Abstract
The nanorods of [Pb(L)Br2]n (1) (L = 1,2-bis (pyridin-3-ylmethylene)hydrazine) underwent ultrasound irradiation and were synthesized as a novel three-dimensional fishbone-like Pb(II)–organic coordination supramolecular compound. The morphology and nanostructure of the synthesized compound were determined through SEM, FTIR, elemental analyses, and XRD. Compound 1 was structurally characterized by single-crystal X-ray diffraction and revealed six-coordinated Pb (II) ions bonded to two N atoms from two L ligands and four bromine anions, forming a one-dimensional fishbone-like coordination polymer, which extended into a 3D supramolecular structure through weak intermolecular interactions. The bulk thermal stability of compound 1 was examined using thermogravimetric analysis (TGA). Moreover, PbO nanoparticles with sizes of 40–80 nm were obtained through the thermolysis of 1 at 180 °C using oleic acid as a surfactant.
1. Introduction
Coordination compounds or metal–organic hybrid materials have remarkably attracted huge attention during the past twenty years. Inorganic–organic hybrid supramolecular materials include the self-assembly of an organic ligand with proper metal ions and functional groups with special directionality and functionality [1,2,3,4,5]. Structural blocks are created based on coordinating interactions and other poor non-covalent inter-molecular forces, including hydrogen bonds and pi stacking, which have key roles in their stability. The attraction to these compounds comes from the effects of basic structural chemistry, and their probable applications in areas including luminescence, catalysis, nonlinear optics, magnetism and molecular sensing and adsorption, which do not exist for mononuclear compounds [6,7,8]. Lowering the size of nano-scaled coordination supramolecular compounds increases the surface area. Preparing any types of nano-scaled coordination supramolecular compounds is therefore the main path toward the technological application of these novel materials [9].
Pb(II) frameworks have attracted interest because of their large ion radius, variable coordination number, and possible occurrence of a stereochemically active lone pair of 6s2 outer electrons with a novel network of topologies and interesting properties [10,11,12]. According to the hard–soft acid–base theory, the intermediate coordination ability of Pb(II) means that it can flexibly coordinate small nitrogen or oxygen atoms as well as large sulfur atoms [13]. Investigation of the stereo-chemical activity of valence shell lone electron pairs in the polymeric and supramolecular compounds may be more interesting. The spontaneous aggregation of several bridging ligands causes the gap to disappear, and the coordination of Pb (II) assumes a less common holodirected arrangement [14].
The present study introduces an eco-friendly and simple nanocrystal synthesis of a three-dimensional coordination supramolecular compound via ultrasonic irradiation. Organic synthesis was performed, and nanomaterials prepared using sonochemical techniques [15]. Further studies are still required for clarifying the application of the Ultrasound technique (UST) to constructing coordination supramolecular compounds. The present study investigated the fast synthesis of [Pb(L)Br2]n (1) [L = 1,2-bis (pyridin-3-ylmethylene)hydrazine] as the nanocrystals of a one-dimensional fishbone-like Pb(II) coordination polymer. According to the findings obtained, Ultrasound technique (UST) synthesis is an efficient, low cost, simple and eco-friendly method for coordination supramolecular nanocompounds compared to conventional synthetic methods such as the solvent diffusion method and solvothermal and hydrothermal approaches [16].
Ultrasound technique (UST) leads to high-energy chemistry by processing acoustic cavitation, including bubbles, expansion, and their implosive collapse within a liquid intermediate [17]. UST results in alternative compressive and expansive acoustic waves to make and oscillate bubbles by irradiating liquids. Bubble can invade and consequently collapse fast, i.e., with cooling and heating rates of over 1010 Ks−1. The huge energy concentration obtained over the collapse leads to a pressure of about one thousand bar and a local temperature of approximately 5000 K. The energy distribution to the surroundings during collapse and after bounce causes the hot spot gas temperature to swiftly reach the temperature of the surrounding area [18,19]. Ultrasonic irradiation can therefore be used to assist various chemical reactions progress at room temperature, and even certain reactions formerly difficult to actualize using conventional approaches [20,21,22,23]. The present study employed oleic acid as a surfactant and performed thermolysis of 1 at 180 °C to synthesize and structurally characterize a novel nano lead(II) metal–organic supramolecular compound with 1,2-bis (pyridine-3-ylmethylene)hydrazine ligand (Scheme 1) and simply synthesize PbO nanoparticles.
Scheme 1.
1,2-bis (pyridine-3-ylmethylene)hydrazine ligand.
2. Experiments
2.1. Measurements and Materials
Chemicals were obtained from Sigma-Aldrich (Seoul, South Korea) and used as received without further purification. Elemental analyses of the samples were carried out using a Vario Microanalyzer. FTIR was carried out by employing Bruker Vector 22 and KBr disks at 4000–400 cm−1. Thermogravimetric analysis (TGA) was conducted under argon flow at 20–600 degrees Celsius and a heating rate of 3 °C/min using LABSYS evo (SETARAM).
X-ray powder diffraction (XRD) measurements were carried out using an X’pert diffractometer (Panalytical) with monochromatized Cu-Kα radiation. In addition, XRD patterns were simulated in Mercury to acquire single-crystal information [24]. The morphology of the nanostructured compound was determined by scanning electron microscopy (SEM) (S-4200, Hitachi, Japan) and TEM (JEM-2200FS, JEOL). A 40 kHz Sonicator_3000 multiwave ultrasonic generator made by Misonix Inc. in the UST equipped with a titanium oscillator (horn) and a converter/transducer 12.5 mm in diameter was utilized to perform ultrasound. The minimum power output of the generator was 600 W for one hour at ambient temperature. The chemical composition and status of the product were evaluated by X-ray photoelectron spectroscopy (XPS) (K-ALPHA, UK).
2.2. Preparation of 1,2-bis (pyridine-3-ylmethylene)hydrazine (L)
A mixture of 5 mmol (0.45 mL, 0.45 g) of hydrazine hydrate (35 wt.% in water) and a solution of 10 mmol (1.07 g) of nicotinaldehyde in 100 mL of methanol was refluxed for one day. Afterwards, the obtained yellow solids were filtered and then rinsed in methanol and dried in a vacuum (yield: 65%, 0.98 g), m.p. 140 °C, FTIR (KBr) = νmax (698, 706, 817, 863, 1021, 1089, 1185, 1225, 1304, 1417, 1482, 1586, 1625, 2929) Cm−1. H NMR (CDCl3, ppm): 8.66 (s, 2H), 8.70 (d, 2H), 8.97 (s, 2H), 7.41 (d, 2H), 8.22 (d, 2H).
2.3. Preparation of Nanostructure and Single Crystal [Pb(L)Br2 ]n (1)
A total of 30 mL of a 0.1 M solution of PbBr2 was added to 30 mL of a 0.1 M solution of the (L) ligand, and sonication performed with a high-density 40 kHz, 600 W UST probe to make the nanostructured [Pb(L)Br2]n (1). The obtained precipitate was filtered and rinsed in water and then dried in the air at the decomposition point: 330 °C. According to the analyses, C: 25.0%, H: 2.0% and N: 10.0% and C: 24.9%, H: 1.5% and N: 9.7% were obtained for C12H10Br2N4Pb. The selected FTIR bands (cm−1) were as follows: 635s, 750s, 864m, 1045m, 1418s, 1482m, 1590s, 1620s, 3020w.
An appropriate single crystal of [Pb(L)Br2]n (1) determining the X-ray structure was made and isolated by inserting 1 mmol of L into an arm of a tube with branches and 1 mmol of PbBr2 to the other arm [25]. Having filled both the arms with methanol and sealing the tube, the arm containing the ligand was submerged in a 60 °C oil bath. Yellow crystals (decomposition point of 333 °C) were precipitated for 9 days on the other arm that was placed at room temperature. Afterwards, the crystals were filtered, rinsed in water, and dried in air with a yield of 70% (0.402 g). The results obtained were as follows: H: 2.0%, C: 25.5% and N: 10.0%. Moreover, C: 24.9%, H: 1.5% and N: 9.7% were obtained for C12H10Br2N4Pb. The selected FTIR bands (cm−1) were as follows: 634s, 750s, 864m, 1045m, 1418s, 1482m, 1590s, 1620s, 3024w.
2.4. Preparation of Nanoparticles of Pb(II) Oxide
After dissolving 0.1 mmol of [Pb(L)Br2]n (1) in 10 mL of oleic acid, the light-yellow solution obtained was degassed for forty-five minutes (with stirring and slow heating) and then heated for one hour at 180 °C, ultimately yielding a black precipitate. After adding a great amount of ethanol and a little toluene to the solution, lead(II) oxide nanoparticles were centrifuged and thereby isolated. The final solid was rinsed in ethanol and dried at room temperature (yield: 43%, 0.01 g).
2.5. X-ray Crystallography
A 0.65 × 0.15 × 0.09 mm3 yellow compound crystal was placed over glass fiber using epoxy adhesives. An X-ray diffractometer (50 kV, 30 mA) with graphite monochromated Mo Kα radiation (λ = 0.71073 Å) was used over 2θ = 4.56–50.08° to collect data. No significant data were missing from the data collected. The data were processed in the crystal structure analysis module of the Bruker AXS [26]. The modules of AXS utilized included SADABS (Bruker) for absorption correction, SHELXS-97 (Sheldrick) and XPREP (Bruker) for structure solution, SHELXTL (Sheldrick) for molecular graphics and publication materials, SHELXL-97 (Sheldrick) for structure refinement, APEX2 (Bruker) for data collection, and SAINT (Bruker) for cell refinement and data reduction. Furthermore, the method proposed by Waber and Cromer was used to obtain scattering factors for neutral atoms [27]. The crystal was of the monoclinic space group P21/c given the systematic deficiency, E statistic, and effective structure refining. Furthermore, the structure was solved by utilizing direct techniques. For the data obtained from the compound, full-matrix least squares refinement was performed by minimizing the function of . Moreover, non-hydrogen atoms were anisotropically refined. The location of the hydrogen atoms was geometrically determined with 0.98Å (CH3) and C–H = 0.95 (aromatic) and refined with Uiso(H) = −1.2 UeqC as riding atoms. Table 1 and Table 2 present the crystallographic data, the bond lengths, and the angles.

Table 1.
Crystal data and structure refinement for [Pb(L)Br2]n (1).

Table 2.
Selected bond lengths (Å) and angles (°) for [Pb(L)Br2]n (1).
3. Results and Discussion
[Pb(L)Br2]n (1) was obtained as a novel one-dimensional fishbone coordination polymer from the reaction of lead(II) bromide with 1,2-bis (pyridine-3-ylmethylene)hydrazine (L). The nanostructure of the compound was determined in an aqueous solution through UST irradiation. The suitable single crystals of compound I for X-ray crystallography were obtained using the heat gradient of an aqueous solution based on the branched-tube method [28].
According to Figure 1, the bulk material developed using the branched-tube approach could not be differentiated from the nanostructured material obtained using the sonochemical approach in terms of FTIR spectrum and the results of elemental analysis. The FTIR spectrum of the single crystal materials and nanostructured material showed the representative absorption bands of the “L” ligand. Furthermore, the aromatic C–H absorption of hydrogen atoms caused a relatively weak band at approximately 3021 cm−1. The aromatic ring vibration of the “L” ligand was also observed at frequencies of 1418–1590 cm−1.
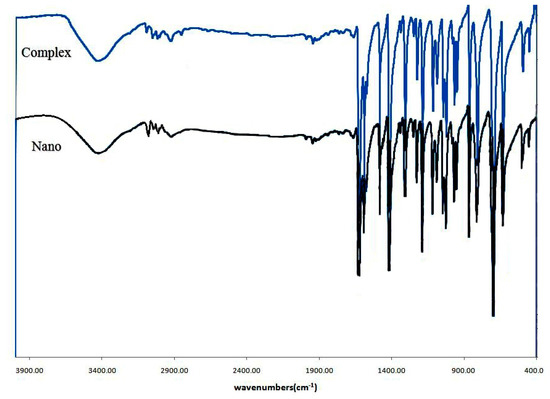
Figure 1.
FT-IR spectrum of crystal and nano structure of 1.
Table 1 and Table 2 show the results of single-crystal XRD for the structure of compound I. According to the single-crystal XRD in Figure 2, the structure of [Pb(L)Br2]n crystallized in a monoclinic system with the space group of P21/c resembled that of solid 1D fishbone metal–organic coordination polymers. Figure 2a shows the structure of the asymmetrical unit of 1 and an atom numbering arrangement selected. A Pb atom is coordinated by two N atoms of the L ligand with the same Pb–N distance of 2.670 Å and four bridged bromine anions with a Pb–Br distance of 2.992 (2.972, 2.992 and 2.972 Å). Figure 2b shows a coordination number of six for lead(II) atom in two modes with a PbN2Br4 donor set. The adjusted Pb····Pb distances belonging to the chain were 4.001 Å. This order demonstrates a symmetry in the coordination geometry surrounding metal ions in a holodirected fashion. Given the labile interactions, the architecture of one-dimensional chains was allowed to interact with neighboring chains, and the structure was also allowed to be extended into three-dimensional supramolecular metal-organic coordination polymers, as shown in Figure 2d.
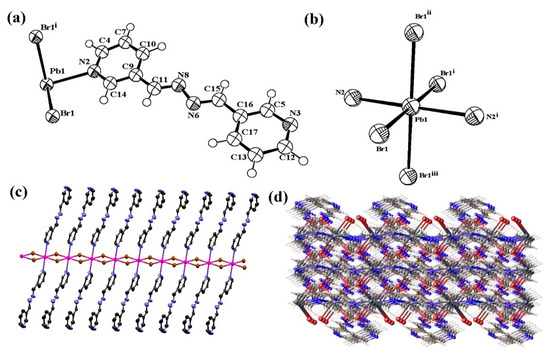
Figure 2.
(a) Molecular structure of asymmetrical unit (1). (b) Coordination sphere of Pb(II) in 1. (c) Fragment of the coordination polymer showing the 1D fish bone metal–organic coordination polymer. (d) From 1D architecture to supramolecular 3D coordination polymer via labile interactions (such as aromatic and π–π interaction, H-bonding).
Figure 3a shows the XRD pattern of compound 1 simulated from the single-crystal X-ray data, and Figure 3b shows the experimental XRD pattern of compound 1 prepared by using the sonochemical process. Except for slight changes in 2θ, the experimental data were consistent with the simulated XRD patterns, suggesting that the compound obtained as a nano-structure using the sonochemical method was the same as that derived through single-crystal XRD. The major peak enlargmenet showed the nano-dimension of the particles. According to D = 0.891λ/βcosθ as the Scherrer equation, the mean size of the grain was estimated at 39 nm, with D representing the mean grain size, λ = 0.15405 nm as the X-ray wavelength, and θ and β as the diffraction and full-width angles, respectively, at 50% of a peak.
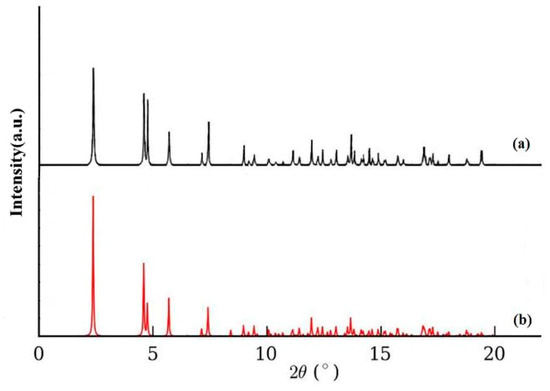
Figure 3.
X-ray Powder Diffraction Pattern (XRPD) of compound 1 (a) computed from single-crystal X-ray data and (b) from the nanorods.
The crystalline substance was provided with [Pb(L)Br2]n (1) through the reaction of 1,2-bis (pyridin-3-ylmethylene)hydrazine (L) with lead(II) bromide. In addition, the size and morphology of compound I prepared by the UST technique were analyzed with SEM. Figure 4 shows the SEM of compound I acquired by using a 600 W ultrasonic generator at a [Pb2+] = [Lˉ] = 0.1 M concentration of primary reagents. Figure 4 also shows the nanorod morphology with a diameter of 45–140 nm for compound I. Further assessments are required for clarifying the formation of these structures. Packing the system at molecular levels, however, morphologically affects the nanostructured compound, suggesting coordination-encouraged creation over the morphology of the nanostructure [29,30,31,32].

Figure 4.
SEM photographs of [Pb(L)Br2]n (1) nanorods.
Figure 5 shows the results of the TGA of the single crystals and nanostructures performed at 25–600 °C in argon to investigate the thermal stability of compound 1. For single crystals of [Pb(L)Br2]n, after removing water molecules from the coordinated molecules and moisture at 80–110 °C, compound 1 was stable at up to 160 °C. Decomposition at 160–425 °C resulted in a mass loss of about 47.8%. In the case of nanostructured [Pb(L)Br2]n, the decomposition started at a lower temperature, around 145 °C, with the similar weight loss. This may be attributed to different crystallographic surfaces of both samples exposed to a thermal gradient [33]. The thermal stability of [Pb(L)Br2]n is much lower than our previous reports on lead (II) coordination polymer, which may be attributable to differences in the structures of ligands coordinated to lead [34,35]. XPS findings showed that the solid residue at about 600 °C was PbO.
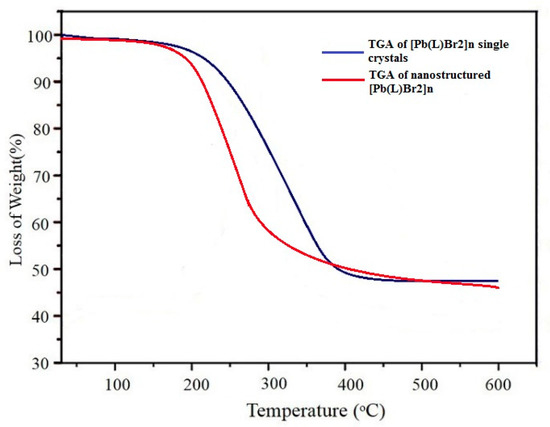
Figure 5.
TGA plot of [Pb(L)Br2]n (1) in single crystal shape and nanostructured mode.
The final products of the decomposition of compound 1 based on their XRD arrangements as per Figure 6a matched the standard design of tetragonal PbO with S.G = P4/nmm, a = 3.947 Å, Z = 2, c = 4.988 Å and JCPDS (Joint Committee on Powder Diffraction Standards) card file No: 85-0711. Figure 6b displays the XPS results of nano PbO. The two asymmetric peaks at 142.28 eV and 137.48 eV were attributed to the transitions of 4f5/2 and 4f7/2 from Pb2+ ions in PbO, respectively, indicating that Pb2+ in the product was the same as PbO in shape. The O1 s spectrum showed a peak at 531.1 eV, which was estimated at 530.9 eV of O1 s in PbO in the literature. Figure 7 shows the SEM and TEM results of the residue obtained from the thermolysis of 1 at 180 °C by employing oleic acid as a surfactant, suggesting the regular shape of Pb(II) oxide nanoparticles. The significant broadening of the peaks indicates that the particles have nanometer dimensions. The average grain size D was estimated as 58 nm using the Scherrer formula, D = 0.891λ/βcosθ, where λ is the X-ray wavelength (0.15405 nm) and θ and β are the diffraction angle and full-width at half maximum of an observed peak, respectively [36].
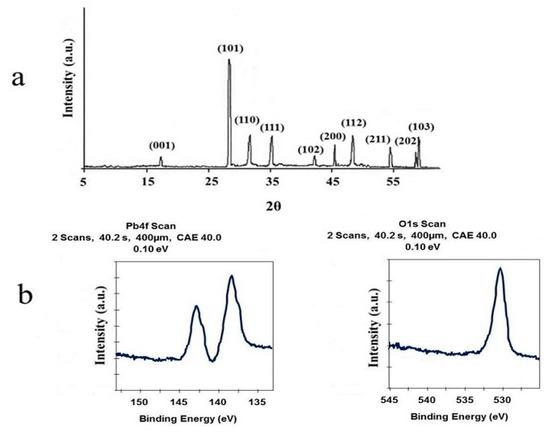
Figure 6.
(a) XRD pattern and (b) XPS spectra of PbO nanoparticles prepared by thermolysis of compound 1.
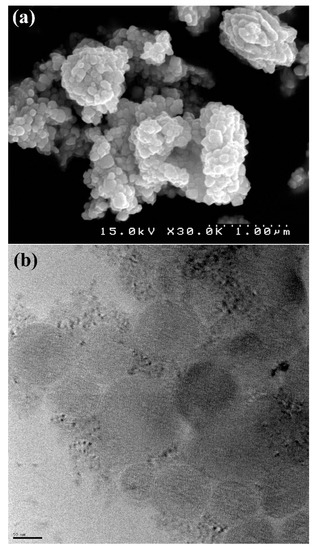
Figure 7.
(a) SEM and (b) TEM images of PbO nano-structures produced by the thermolysis of nano-structures of compound 1.
4. Conclusions
In this study, a sonochemical method was used to synthesize nano-structures of a new 1D fishbone coordination polymer of divalent lead with 1,2-bis (pyridin-3-ylmethylene)hydrazine (L). Single-crystal XRD was performed to structurally determine compound 1. A three-dimensional supramolecular compound included in the crystal structure of compound I demonstrated a coordination number of six in lead (II) ions. The complex takes the form of a 1D metal–organic system in solid state. As a result of several labile interactions with neighboring chains, the 1D chain extended into a 3D supramolecular coordination polymer. The sonochemically made compound 1 had nanorod morphology with diameter of 38–43 nm. Moreover, the calcination of compound I through the thermolysis of 1 at 180 °C by using oleic acid as a surfactant yielded a uniform PbO nanoparticle.
Author Contributions
Y.H., supervision, writing—review and editing; B.M., writing—review and editing; S.W.J., project administration and editing; M.S.-R., writing—original draft preparation; M.A., formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the grant NRF-2019R1A5A8080290 of the National Research Foundation of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Cambridge Crystallographic Data Centre provided the crystallographic information for the structures as supplementary publication CCDC-1567103 for [Pb(L)Br2]n (1). Duplicates of these data may be acquired by contacting deposit@ccdc.cam.ac.uk by email or through official correspondence with CCDC, 12 Union Road, Cambridge CB2 1EZ, United Kingdom, Fax: +441223336033.
Acknowledgments
The authors thank Sayyed Jamaledin Asadabadi University and Qom University for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chakrabarty, R.; Mukherjee, P.S.; Stang, P.J. Supramolecular coordination: Self-assembly of finite two-and three-dimensional ensembles. Chem. Rev. 2011, 111, 6810–6918. [Google Scholar] [CrossRef]
- Hu, M.L.; Morsali, A.; Aboutorabi, L. Lead (II) carboxylate supramolecular compounds: Coordination modes, structures and nano-structures aspects. Coord. Chem. Rev. 2011, 255, 2821–2859. [Google Scholar] [CrossRef]
- Leong, W.L.; Vittal, J.J. One-dimensional coordination polymers: Complexity and diversity in structures, properties, and applications. Chem. Rev. 2011, 111, 688–764. [Google Scholar] [CrossRef] [PubMed]
- Akhbari, K.; Morsali, A. Thallium (I) supramolecular compounds: Structural and properties consideration. Coord. Chem. Rev. 2010, 254, 1977–2006. [Google Scholar] [CrossRef]
- Gharib, M.; Safarifard, V.; Morsali, A. Ultrasound assisted synthesis of amide functionalized metal-organic framework for nitroaromatic sensing. Ultrason. Sonochem. 2018, 42, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O-and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Sonochemical synthesis of amide-functionalized metal-organic framework/graphene oxide nanocomposite for the adsorption of methylene blue from aqueous solution. Ultrason. Sonochem. 2018, 41, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Kitagawa, S. Nanocrystals of coordination polymers. Chem. Lett. 2005, 34, 132–137. [Google Scholar] [CrossRef]
- Shimoni-Livny, L.; Glusker, J.P.; Bock, C.W. Lone pair functionality in divalent lead compounds. Inorg. Chem. 1998, 37, 1853–1867. [Google Scholar] [CrossRef]
- Engelhardt, L.M.; Furphy, B.M.; Harrowfield, J.M.; Patrick, J.M.; White, A.H. 1: 1 Adducts of lead (II) thiocyanate with 1, 10-phenanthroline and 2,2′,6′,2′′-terpyridine. Inorg. Chem. 1989, 28, 1410–1413. [Google Scholar] [CrossRef]
- Akhbari, K.; Morsali, A.; Retailleau, P. Effect of two sonochemical procedures on achieving to different morphologies of lead (II) coordination polymer nano-structures. Ultrason. Sonochem. 2013, 20, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Platas-Iglesias, C.; Esteban-Gómez, D.; Enríquez-Pérez, T.; Avecilla, F.; de Blas, A.; Rodriguez-Blas, T. Lead (II) thiocyanate complexes with bibracchial lariat ethers: An X-ray and DFT study. Inorg. Chem. 2005, 44, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Mojidov, A.; Morsali, A. Two-dimensional Holodirected Lead (II) Coordination Polymer, [Pb (μ-bpp)(μ-SCN) 2] n (bpp= 1, 3-di (4-pyridyl) propane). Z. Anorg. Allg. Chem. 2007, 633, 1949–1951. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Son, W.J.; Kim, J.; Kim, J.; Ahn, W.S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 47, 6336–6338. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. The sonochemical hot spot. J. Acoust. Soc. Am. 1991, 89, 1885–1886. [Google Scholar] [CrossRef]
- Safarifard, V.; Morsali, A. Facile preparation of nanocubes zinc-based metal-organic framework by an ultrasound-assisted synthesis method; precursor for the fabrication of zinc oxide octahedral nanostructures. Ultrason. Sonochem. 2018, 40, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Koda, S.; Nomura, H. Effects of sample volume and frequency on ultrasonic power in solutions on sonication. Jpn. J. Appl. Phys. 1998, 37, 2992. [Google Scholar] [CrossRef]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Safarifard, V.; Morsali, A. Applications of ultrasound to the synthesis of nanoscale metal–organic coordination polymers. Coord. Chem. Rev. 2015, 292, 1–14. [Google Scholar] [CrossRef]
- Mercury 1.4.1; Copyright Cambridge Crystallographic Data Centre: Cambridge, UK, 2001–2005.
- Harrowfield, J.M.; Miyamae, H.; Skelton, B.W.; Soudi, A.A.; White, A.H. Lewis-base adducts of lead (II) compounds. XX. synthesis and structure of the 1: 1 adduct of pyridine with lead (II) thiocyanate. Aust. J. Chem. 1996, 49, 1165–1169. [Google Scholar] [CrossRef]
- SHELXTL, N.T. Crystal Structure Analysis Package, Version 6.14; Bruker AXS: Madison, WI, USA, 2000. [Google Scholar]
- Cromer, D.T.; Waber, J.T. International Tables for X-ray Crystallography; Table 2.2 A; Kynoch Press: Birmingham, UK, 1974; Volume 4. [Google Scholar]
- Morsali, A.; Masoomi, M.Y. Structures and properties of mercury (II) coordination polymers. Coord. Chem. Rev. 2009, 253, 1882–1905. [Google Scholar] [CrossRef]
- Fard, M.J.S.; Morsali, A. Sonochemical Synthesis of New Nano-Belt One-Dimensional Double-Chain Lead (II) Coordination Polymer; As Precursor for Preparation of PbBr (OH) Nano-Structure. J. Inorg. Organomet. Polym. Mater. 2010, 20, 727–732. [Google Scholar] [CrossRef]
- Ranjbar, Z.R.; Morsali, A.; Retailleau, P. Thermolysis preparation of zinc (II) oxide nanoparticles from a new micro-rods one-dimensional zinc (II) coordination polymer synthesized by ultrasonic method. Inorg. Chim. Acta 2011, 376, 486–491. [Google Scholar] [CrossRef]
- Ranjbar, Z.R.; Morsali, A. Preparation of Zinc (II) Oxide Nanoparticles from a New Nano-Size Coordination Polymer Constructed from a Polypyridyl Amidic Ligand; Spectroscopic, Photoluminescence and Thermal Analysis Studies. J. Inorg. Organomet. Polym. Mater. 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Mahmoudi, G.; Morsali, A. Sonochemical syntheses and characterization of new nanorod crystals of mercury (II) metal–organic polymer generated from polyimine ligands. J. Coord. Chem. 2010, 63, 1186–1193. [Google Scholar] [CrossRef]
- Marzbanrad, E.; Rivers, G.; Peng, P.; Zhao, B.; Zhou, N.Y. How morphology and surface crystal texture affect thermal stability of a metallic nanoparticle: The case of silver nanobelts and pentagonal silver nanowires. Phys. Chem. Chem. Phys. 2015, 17, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hanifehpour, Y.; Mirtamizdoust, B.; Ahmadi, H.; Wang, R.; Joo, S.W. Ultrasonic-assisted synthesis, characterizing the structure and DFT calculation of a new Pb(II)-chloride metal-ligand coordination polymer as a precursor for preparation of α-PbO nanoparticles. J. Mol. Struct. 2021, 1124, 129031. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Mirtamizdoust, B.; Ahmadi, H.; Wang, R.; Joo, S.W. Sonochemical synthesis, crystal structure, and DFT calculation of an innovative nanosized Pb(II)-azido metal–organic coordination polymer as a precursor for preparation of PbO nanorod. Chem. Pap. 2020, 74, 3651–3660. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).