Scholar]
- Feduik, R. Reducing permeability of fiber concrete using composite binders. Spec. Top. Rev. Porous Media 2018, 9, 79–89. [Google Scholar] [CrossRef]
- Fediuk, R.; Yushin, A. Composite binders for concrete with reduced permeability. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bristol, UK, February 2016; Volume 116. [Google Scholar]
- Mukhopadhyaya, P.; Kumaran, K.; Plescia, S.; Lackey, J.; Normandin, N.; Van Reenen, D. High performance stucco to optimize moisture management in wood-frame stucco walls. J. Test. Eval. 2008, 36, 506–515. [Google Scholar]
- Vejmelková, E.; Keppert, M.; Sovják, R.; Konvalinka, P.; Černý, R. Material properties of plasters for façade renovation of historical buildings. In Proceedings of the 10th International Conference Modern Building Materials, Structures and Techniques, Vilnius, Lithuania, 19–21 May 2010. [Google Scholar]
- Robador, M.D.; Arroyo, F. Characterisation of Roman coatings from the a Roman house in Mérida (Spain). J. Cult. Herit. 2013, 14, S52–S58. [Google Scholar] [CrossRef]
- Galván-Ruiz, M.; Hernández, J.; Baños, L.; Noriega-Montes, J.; Rodríguez-García, M.E. Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction. J. Mater. Civ. Eng. 2009, 21, 694–698. [Google Scholar] [CrossRef]
- Fediuk, R.; Pak, A.; Kuzmin, D. Fine-Grained Concrete of Composite Binder. In Proceedings of the IOP Conference Series: Materials Science and Engineering, International Conference on Construction, Architecture and Technosphere Safety (ICCATS 2017), Chelyabinsk, Russian, 21–22 September 2017. [Google Scholar]
- Monkman, S.; Shao, Y. Assessing the Carbonation Behavior of Cementitious Materials. J. Mater. Civ. Eng. 2006, 18, 768–776. [Google Scholar] [CrossRef]
- Ibragimov, R.; Fediuk, R. Improving the early strength of concrete: Effect of mechanochemical activation of the cementitious suspension and using of various superplasticizers. Constr. Build. Mater. 2019, 226, 839–848. [Google Scholar] [CrossRef]
- Das, S.K. Yudhbir Geotechnical Characterization of Some Indian Fly Ashes. J. Mater. Civ. Eng. 2005, 17, 544–552. [Google Scholar] [CrossRef]
- Morin, V.; Garrault, S.; Begarin, F.; Dubois-Brugger, I. The influence of an ion-exchange resin on the kinetics of hydration of tricalcium silicate. Cem. Concr. Res. 2010, 40, 1459–1464. [Google Scholar] [CrossRef]
- Fediuk, R.S. Mechanical Activation of Construction Binder Materials by Various Mills. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bristol, UK, 1 April 2016; Volume 125. [Google Scholar]
- Salamanova, M.S.; Murtazaev, S.-A.Y.; Alaskhanov, A.K.; Ismailova, Z.K.; Salamanova, M.S. Development of Multicomponent Binders Using Fine Powders. In Proceedings of the International Symposium “Engineering and Earth Sciences: Applied and Fundamental Research” Dedicated to the 85th Anniversary of H.I. Ibragimov (ISEES 2019), Grozny, Russia, 10–13 June 2019. [Google Scholar]
- Lukuttsova, N.; Ustinov, A. Concrete modified by additive based on biosilicated nanotubes. Int. J. Appl. Eng. Res. 2015, 10, 40457. [Google Scholar]
- Tolstoy, A.D.; Lesovik, V.S.; Glagolev, E.S.; Krymova, A.I. Synergetics of hardening construction systems. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2018. [Google Scholar]
- Molodtsov, M.V.; Vshivkov, E.P.; Molodtsova, V.E. Behavior of concrete beams reinforced with fiberglass composite rebar under load. Mag. Civ. Eng. 2020, 97, 9703. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
