Halogen Bonding Involving I2 and d8 Transition-Metal Pincer Complexes
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
4. Conclusions
- According to our results, the catalytic activity of the original pincer complex is related to the 3c–4e character of the non-classical three-center M–I–I bond, which is involved in the first step of the oxidative addition of molecular iodine I2 to the metal. The charge transfer from the metal to the antibonding orbital of the I–I bond changes the 3c–4e character of the three-center M–I–I bond, which in turn leads to a weakening of the I–I bond and a strengthening of the M–I bond.
- The largest change in charge transfer with regard to the original van Koten complex 1 was observed for the complexes with Co, Rh and Ir transition metals and a pyridine instead of a benzene ligand, for which we observed an inverse 3c–4e character of the three-center M–I–I bond, i.e., the M–I bond becomes stronger than the I–I bond. The large 3c–4e character in these three pincer complexes is attributed to relativistic effects which expand the d orbitals of the metal leading to a larger charge transfer to the antibonding orbital of the I–I ligand.
- According to solvent calculations, the charge transfer is increased in a polar solvent, which leads to a larger polarization of the M–I–I three-center bond, increasing its 3c–4e character and decreasing the strength of the I–I bond.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chipperfield, J.R.; Ford, J.; Hayter, A.C.; Lee, D.J.; Webster, D.E. Reactivity of Main-Group-Transition-Metal Bonds. Part V. Kinetics of Reactions of Halogens and Interhalogens with Pentacarbonyl(Trimethylstannyl)Manganese, Tricarbonyl(η-Cyclopentadienyl)(Trimethylstannyl)Molybdenum, and Dicarbonyl(η-Cyclopentadienyl)(Trimethylstannyl)Iron, Including the Effects of Solvents on Reactivity. J. Chem. Soc. Dalton Trans. 1976, 11, 1024–1028. [Google Scholar] [CrossRef]
- Piccirilli, L.; Pinheiro, D.L.J.; Nielsen, M. Recent Progress with Pincer Transition Metal Catalysts for Sustainability. Catalysts 2020, 10, 773. [Google Scholar] [CrossRef]
- Budweg, S.; Junge, K.; Beller, M. Catalytic oxidations by dehydrogenation of alkanes, alcohols and amines with defined (non)-noble metal pincer complexes. Catal. Sci. Technol. 2020, 10, 3825–3842. [Google Scholar] [CrossRef]
- Lawrence, M.; Green, K.; Nelson, P.; Lorraine, S. Review: Pincer ligands—Tunable, versatile and applicable. Polyhedron 2018, 143, 11–27. [Google Scholar] [CrossRef]
- Morales-Morales, D. Pincer Compounds: Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Asay, M.; Morales-Morales, D. Non-symmetric pincer ligands: Complexes and applications in catalysis. Dalton Trans. 2015, 44, 17432–17447. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; van Koten, G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3750–3781. [Google Scholar] [CrossRef]
- Van der Boom, M.E.; Milstein, D. Cyclometalated Phosphine-Based Pincer Complexes: Mechanistic Insight in Catalysis, Coordination, and Bond Activation. Chem. Rev. 2003, 103, 1759–1792. [Google Scholar] [CrossRef] [PubMed]
- Kubas, G.J. Molecular Hydrogen Complexes: Coordination of a σ Bond to Transition Metals. Acc. Chem. Res. 1988, 21, 120–128. [Google Scholar] [CrossRef]
- Musa, S.; Shaposhnikov, I.; Cohen, S.; Gelman, D. Ligand-Metal Cooperation in PCP Pincer Complexes: Rational Design and Catalytic Activity in Acceptorless Dehydrogenation of Alcohols. Angew. Chem. Int. Ed. 2011, 50, 3533–3537. [Google Scholar] [CrossRef]
- Dani, P.; Karlen, T.; Gossage, R.A.; Gladiali, S.; van Koten, G. Hydrogen-Transfer Catalysis with Pincer-Aryl Ruthenium(II) Complexes. Angew. Chem. Int. Ed. 2000, 39, 743–745. [Google Scholar] [CrossRef]
- Jensen, C.M. Iridium PCP Pincer Complexes: Highly Active and Robust Catalysts for Novel Homogeneous Aliphatic Dehydrogenations. Chem. Commun. 1999, 2443–2449. [Google Scholar] [CrossRef]
- Choi, J.; MacArthur, A.H.R.; Brookhart, M.; Goldman, A.S. Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes. Chem. Rev. 2011, 111, 1761–1779. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)-Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Pak, E.B.; Singh, B.; Jensen, C.M.; Goldman, A.S. Dehydrogenation of n-Alkanes Catalyzed by Iridium “Pincer” Complexes: Regioselective Formation of α-Olefins. J. Am. Chem. Soc. 1999, 121, 4086–4087. [Google Scholar] [CrossRef]
- Jongbloed, L.S.; Vogt, N.; Sandleben, A.; de Bruin, B.; Klein, A.; van der Vlugt, J.I. Nickel-Alkyl Complexes with a Reactive PNC-Pincer Ligand. Eur. J. Inorg. Chem. 2018, 2018, 2408–2418. [Google Scholar] [CrossRef]
- Fang, S.; Chen, H.; Wei, H. Insight into Catalytic Reduction of CO2 to Methane with Silanes using Brookhart’s Cationic Ir(III) Pincer Complex. RSC Adv. 2018, 8, 9232–9242. [Google Scholar] [CrossRef]
- Yao, L.; Li, Y.; Huang, L.; Guo, K.; Ren, G.; Wu, Z.; Lei, Q.; Fang, W.; Xie, H. A DFT Study on the Mechanisms of Hydrogenation and Hydrosilylation of Nitrous Oxide Catalyzed by a Ruthenium PNP Pincer Complex. Comput. Theor. Chem. 2018, 1128, 48–55. [Google Scholar] [CrossRef]
- Murphy, L.J.; Hollenhorst, H.; McDonald, R.; Ferguson, M.; Lumsden, M.D.; Turculet, L. Selective Ni-Catalyzed Hydroboration of CO2 to the Formaldehyde Level Enabled by New PSiP Ligation. Organometallics 2017, 36, 3709–3720. [Google Scholar] [CrossRef]
- Ramaraj, A.; Reddy, K.H.K.; Keil, H.; Herbst-Irmer, R.; Stalke, D.; Jemmis, E.D.; Jagirdar, B.R. Approaches to Sigma Complexes via Displacement of Agostic Interactions: An Experimental and Theoretical Investigation. Organometallics 2017, 36, 2736–2745. [Google Scholar] [CrossRef]
- Imayoshi, R.; Nakajima, K.; Takaya, J.; Iwasawa, N.; Nishibayashi, Y. Synthesis and Reactivity of Iron- and Cobalt-Dinitrogen Complexes Bearing PSiP-Type Pincer Ligands toward Nitrogen Fixation. Eur. J. Inorg. Chem. 2017, 2017, 3769–3778. [Google Scholar] [CrossRef]
- Mazzotta, M.G.; Xiong, M.; Abu-Omar, M.M. Carbon Dioxide Reduction to Silyl-Protected Methanol Catalyzed by an Oxorhenium Pincer PNN Complex. Organometallics 2017, 36, 1688–1691. [Google Scholar] [CrossRef]
- Zeng, R.; Feller, M.; Ben-David, Y.; Milstein, D. Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex. J. Am. Chem. Soc. 2017, 139, 5720–5723. [Google Scholar] [CrossRef] [PubMed]
- Wenz, J.; Wadepohl, H.; Gade, L.H. Regioselective Hydrosilylation of Epoxides Catalysed by Nickel(II) Hydrido Complexes. Chem. Commun. 2017, 53, 4308–4311. [Google Scholar] [CrossRef]
- Fang, H.; Guo, L.; Zhang, Y.; Yao, W.; Huang, Z. A Pincer Ruthenium Complex for Regioselective C–H Silylation of Heteroarenes. Org. Lett. 2016, 18, 5624–5627. [Google Scholar] [CrossRef] [PubMed]
- Charboneau, D.J.; Balcells, D.; Hazari, N.; Lant, H.M.C.; Mayer, J.M.; Melvin, P.R.; Mercado, B.Q.; Morris, W.D.; Repisky, M.; Suh, H.W. Dinitrogen-Facilitated Reversible Formation of a Si–H Bond in a Pincer-Supported Ni Complex. Organometallics 2016, 35, 3154–3162. [Google Scholar] [CrossRef]
- Buslov, I.; Keller, S.C.; Hu, X. Alkoxy Hydrosilanes As Surrogates of Gaseous Silanes for Hydrosilylation of Alkenes. Org. Lett. 2016, 18, 1928–1931. [Google Scholar] [CrossRef] [PubMed]
- Comanescu, C.C.; Iluc, V.M. E–H (E = B, Si, Ge) Bond Activation of Pinacolborane, Silanes, and Germanes by Nucleophilic Palladium Carbene Complexes. Chem. Commun. 2016, 52, 9048–9051. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, X.; Zhang, S.; Shi, Y.; Sun, H. Synthesis and Reactivity of N-Heterocyclic PSiP Pincer Iron and Cobalt Complexes and Catalytic Application of Cobalt Hydride in Kumada Coupling Reactions. Organometallics 2016, 35, 357–363. [Google Scholar] [CrossRef]
- Mucha, N.T.; Waterman, R. Correction to Iridium Pincer Catalysts for Silane Dehydrocoupling: Ligand Effects on Selectivity and Activity. Organometallics 2015, 34, 5682. [Google Scholar] [CrossRef]
- LaPierre, E.A.; Piers, W.E.; Spasyuk, D.M.; Bi, D.W. Activation of Si–H Bonds across the Nickel Carbene Bond in Electron Rich Nickel PCcarbeneP Pincer Complexes. Chem. Commun. 2016, 52, 1361–1364. [Google Scholar] [CrossRef]
- Hao, J.; Vabre, B.; Zargarian, D. Reactions of Phenylhydrosilanes with Pincer–Nickel Complexes: Evidence for New Si–O and Si–C Bond Formation Pathways. J. Am. Chem. Soc. 2015, 137, 15287–15298. [Google Scholar] [CrossRef]
- Buslov, I.; Becouse, J.; Mazza, S.; Montandon-Clerc, M.; Hu, X. Chemoselective Alkene Hydrosilylation Catalyzed by Nickel Pincer Complexes. Angew. Chem. Int. Ed. 2015, 54, 14523–14526. [Google Scholar] [CrossRef]
- Puddephatt, R.J. Coordinative Unsaturation in Platinum(IV) Chemistry: From Proposed Reaction Intermediates to the First Structurally Characterized Complexes. Angew. Chem. Int. Ed. 2002, 41, 261. [Google Scholar] [CrossRef]
- Titova, E.M.; Osipova, E.S.; Pavlov, A.A.; Filippov, O.A.; Safronov, S.V.; Shubina, E.S.; Belkova, N.V. Mechanism of Dimethylamine–Borane Dehydrogenation Catalyzed by an Iridium(III) PCP-Pincer Complex. ACS Catal. 2017, 7, 2325–2333. [Google Scholar] [CrossRef]
- Rogachev, A.Y.; Hoffmann, R. Iodine (I2) as a Janus–Faced Ligand in Organometallics. J. Am. Chem. Soc. 2013, 135, 3262–3275. [Google Scholar] [CrossRef] [PubMed]
- Gossage, R.A.; Ryabov, A.D.; Spek, A.L.; Stufkens, D.J.; van Beek, J.A.M.; van Eldik, R.; van Koten, G. Models for the Initial Stages of Oxidative Addition. Synthesis, Characterization, and Mechanistic Investigation of η1–I2 Organometallic “Pincer” Complexes of Platinum. X-ray Crystal Structures of [PtI(C6H3{CH2NMe2}2–2,6)(η1–I2)] and exo-meso-[Pt(η1–I3)(η1–I2)(C6H3{CH2N(t–Bu)Me}2–2,6)]. J. Am. Chem. Soc. 1999, 121, 2488–2497. [Google Scholar] [CrossRef]
- Beek, J.A.M.V.; Koten, G.V.; Smeets, W.J.J.; Spek, A.L. Model for the Initial Stage in the Oxidative Addition of I2 to Organoplatinum(II) Compounds. X-Ray Structure of Square-Pyramidal [PtIII{C6H3(CH2NMe2)2−o,o′}(η1–I2)] containing a Linear Pt–I–I Arrangement. J. Am. Chem. Soc. 1986, 108, 5010–5011. [Google Scholar] [CrossRef]
- Van Koten, G.; Hollis, T.K.; Morales-Morales, D. Pincer Chemistry and Catalysis. Eur. J. Inorg. Chem. 2020, 2020, 4416–4417. [Google Scholar] [CrossRef]
- Van Koten, G. Novel Aspects of η’-Diiodine Coordination and Diiodine Oxidative Addition to Platinum(II) and Halide Transfer Oxidation Reactions of Organo-Platinum(II) with CuIIX2. Pure Appl. Chem. 1990, 62, 1155–1159. [Google Scholar] [CrossRef]
- Van Koten, G. Highlights of 45 years of research: A personal account. J. Organomet. Chem. 2017, 845, 4–18. [Google Scholar] [CrossRef]
- Van Beek, J.A.; van Koten, G.; Dekker, G.P.; Wissing, E.; Zoutberg, M.C.; Stam, C.H. Synthesis and Reactivity Towards Diiodine of Palladium(II) and Platinum(II) Complexes with Non-Cyclic and Cyclic Ligands (C6H3{CH2NR1R2}2–2,6)−. End-on Diiodine-Platinum(II) Bonding in Macrocyclic [PtI(C6H3{CH2NMe(CH2)7MeNCH2}–2,6)(η1–I2)]. J. Organomet. Chem. 1990, 394, 659–678. [Google Scholar] [CrossRef]
- Li, Y.H.; Ding, X.H.; Zhang, Y.; He, W.R.; Huang, W. Synthesis, characterization, and catalytic behavior of a PSiP pincer-type ruthenium(II) complex. Inorg. Chem. Commun. 2012, 15, 194–197. [Google Scholar] [CrossRef]
- Scharf, A.; Goldberg, I.; Vigalok, A. Evidence for Metal–Ligand Cooperation in a Pd–PNF Pincer-Catalyzed Cross-Coupling. J. Am. Chem. Soc. 2012, 135, 967–970. [Google Scholar] [CrossRef]
- Robinson, T.P.; De Rosa, D.; Aldridge, S.; Goicoechea, J.M. On the Redox Reactivity of a Geometrically Constrained Phosphorus(III) Compound. Chem. Eur. J. 2017, 23, 15455–15465. [Google Scholar] [CrossRef] [PubMed]
- Fleckhaus, A.; Mousa, A.H.; Lawal, N.S.; Kazemifar, N.K.; Wendt, O.F. Aromatic PCN Palladium Pincer Complexes. Probing the Hemilability through Reactions with Nucleophiles. Organometallics 2015, 34, 1627–1634. [Google Scholar] [CrossRef]
- Clark, W.D.; Cho, J.; Valle, H.U.; Hollis, T.K.; Valente, E.J. Metal and Halogen Dependence of the Rate Effect in Hydroamination/Cyclization of Unactivated Aminoalkenes: Synthesis, Characterization, and Catalytic Rates of CCC-NHC Hafnium and Zirconium Pincer Complexes. J. Organomet. Chem. 2014, 751, 534–540. [Google Scholar] [CrossRef]
- De Aguiar, S.R.M.M.; Stöger, B.; Pittenauer, E.; Allmaier, G.; Veiros, L.F.; Kirchner, K. Structural Diversity of Halocarbonyl Molybdenum and Tungsten Pnp Pincer Complexes through Ligand Modifications. Dalton Trans. 2016, 45, 13834–13845. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.E.; Ocampo, C.; Park, Y.J.; Fout, A.R. Accessing Pincer Bis(carbene) Ni(IV) Complexes from Ni(II) via Halogen and Halogen Surrogates. J. Am. Chem. Soc. 2016, 138, 4290–4293. [Google Scholar] [CrossRef]
- Sitek, P.; Jaworska, M.; Lodowski, P.; Chmielowska, A. Methyl Transfer Reaction between MeI and Ni(PPh2CH2CH2SEt)2 complex. A DFT study. Inorg. Chem. Commun. 2013, 29, 65–69. [Google Scholar] [CrossRef]
- Adcock, R.J.; Nguyen, D.H.; Ladeira, S.; Berre, C.L.; Serp, P.; Kalck, P. Reactivity of Rhodium(I) Complexes Bearing Nitrogen-Containing Ligands toward CH3I: Synthesis and Full Characterization of Neutral cis-[RhX(CO)2(L)] and Acetyl [RhI(μ-I)(COMe)(CO)(L)]2 Complexes. Inorg. Chem. 2012, 51, 8670–8685. [Google Scholar] [CrossRef]
- Gaunt, J.A.; Gibson, V.C.; Haynes, A.; Spitzmesser, S.K.; White, A.J.P.; Williams, D.J. Bis(imino)carbazolide Complexes of Rhodium: Highly Nucleophilic Ligands Exerting a Dramatic Accelerating Effect on MeI Oxidative Addition. Organometallics 2004, 23, 1015–1023. [Google Scholar] [CrossRef]
- Cheong, M.; Ziegler, T. Density Functional Study of the Oxidative Addition Step in the Carbonylation of Methanol Catalyzed by [M(CO)2I2]−(M = Rh, Ir). Organometallics 2005, 24, 3053–3058. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Interaction Nature and Computational Methods for Halogen Bonding: A Perspective. J. Chem. Inf. Model. 2020, 60, 268–2696. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Catalano, L.; Cavallo, G.; Metrangolo, P.; Resnati, G.; Terraneo, G. Halogen Bonding in Hypervalent Iodine Compounds. In Hypervalent Iodine Chemistry; Wirth, T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 289–309. [Google Scholar]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Shixaliyev, N.Q.; Gurbanov, A.V.; Mahmudov, K.T.; Nenajdenko, V.G.; Pombeiro, A.J.L.; Kopylovich, M.N. Halogen Bonding in the Synthesis and Design of Coordination and Organometallic Compounds. In Non-covalent Interactions in the Synthesis and Design of New Compounds; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 145–162. [Google Scholar]
- Politzer, P.; Murray, J.S.; Clark, T. η-Hole Bonding: A Physical Interpretation. In Halogen Bonding I: Impact on Materials Chemistry and Life Sciences; Metrangolo, P., Resnati, G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–42. [Google Scholar]
- Scheiner, S.; Lu, J. Halogen, Chalcogen, and Pnicogen Bonding Involving Hypervalent Atoms. Chem. Eur. J. 2018, 24, 8167–8177. [Google Scholar] [CrossRef]
- Grabowski, S. New Type of Halogen Bond: Multivalent Halogen Interacting with π- and σ-Electrons. Molecules 2017, 22, 2150. [Google Scholar] [CrossRef]
- Engelage, E.; Reinhard, D.; Huber, S.M. Is There a Single Ideal Parameter for Halogen-Bonding-Based Lewis Acidity? Chem. Eur. J. 2020, 26, 3843–3861. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Wijethunga, T.K.; Desper, J.; Dakovic, M. Electrostatic Potential Differences and Halogen-Bond Selectivity. Cryst. Growth Des. 2016, 16, 2662–2670. [Google Scholar] [CrossRef]
- Wang, C.; Danovich, D.; Mo, Y.; Shaik, S. On The Nature of the Halogen Bond. J. Chem. Theory Comput. 2014, 10, 3726–3737. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Frangville, P.; Meyer, F. Halogen bonding for molecular recognition: New developments in materials and biological sciences. Chem. Commun. 2020, 56, 4970–4981. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E.; Cremer, D. The Intrinsic Strength of the Halogen Bond: Electrostatic and Covalent Contributions Described by Coupled Cluster Theory. Phys. Chem. Chem. Phys. 2016, 18, 33031–33046. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E.; Cremer, D. Quantitative Assessment of Halogen Bonding Utilizing Vibrational Spectroscopy. Inorg. Chem. 2016, 56, 488–502. [Google Scholar] [CrossRef]
- Tao, Y.; Qiu, Y.; Zou, W.; Nanayakkara, S.; Yannacone, S.; Kraka, E. In Situ Assessment of Intrinsic Strength of X-I⋯OA Type Halogen Bonds in Molecular Crystals with Periodic Local Vibrational Mode Theory. Molecules 2020, 25, 1589. [Google Scholar] [CrossRef]
- Oliveira, V.; Cremer, D. Transition from Metal-Ligand Bonding to Halogen Bonding Involving a Metal as Halogen Acceptor: A Study of Cu, Ag, Au, Pt, and Hg Complexes. Chem. Phys. Lett. 2017, 681, 56–63. [Google Scholar] [CrossRef]
- Oliveira, V.P.; Marcial, B.L.; Machado, F.B.C.; Kraka, E. Metal-Halogen Bonding Seen through the Eyes of Vibrational Spectroscopy. Materials 2020, 13, 55. [Google Scholar] [CrossRef]
- Eliseeva, A.A.; Ivanov, D.M.; Rozhkov, A.V.; Ananyev, I.V.; Frontera, A.; Kukushkin, V.Y. Bifurcated Halogen Bonding Involving Two Rhodium(I) Centers as an Integrated σ-Hole Acceptor. J. Am. Chem. Soc. Au 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guerin, V.; Legault, C.Y.; Kirsch, S.F.; Huber, S.M. Activation of a Metal-Halogen Bond by Halogen Bonding. Angew. Chem. Int. Ed. 2020, 59, 16496–16500. [Google Scholar] [CrossRef]
- Yang, H.; Wong, M.W. Application of Halogen Bonding to Organocatalysis: A Theoretical Perspective. Molecules 2020, 25, 1045. [Google Scholar] [CrossRef]
- Sousa e Silva, F.C.; Tierno, A.F.; Wengryniuk, S.E. Hypervalent Iodine Reagents in High Valent Transition Metal Chemistry. Molecules 2017, 22, 780. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Gurbanov, A.V.; Guseinov, F.I.; Guedes da Silva, M.F.C. Noncovalent Interactions in Metal Complex Catalysis. Coord. Chem. Rev. 2019, 387, 32–46. [Google Scholar] [CrossRef]
- Benz, S.; Poblador-Bahamonde, A.I.; Low-Ders, N.; Matile, S. Catalysis with Pnictogen, Chalcogen, and Halogen Bonds. Angew. Chem. Int. Ed. 2018, 57, 5408–5412. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Kirina, Y.V.; Kukushkin, V.Y. Halogen Bonding between Metal Centers and Halocarbons. Chem. Commun. 2016, 52, 5565–5568. [Google Scholar] [CrossRef]
- Rogachev, A.Y.; Hoffmann, R. Hypervalent compounds as ligands: I3–anion adducts with rransition metal pentacarbonyls. Inorg. Chem. 2013, 52, 7161–7171. [Google Scholar] [CrossRef]
- Cotton, F.A.; Dikarev, E.V.; Petrukhina, M.A. Coordinated and Clathrated Molecular Diiodine in [Rh2(O2CCF3)4I2]·I2. Angew. Chem. Int. Ed. 2000, 39, 2362–2364. [Google Scholar] [CrossRef]
- Yannacone, S.; Oliveira, V.; Verma, N.; Kraka, E. A Continuum from Halogen Bonds to Covalent Bonds: Where Do λ3 Iodanes Fit? Inorganics 2019, 7, 47. [Google Scholar] [CrossRef]
- Konkoli, Z.; Cremer, D. A New Way of Analyzing Vibrational Spectra. I. Derivation of Adiabatic Internal Modes. Int. J. Quantum Chem. 1998, 67, 1–9. [Google Scholar] [CrossRef]
- Konkoli, Z.; Larsson, J.A.; Cremer, D. A New Way of Analyzing Vibrational Spectra. II. Comparison of Internal Mode Frequencies. Int. J. Quantum Chem. 1998, 67, 11–27. [Google Scholar] [CrossRef]
- Konkoli, Z.; Cremer, D. A New Way of Analyzing Vibrational Spectra. III. Characterization of Normal Vibrational Modes in terms of Internal Vibrational Modes. Int. J. Quantum Chem. 1998, 67, 29–40. [Google Scholar] [CrossRef]
- Konkoli, Z.; Larsson, J.A.; Cremer, D. A New Way of Analyzing Vibrational Spectra. IV. Application and Testing of Adiabatic Modes within the Concept of the Characterization of Normal Modes. Int. J. Quantum Chem. 1998, 67, 41–55. [Google Scholar] [CrossRef]
- Cremer, D.; Larsson, J.A.; Kraka, E. New Developments in the Analysis of Vibrational Spectra on the Use of Adiabatic Internal Vibrational Modes. In Theoretical and Computational Chemistry; Parkanyi, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 259–327. [Google Scholar] [CrossRef]
- Kraka, E.; Zou, W.; Tao, Y. Decoding chemical information from vibrational spectroscopy data: Local vibrational mode theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1480. [Google Scholar] [CrossRef]
- Landis, C.R.; Weinhold, F. The NBO View of Chemical Bonding. In The Chemical Bond: Fundamental Aspects of Chemical Bonding; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 91–120. [Google Scholar]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Bousquet, D.; Brémond, E.; Sancho-García, J.C.; Ciofini, I.; Adamo, C. Is There Still Room for Parameter Free Double Hybrids? Performances of PBE0-DH and B2PLYP over Extended Benchmark Sets. J. Chem. Theory Comput. 2013, 9, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for Mixing Exact Exchange with Density Functional Approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian Basis Sets for use in Correlated Molecular Calculations. IV. Calculation of Static Electrical Response Properties. J. Chem. Phys. 1994, 100, 2975–2988. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian Basis Sets for use in Correlated Molecular Calculations. III. The Atoms Aluminum through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron Affinities of the First Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post-d Group 16-18 Elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Peterson, K.A. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. I. Correlation Consistent Basis Sets for the Post-d Group 13–15 Elements. J. Chem. Phys. 2003, 119, 11099–11112. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Zou, W.; Tao, Y.; Freindorf, M.; Makoś, M.Z.; Verma, N.; Kraka, E. Local Vibrational Mode Analysis (LModeA); Computational and Theoretical Chemistry Group (CATCO), Southern Methodist University: Dallas, TX, USA, 2020. [Google Scholar]
- Keith, T.A. AIMAll, Version 17.11.14; TK Gristmill Software: Overland Park, KS, USA, 2017; Available online: aim.tkgristmill.com (accessed on 15 February 2021).
- Bader, R.F.W. The Quantum Mechanical Basis of Conceptual Chemistry. Monatshefte Chem. 2005, 136, 819–854. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density? Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Kraka, E.; Cremer, D. Chemical Implication of Local Features of the Electron Density Distribution. In Theoretical Models of Chemical Bonding. The Concept of the Chemical Bond; Maksic, Z.B., Ed.; Springer: Heidelberg, Germany, 1990; Volume 2, p. 453. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor–Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R.; Glendening, E.D. What is NBO Analysis and How is it Useful? Int. Rev. Phys. Chem. 2016, 35, 39–440. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cremer, D.; Kraka, E. From Molecular Vibrations to Bonding, Chemical Reactions, and Reaction Mechanism. Curr. Org. Chem. 2010, 14, 1524–1560. [Google Scholar] [CrossRef]
- Kraka, E.; Larsson, J.A.; Cremer, D. Generalization of the Badger Rule Based on the Use of Adiabatic Vibrational Modes. In Computational Spectroscopy; Grunenberg, J., Ed.; Wiley: New York, NY, USA, 2010; pp. 105–149. [Google Scholar] [CrossRef]
- Oliveira, V.P.; Kraka, E.; Machado, F.B.C. Pushing 3c–4e Bonds to the Limit: A Coupled Cluster Study of Stepwise Fluorination of First-Row Atoms. Inorg. Chem. 2019, 58, 14777–14789. [Google Scholar] [CrossRef]
- Boys, S.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Wu, G. Vibrational Spectroscopy; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Huang, Y.; Chang, C.; Yuan, J.; Zhao, Z. High-Harmonic and Terahertz Spectroscopy (HATS): Methods and Applications. Appl. Sci. 2019, 9, 853. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach; Wiley: New York, NY, USA, 2019. [Google Scholar]
- Wilson, E.B.; Decius, J.C.; Cross, P.C. Molecular Vibrations; McGraw-Hill: New York, NY, USA, 1955. [Google Scholar]
- Wilson, E.B., Jr. A Method of Obtaining the Expanded Secular Equation for the Vibration Frequencies of a Molecule. J. Chem. Phys. 1939, 7, 1047–1052. [Google Scholar] [CrossRef]
- Woodward, L.A. Introduction to the Theory of Molecular Vibrations and Vibrational Spectroscopy; Oxford University Press: Oxford, UK, 1972. [Google Scholar]
- Califano, S. Vibrational States; Wiley: London, UK, 1976. [Google Scholar]
- Kelley, J.D.; Leventhal, J.J. Problems in Classical and Quantum Mechanics: Normal Modes and Coordinates; Springer: Cham, Switzerland, 2017; pp. 95–117. [Google Scholar]
- Groner, P. Normal Coordinate Analysis; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Neto, N. Tensor Formalism in Anharmonic Calculations. Chem. Phys. 1984, 91, 89–100. [Google Scholar] [CrossRef]
- Stare, J. First-Principle Calculation of Reduced Masses in Vibrational Analysis Using Generalized Internal Coordinates: Some Crucial Aspects and Examples. J. Chem. Inf. Model. 2007, 47, 840–850. [Google Scholar] [CrossRef]
- Zou, W.; Cremer, D. C2 in a Box: Determining its Intrinsic Bond Strength for the X1Σ+g Ground State. Chem. Eur. J. 2016, 22, 4087–4097. [Google Scholar] [CrossRef]
- Oomens, J.; Kraka, E.; Nguyen, M.K.; Morton, T.M. Structure, Vibrational Spectra, and Unimolecular Dissociation of Gaseous 1-Fluoro-1-phenethyl Cations. J. Phys. Chem. A 2008, 112, 10774–10783. [Google Scholar] [CrossRef]
- Zou, W.; Kalescky, R.; Kraka, E.; Cremer, D. Relating Normal Vibrational Modes to Local Vibrational Modes: Benzene and Naphthalene. J. Mol. Model. 2012, 19, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. Identification of the Strongest Bonds in Chemistry. J. Phys. Chem. A 2013, 117, 8981–8995. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. Description of Aromaticity with the Help of Vibrational Spectroscopy: Anthracene and Phenanthrene. J. Phys. Chem. A 2013, 118, 223–237. [Google Scholar] [CrossRef]
- Kalescky, R.; Kraka, E.; Cremer, D. New Approach to Tolman’s Electronic Parameter Based on Local Vibrational Modes. Inorg. Chem. 2013, 53, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. Are Carbon-Halogen Double and Triple Bonds Possible? Int. J. Quantum Chem. 2014, 114, 1060–1072. [Google Scholar] [CrossRef]
- Kalescky, R.; Zou, W.; Kraka, E.; Cremer, D. Quantitative Assessment of the Multiplicity of Carbon-Halogen Bonds: Carbenium and Halonium Ions with F, Cl, Br, and I. J. Phys. Chem. A 2014, 118, 1948–1963. [Google Scholar] [CrossRef]
- Humason, A.; Zou, W.; Cremer, D. 11,11-Dimethyl-1,6-methano[10]annulene-An Annulene with an Ultralong CC Bond or a Fluxional Molecule? J. Phys. Chem. A 2014, 119, 1666–1682. [Google Scholar] [CrossRef]
- Sethio, D.; Daku, L.M.L.; Hagemann, H.; Kraka, E. Quantitative Assessment of B–B–B, B–Hb–B, and B–Ht Bonds: From BH3 to B12H122-. ChemPhysChem 2019, 20, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Makoś, M.Z.; Freindorf, M.; Sethio, D.; Kraka, E. New Insights into Fe–H2 and Fe–H− Bonding of a [NiFe] Hydrogenase Mimic—A Local Vibrational Mode Study. Theor. Chem. Acc. 2019, 138, 76. [Google Scholar] [CrossRef]
- Makoś, M.Z.; Zou, W.; Freindorf, M.; Kraka, E. Metal-Ring Interactions in Actinide Sandwich Compounds: A Combined Normalized Elimination of the Small Component and Local Vibrational Mode Study. Mol. Phys. 2020, 118, e1768314. [Google Scholar] [CrossRef]
- Verma, N.; Tao, Y.; Zou, W.; Chen, X.; Chen, X.; Freindorf, M.; Kraka, E. A Critical Evaluation of Vibrational Stark Effect (VSE) Probes with the Local Vibrational Mode Theory. Sensors 2020, 20, 2358. [Google Scholar] [CrossRef]
- Freindorf, M.; Kraka, E. Critical Assessment of the FeC and CO Bond strength in Carboxymyoglobin—A QM/MM Local Vibrational Mode Study. J. Mol. Model. 2020, 26, 281. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Freindorf, M. Characterizing the Metal Ligand Bond Strength via Vibrational Spectroscopy: The Metal Ligand Electronic Parameter (MLEP). In Topics in Organometallic Chemistry—New Directions in the Modeling of Organometallic Reactions; Lledós, A., Ujaque, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 67, pp. 1–43. [Google Scholar]
- Freindorf, M.; Kraka, E.; Cremer, D. A Comprehensive Analysis of Hydrogen Bond Interactions Based on Local Vibrational Modes. Int. J. Quantum Chem. 2012, 112, 3174–3187. [Google Scholar] [CrossRef]
- Kalescky, R.; Zou, W.; Kraka, E.; Cremer, D. Local Vibrational Modes of the Water Dimer—Comparison of Theory and Experiment. Chem. Phys. Lett. 2012, 554, 243–247. [Google Scholar] [CrossRef]
- Kalescky, R.; Kraka, E.; Cremer, D. Local Vibrational Modes of the Formic Acid Dimer—The Strength of the Double H-Bond. Mol. Phys. 2013, 111, 1497–1510. [Google Scholar] [CrossRef]
- Kraka, E.; Freindorf, M.; Cremer, D. Chiral Discrimination by Vibrational Spectroscopy Utilizing Local Modes. Chirality 2013, 25, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Kraka, E.; Cremer, D. Description of Pnicogen Bonding with the help of Vibrational Spectroscopy—The Missing Link Between Theory and Experiment. Chem. Phys. Lett. 2014, 614, 136–142. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Strength of the Pnicogen Bond in Complexes Involving Group VA Elements N, P, and As. J. Phys. Chem. A 2014, 119, 1642–1656. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Hidden Bond Anomalies: The Peculiar Case of the Fluorinated Amine Chalcogenides. J. Phys. Chem. A 2015, 119, 9541–9556. [Google Scholar] [CrossRef]
- Kraka, E.; Setiawan, D.; Cremer, D. Re-Evaluation of the Bond Length-Bond Strength Rule: The Stronger Bond Is not Always the Shorter Bond. J. Comp. Chem. 2015, 37, 130–142. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, H.; Yan, H.; Zou, W.; Cremer, D. B-H π Interaction: A New Type of Nonclassical Hydrogen Bonding. J. Am. Chem. Soc. 2016, 138, 4334–4337. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Cremer, D. Super-Pnicogen Bonding in the Radical Anion of the Fluorophosphine Dimer. Chem. Phys. Lett. 2016, 662, 182–187. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Jia, J.; Li, W.; Cremer, D. Different Ways of Hydrogen Bonding in Water—Why Does Warm Water Freeze Faster than Cold Water? J. Chem. Theory Comput. 2016, 13, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Cremer, D.; Kraka, E. The Many Facets of Chalcogen Bonding: Described by Vibrational Spectroscopy. J. Phys. Chem. A 2017, 121, 6845–6862. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E. Systematic Coupled Cluster Study of Noncovalent Interactions Involving Halogens, Chalcogens, and Pnicogens. J. Phys. Chem. A 2017, 121, 9544–9556. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, X.; Dai, H.; Yan, H.; Cremer, D.; Kraka, E. Description of an Unusual Hydrogen Bond Between Carborane and a Phenyl Group. J. Organomet. Chem. 2018, 865, 114–127. [Google Scholar] [CrossRef]
- Lyu, S.; Beiranvand, N.; Freindorf, M.; Kraka, E. Interplay of Ring Puckering and Hydrogen Bonding in Deoxyribonucleosides. J. Phys. Chem. A 2019, 123, 7087–7103. [Google Scholar] [CrossRef]
- Yannacone, S.; Sethio, D.; Kraka, E. Quantitative Assessment of Intramolecular Hydrogen Bonds in Neutral Histidine. Theor. Chem. Acc. 2020, 139, 125. [Google Scholar] [CrossRef]
- Martins, J.; Quintino, R.P.; Politi, J.R.S.; Sethio, D.; Gargano, R.; Kraka, E. Computational Analysis of Vibrational Frequencies and Rovibrational Spectroscopic Constants of Hydrogen Sulfide Dimer using MP2 and CCSD(T). Spectrochim. Acta A 2020, 239, 118540. [Google Scholar] [CrossRef]
- Yannacone, S.; Freindorf, M.; Tao, Y.; Zou, W.; Kraka, E. Local Vibrational Mode Analysis of π-Hole Interactions between Aryl Donors and Small Molecule Acceptors. Crystals 2020, 10, 556. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Wong, G.W.; Brastow, K.E.; Kaminsky, W.; Goldberg, K.I.; Heinekey, D.M. The Importance of Steric Factors in Iridium Pincer Complexes. Organometallics 2015, 34, 753–762. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Cherry, S.D.T.; Guard, L.M.; Kaminsky, W.; Goldberg, K.I.; Heinekey, D.M. Hydrogen Addition to (pincer)IrI(CO) Complexes: The Importance of Steric and Electronic Factors. Organometallics 2016, 35, 3546–3556. [Google Scholar] [CrossRef]
- Makiura, R.; Nagasawa, I.; Kimura, N.; Ishimaru, S.; Kitagawa, H.; Ikeda, R. An unusual six–co–ordinate platinum(II) complex containing a neutral I2 ligand. Chem. Commun. 2001, 2001, 1642–1643. [Google Scholar]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; Taylor and Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Moltved, K.A.; Kepp, K.P. Chemical Bond Energies of 3d Transition Metals Studied by Density Functional Theory. J. Chem. Theory Comput. 2018, 14, 3479–3492. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.D. Predissociation measurements of bond dissociation energies. Acc. Chem. Res. 2018, 52, 119–126. [Google Scholar] [CrossRef]
- Setiawan, D.; Sethio, D.; Cremer, D.; Kraka, E. From Strong to Weak NF Bonds: On the Design of a New Class of Fluorinating Agents. Phys. Chem. Chem. Phys. 2018, 20, 23913–23927. [Google Scholar] [CrossRef]
- Sethio, D.; Oliveira, V.; Kraka, E. Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules 2018, 23, 2763. [Google Scholar] [CrossRef]
- Pyykkö, P. Relativistic Effects in Chemistry: More Common Than You Thought. Annu. Rev. Phys. Chem. 2012, 63, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Filatov, M.; Cremer, D. Comparison of Gold Bonding with Mercury Bonding. Croat. Chim. Acta 2009, 82, 233–243. [Google Scholar]
- Kaupp, M.; Danovich, D.; Shaik, S. Chemistry is about energy and its changes: A critique of bond-length/bond-strength correlations. Coord. Chem. Rev. 2017, 344, 355–362. [Google Scholar] [CrossRef]
- Kraka, E.; Cremer, D. Weaker Bonds with Shorter Bond Lengths. Revista Processos Químicos 2012, 6, 31–34. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Donor–Acceptor Properties of Ligands from the Natural Orbitals for Chemical Valence. Organometallics 2007, 26, 6576–6580. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A. σ–Donor and π–Acceptor Properties of Phosphorus Ligands: An Insight from the Natural Orbitals for Chemical Valence. Inorg. Chem. 2010, 49, 578–582. [Google Scholar] [CrossRef]
- Iliaš, M.; Pershina, V. Carbonyl compounds of Rh, Ir, and Mt: Electronic structure, bonding and volatility. Phys. Chem. Chem. Phys. 2020, 22, 18681–18694. [Google Scholar] [CrossRef]
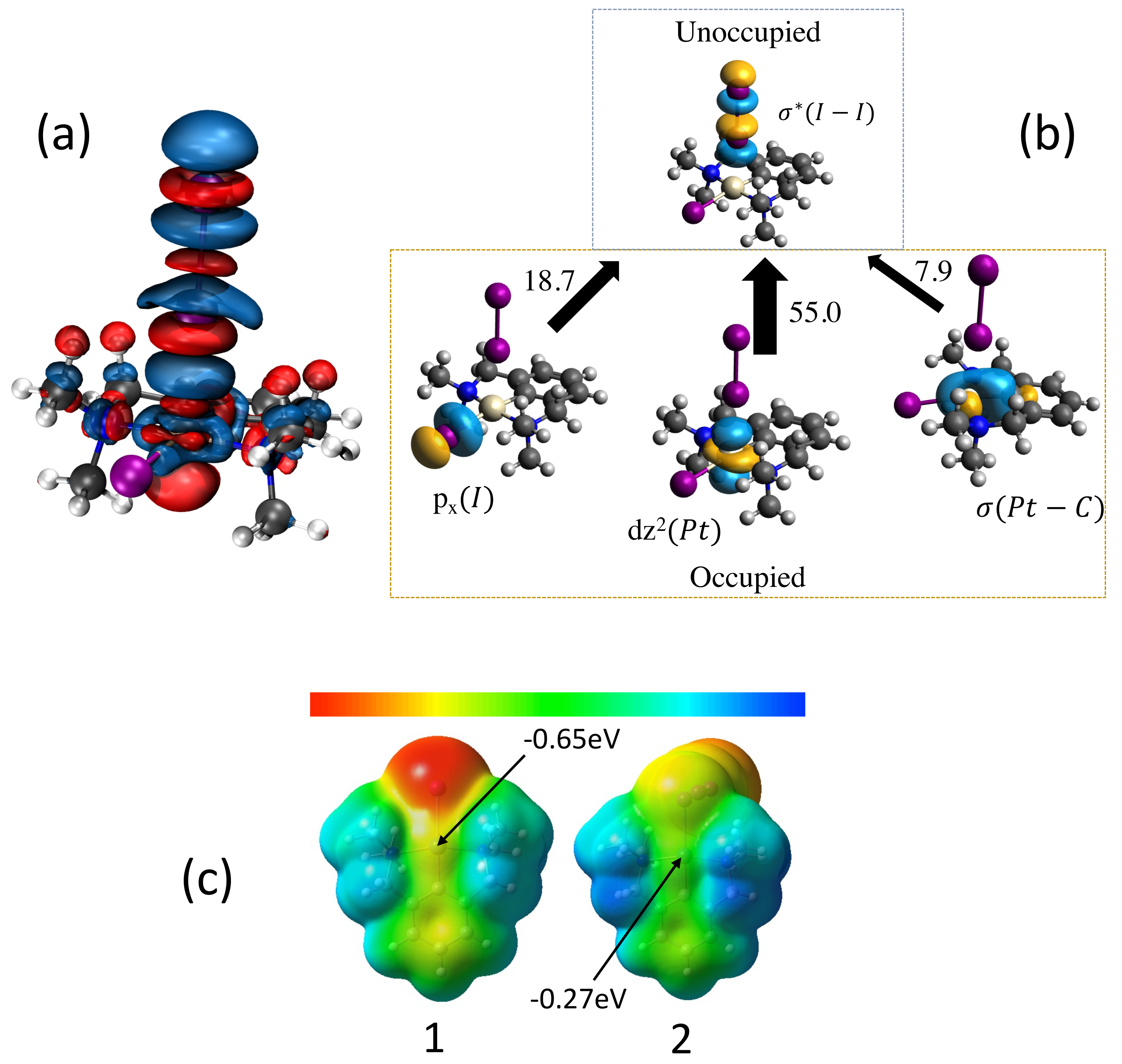
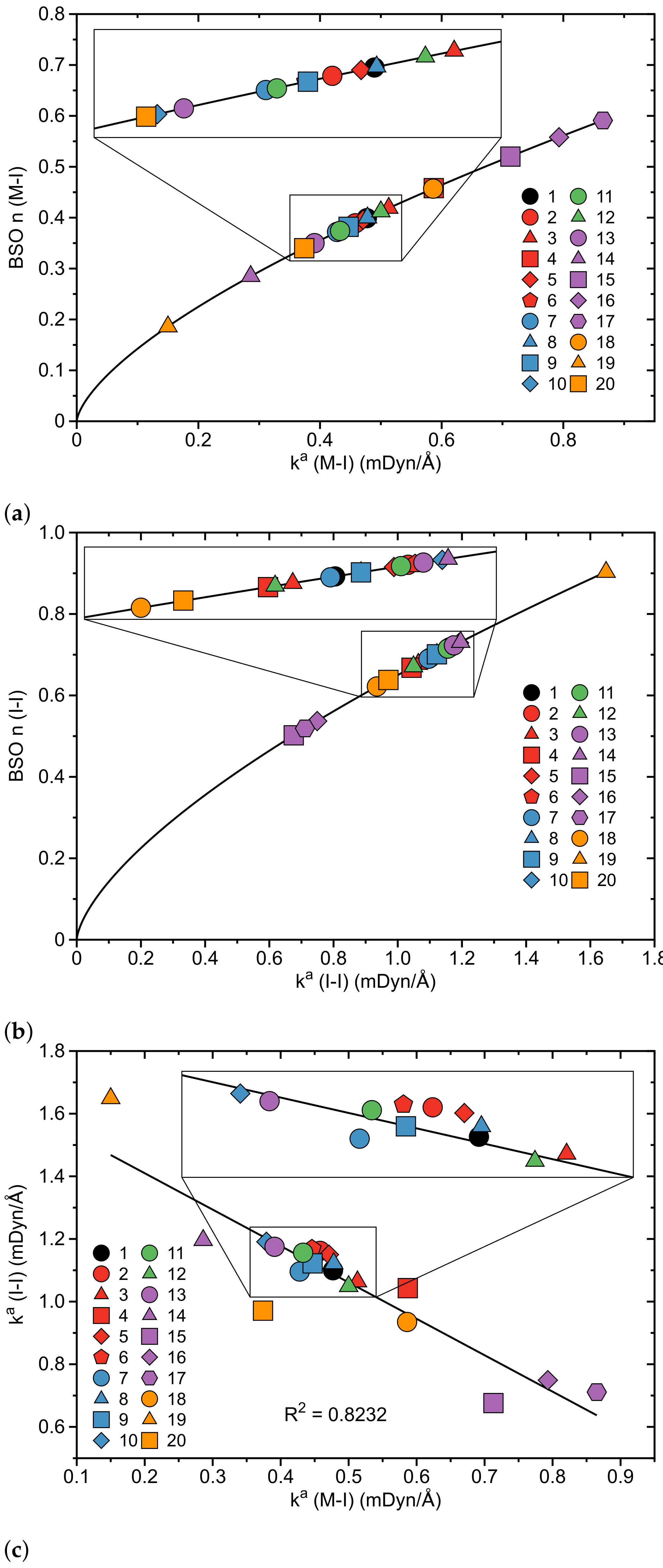

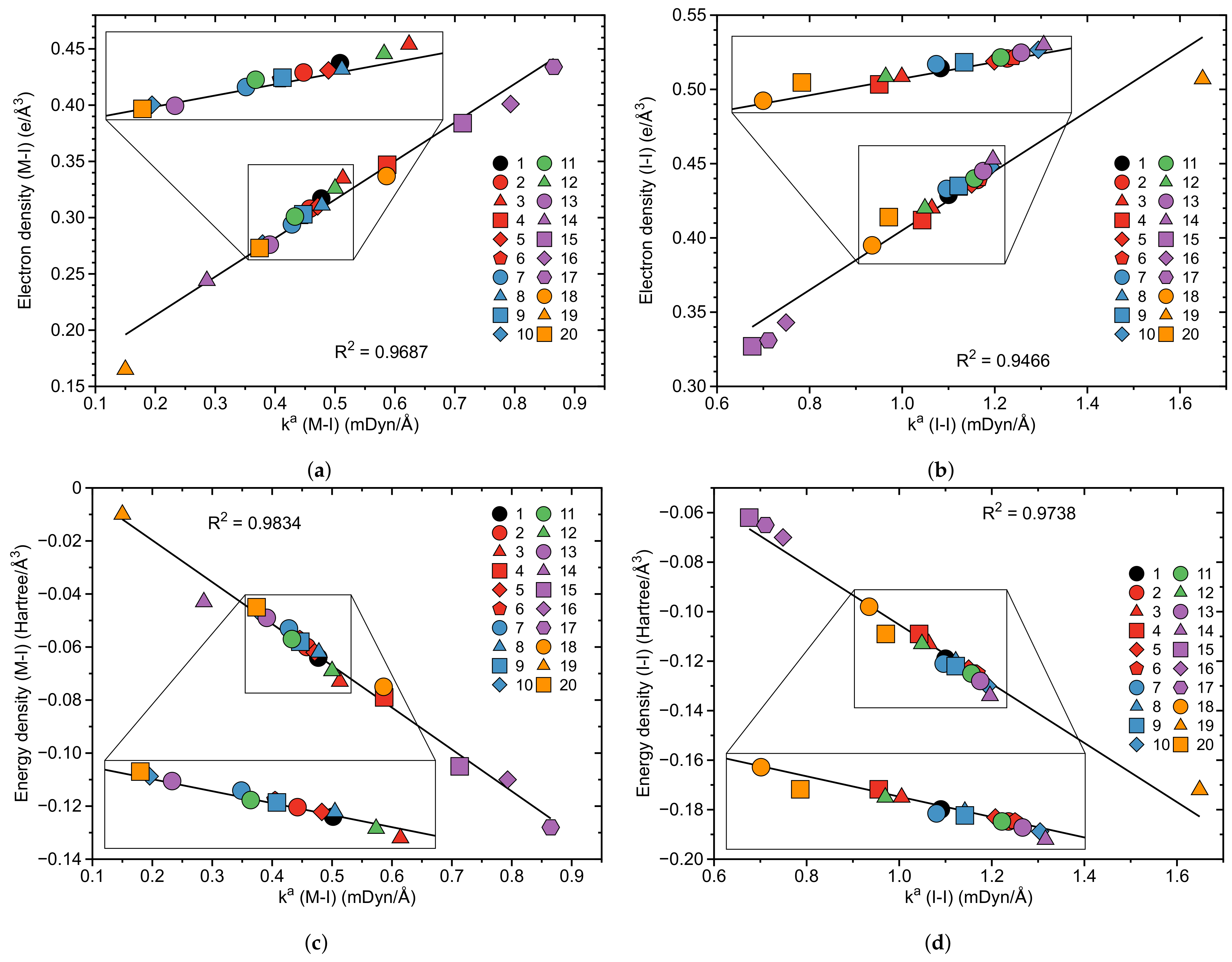
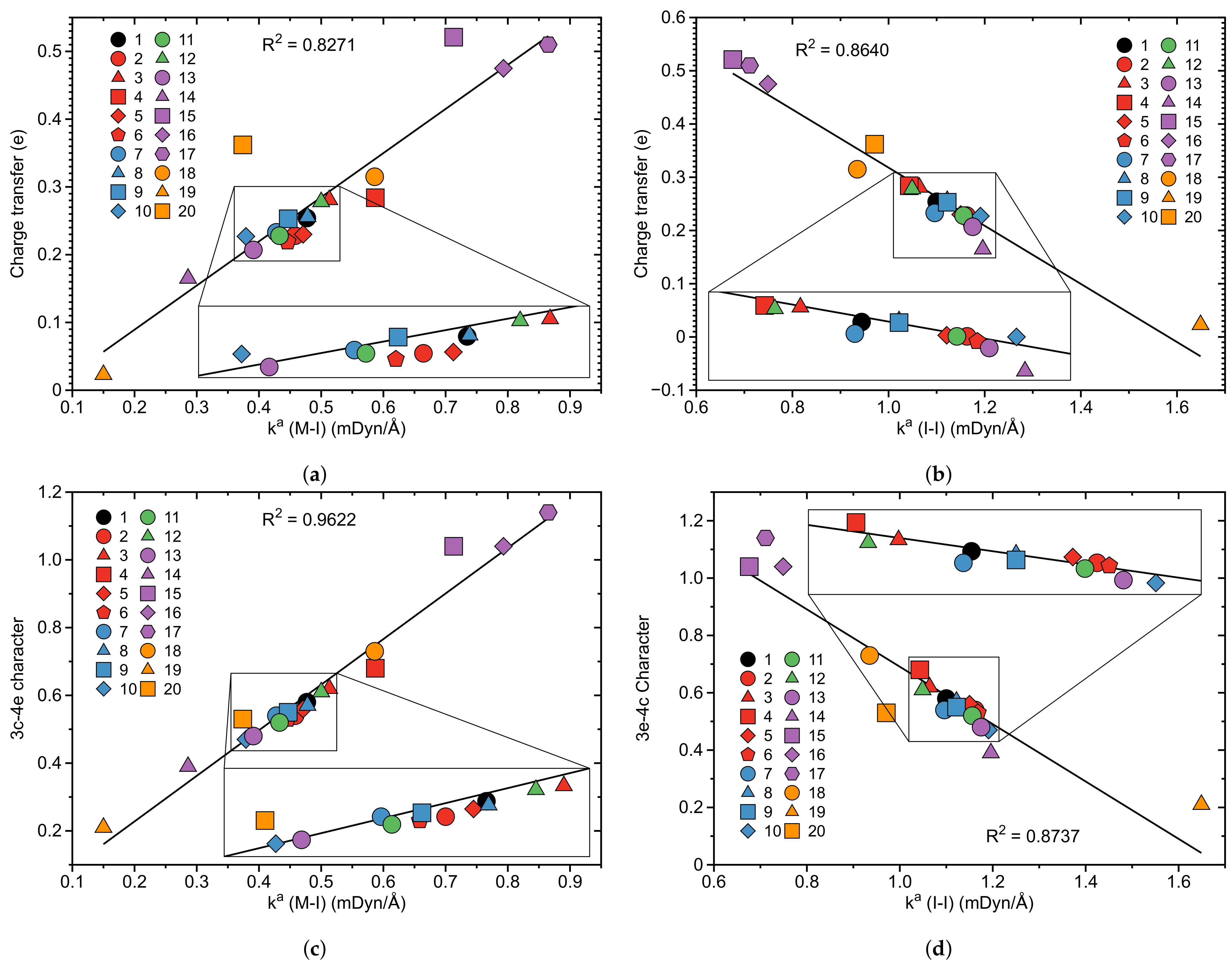
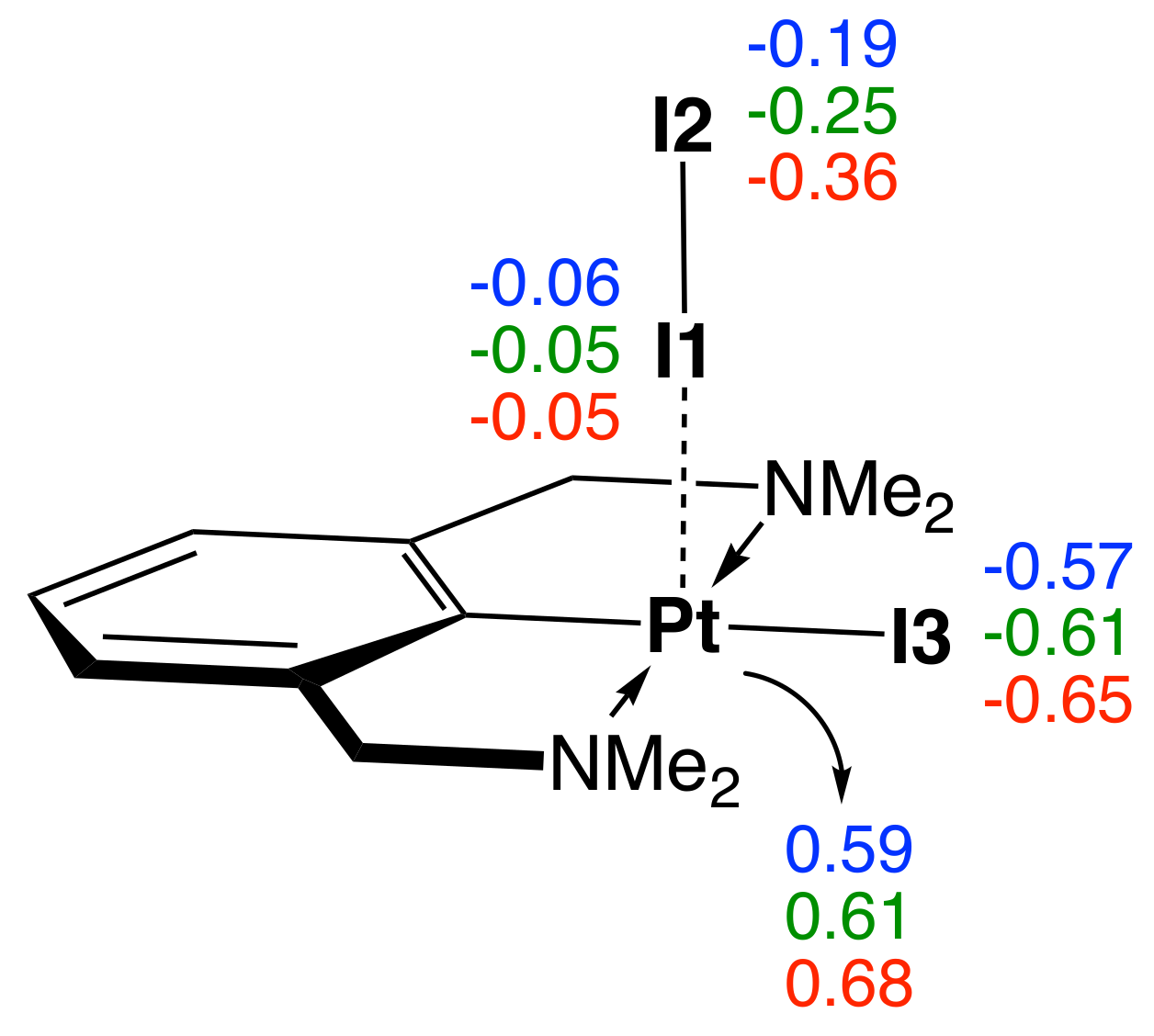
| Nr | r(M–I) | r(I–I) | (M–I) | (I–I) | BSO n(M–I) | BSO n(I–I) | (M–I) | (M–I) | (I–I) | (I–I) | CT | 3c–4e | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19.0 | 2.887 | 2.788 | 0.477 | 1.100 | 0.399 | 0.692 | 0.317 | −0.064 | 0.429 | −0.119 | 0.254 | 0.58 |
| 2 | 16.6 | 2.900 | 2.774 | 0.458 | 1.162 | 0.389 | 0.718 | 0.308 | −0.060 | 0.439 | −0.125 | 0.228 | 0.54 |
| 3 | 20.3 | 2.861 | 2.800 | 0.513 | 1.064 | 0.419 | 0.677 | 0.335 | −0.073 | 0.420 | −0.113 | 0.281 | 0.62 |
| 4 | 22.8 | 2.838 | 2.81 | 0.587 | 1.043 | 0.458 | 0.668 | 0.347 | −0.079 | 0.412 | −0.109 | 0.284 | 0.68 |
| 5 | 18.5 | 2.895 | 2.778 | 0.471 | 1.150 | 0.396 | 0.713 | 0.310 | −0.062 | 0.436 | −0.123 | 0.230 | 0.56 |
| 6 | 17.7 | 2.911 | 2.773 | 0.446 | 1.168 | 0.382 | 0.720 | 0.301 | −0.057 | 0.440 | −0.125 | 0.219 | 0.53 |
| 7 | 18.1 | 2.919 | 2.781 | 0.428 | 1.096 | 0.372 | 0.690 | 0.294 | −0.053 | 0.433 | −0.121 | 0.233 | 0.54 |
| 8 | 17.7 | 2.892 | 2.783 | 0.478 | 1.122 | 0.400 | 0.701 | 0.311 | −0.062 | 0.432 | −0.12 | 0.256 | 0.57 |
| 9 | 16.6 | 2.905 | 2.779 | 0.447 | 1.122 | 0.382 | 0.701 | 0.303 | −0.058 | 0.435 | −0.122 | 0.253 | 0.55 |
| 10 | 14.9 | 2.956 | 2.763 | 0.379 | 1.191 | 0.343 | 0.729 | 0.277 | −0.047 | 0.448 | −0.130 | 0.227 | 0.47 |
| 11 | 17.4 | 2.913 | 2.774 | 0.433 | 1.156 | 0.374 | 0.715 | 0.301 | −0.057 | 0.440 | −0.125 | 0.228 | 0.52 |
| 12 | 20.4 | 2.873 | 2.800 | 0.500 | 1.049 | 0.412 | 0.671 | 0.326 | −0.069 | 0.420 | −0.113 | 0.278 | 0.61 |
| 13 | 16.3 | 2.884 | 2.767 | 0.391 | 1.175 | 0.350 | 0.723 | 0.276 | −0.049 | 0.445 | −0.128 | 0.207 | 0.48 |
| 14 | 14.5 | 2.818 | 2.757 | 0.286 | 1.196 | 0.285 | 0.731 | 0.244 | −0.043 | 0.453 | −0.134 | 0.165 | 0.39 |
| 15 | 33.6 | 2.640 | 2.942 | 0.713 | 0.676 | 0.520 | 0.502 | 0.384 | −0.105 | 0.327 | −0.062 | 0.521 | 1.04 |
| 16 | 33.9 | 2.741 | 2.915 | 0.793 | 0.749 | 0.558 | 0.537 | 0.401 | −0.110 | 0.343 | −0.070 | 0.475 | 1.04 |
| 17 | 36.3 | 2.761 | 2.934 | 0.865 | 0.711 | 0.591 | 0.519 | 0.434 | −0.128 | 0.331 | −0.065 | 0.510 | 1.14 |
| 18 | 22.6 | 2.881 | 2.831 | 0.586 | 0.935 | 0.457 | 0.622 | 0.337 | −0.075 | 0.395 | −0.098 | 0.315 | 0.73 |
| 19 | 11.4 | 3.207 | 2.690 | 0.150 | 1.649 | 0.186 | 0.903 | 0.165 | −0.010 | 0.507 | −0.172 | 0.023 | 0.21 |
| 20 | 19.3 | 3.016 | 2.813 | 0.374 | 0.971 | 0.340 | 0.638 | 0.273 | −0.045 | 0.414 | −0.109 | 0.362 | 0.53 |
| Atoms | Gas Phase | Benzene | Acetone | |||
|---|---|---|---|---|---|---|
| r | r | r | ||||
| I1–I2 | 2.788 | 1.100 | 2.815 | 0.955 | 2.880 | 0.640 |
| Pt–I1 | 2.887 | 0.477 | 2.838 | 0.564 | 2.768 | 0.595 |
| Pt–I3 | 2.707 | 1.161 | 2.723 | 1.037 | 2.750 | 0.856 |
| Pt–N | 2.099 | 1.910 | 2.102 | 1.902 | 2.105 | 1.845 |
| Pt–C | 1.933 | 4.186 | 1.934 | 4.160 | 1.935 | 4.113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freindorf, M.; Yannacone, S.; Oliveira, V.; Verma, N.; Kraka, E. Halogen Bonding Involving I2 and d8 Transition-Metal Pincer Complexes. Crystals 2021, 11, 373. https://doi.org/10.3390/cryst11040373
Freindorf M, Yannacone S, Oliveira V, Verma N, Kraka E. Halogen Bonding Involving I2 and d8 Transition-Metal Pincer Complexes. Crystals. 2021; 11(4):373. https://doi.org/10.3390/cryst11040373
Chicago/Turabian StyleFreindorf, Marek, Seth Yannacone, Vytor Oliveira, Niraj Verma, and Elfi Kraka. 2021. "Halogen Bonding Involving I2 and d8 Transition-Metal Pincer Complexes" Crystals 11, no. 4: 373. https://doi.org/10.3390/cryst11040373
APA StyleFreindorf, M., Yannacone, S., Oliveira, V., Verma, N., & Kraka, E. (2021). Halogen Bonding Involving I2 and d8 Transition-Metal Pincer Complexes. Crystals, 11(4), 373. https://doi.org/10.3390/cryst11040373








