Effect of Mixing Light-Burned MgO with Different Activity on the Expansion of Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
2.3.1. Expansion Rate Test
2.3.2. Compressive Strength Test
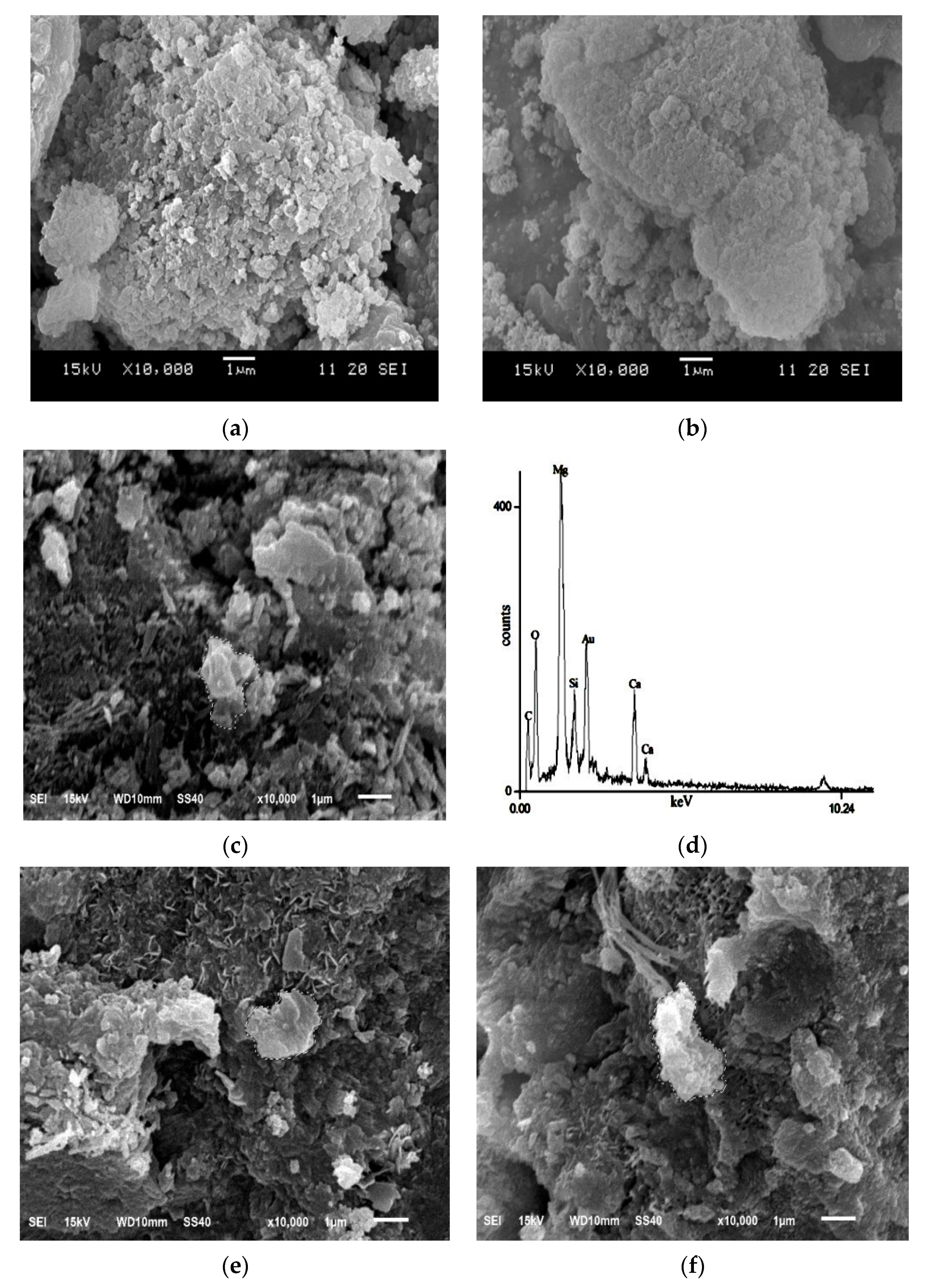
2.3.3. Scanning Electron Microscopy (SEM) Characterization
2.3.4. Measurement of Hydration Degree of Periclase in Cement Paste
3. Results and Discussion
3.1. Expansion Property of Specimens
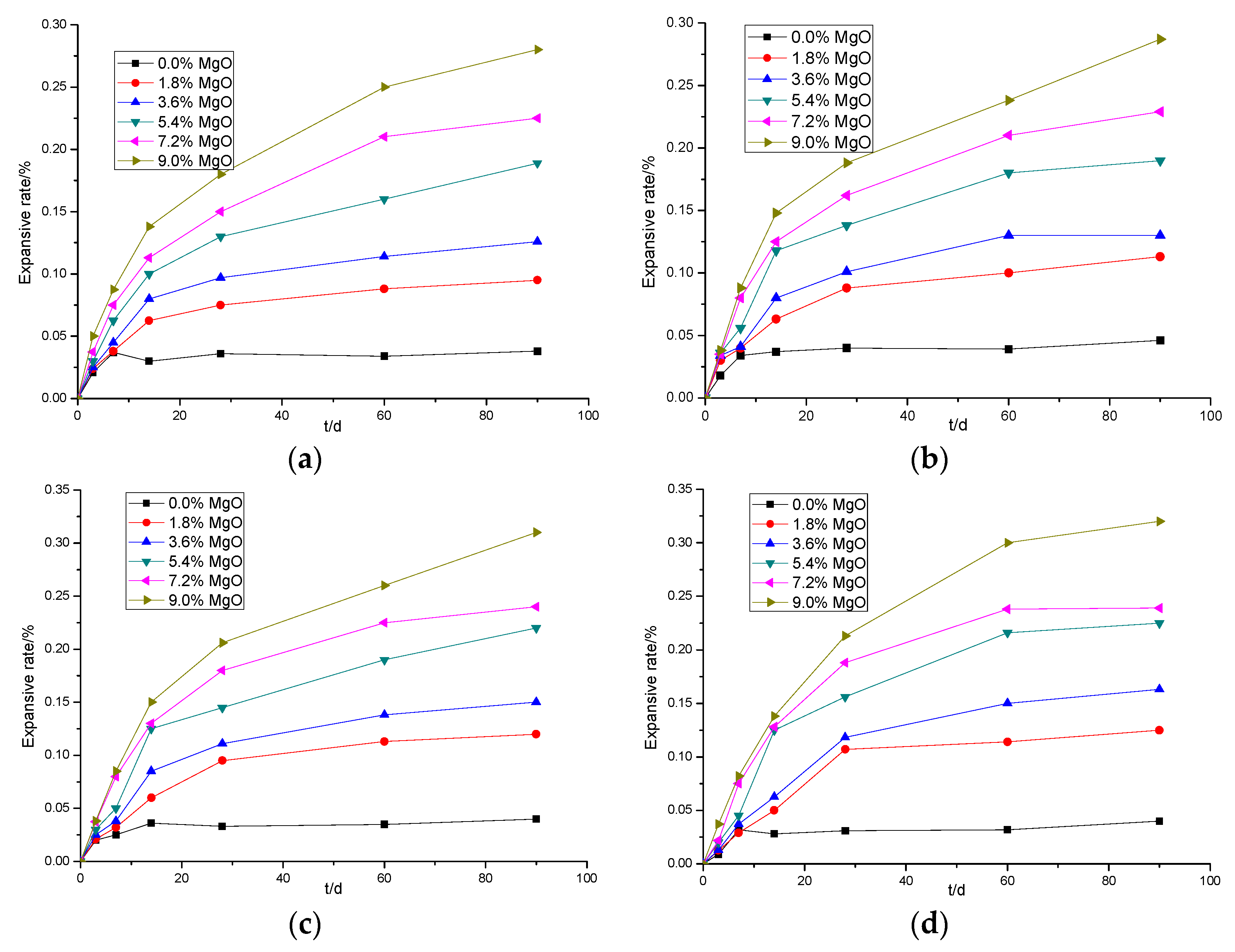
3.1.1. The Effects of Added MgO Content on the Expansion of Cement Pastes Mixed with Light-Burned MgO
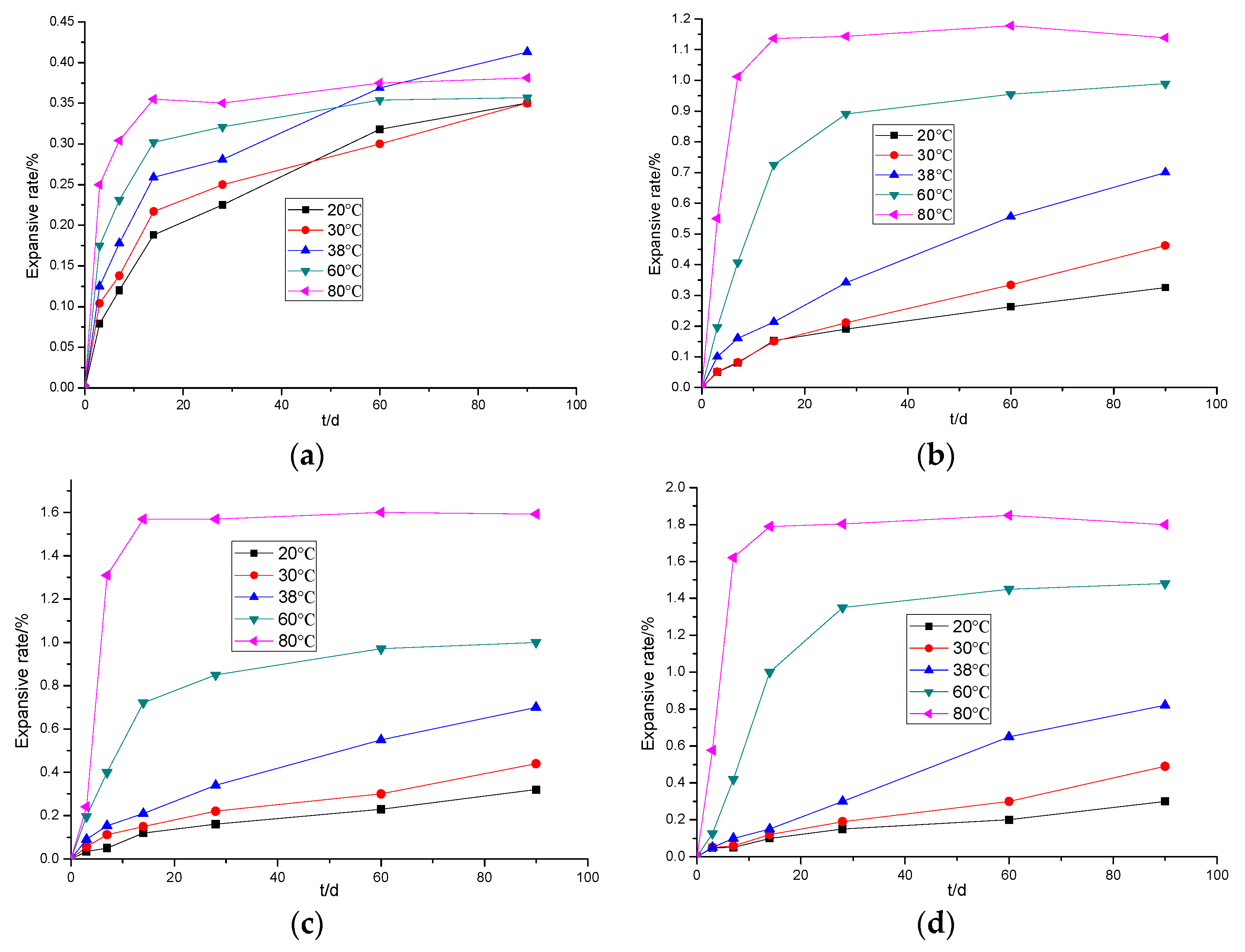
3.1.2. The Effects of Temperature on the Expansion of Cement Paste Mixed with Light-Burned MgO
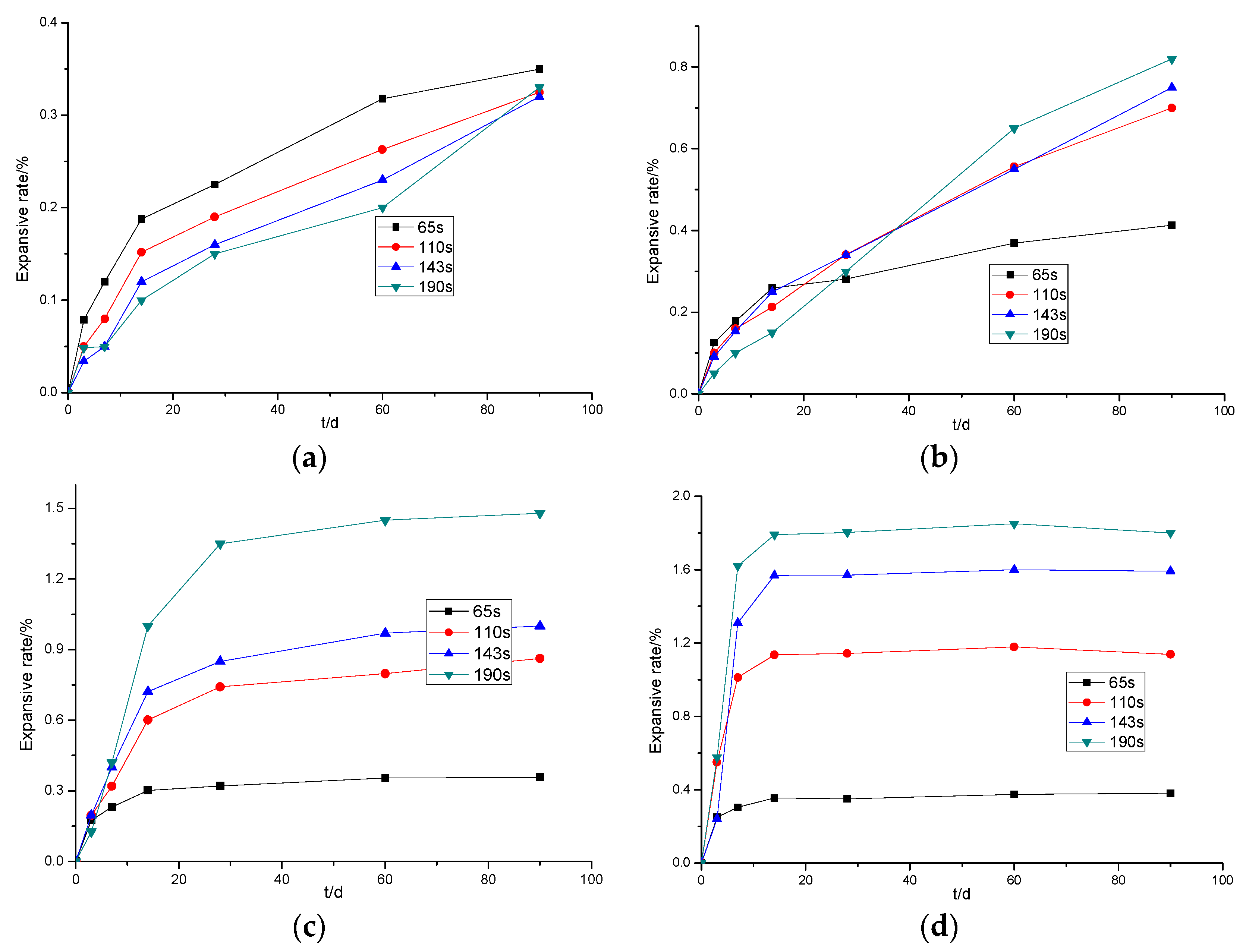
3.1.3. The Effects of MgO Activity on the Expansion of Cement Paste Mixed with Light-Burned MgO
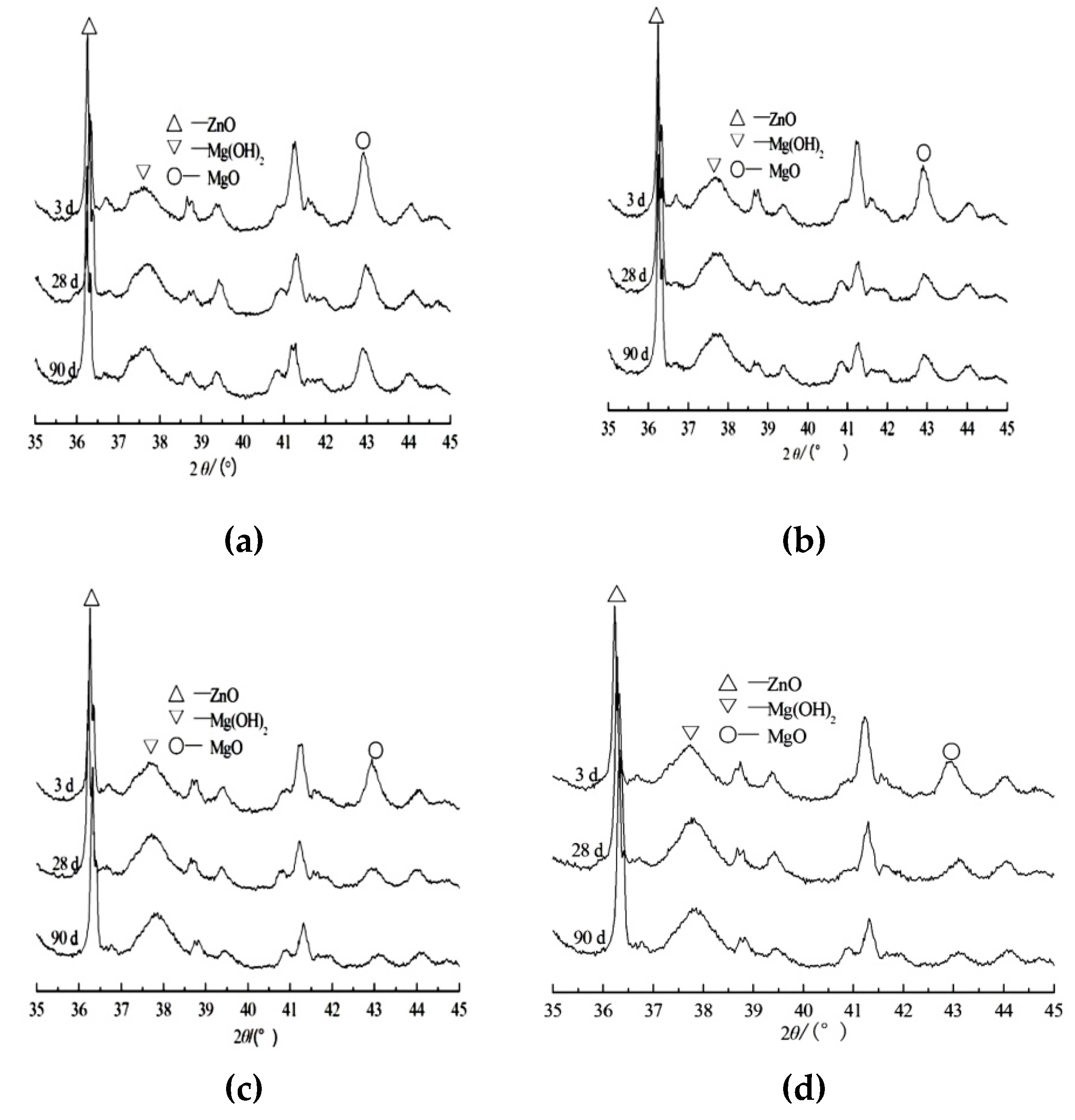
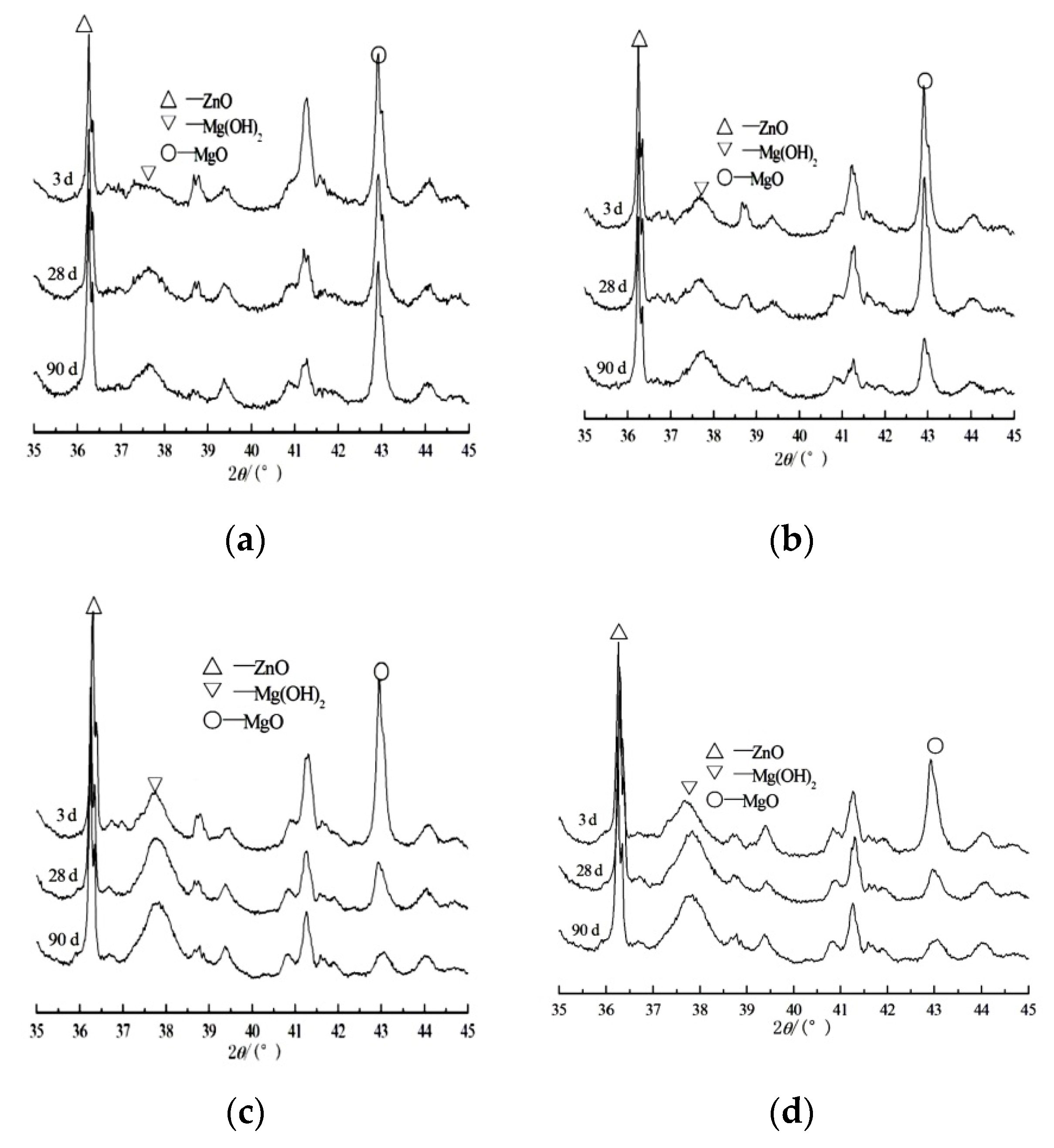
3.2. Measurement of Hydration Degree of Periclase in Cement Paste
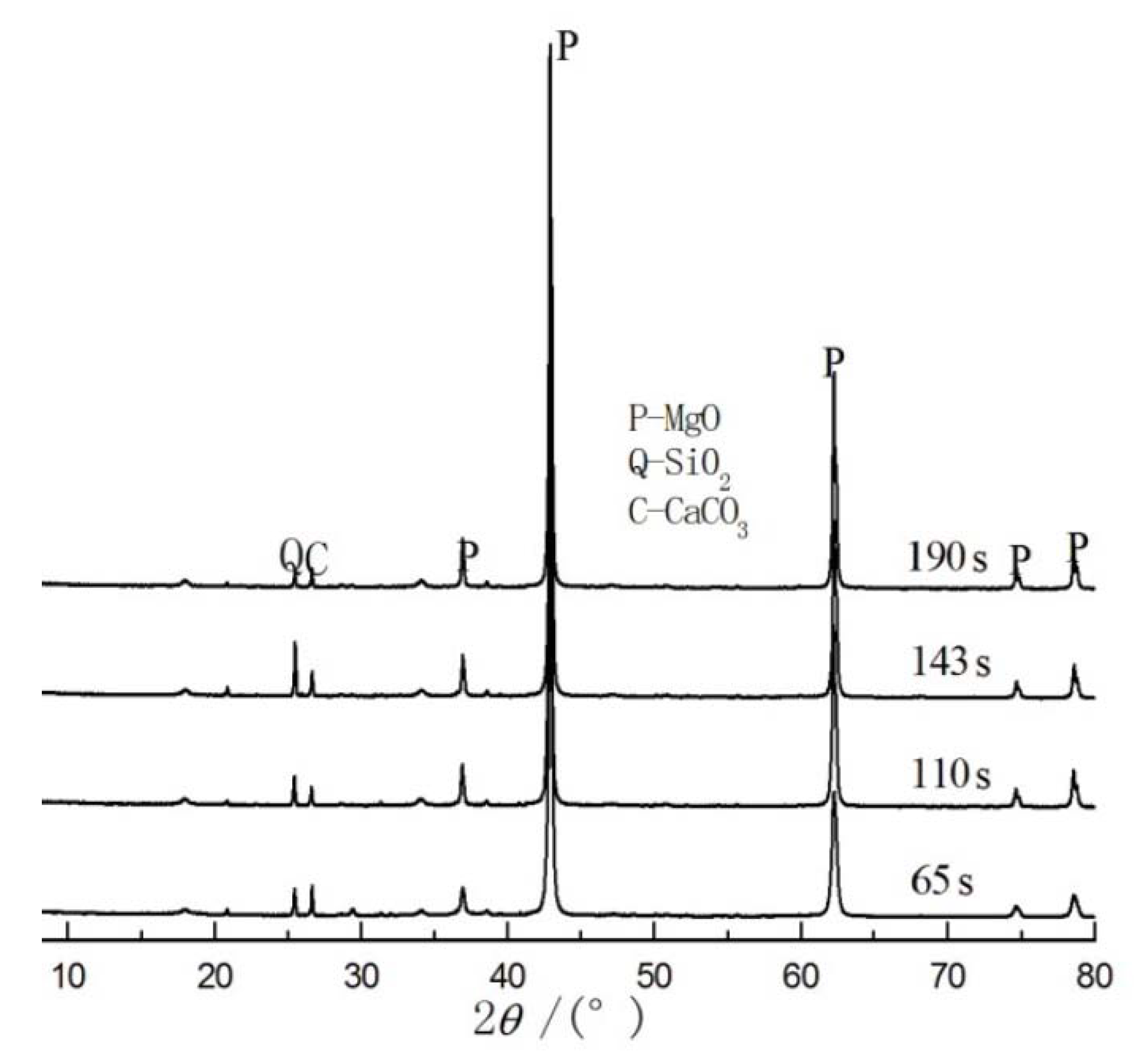
3.3. Microstructure of Specimens
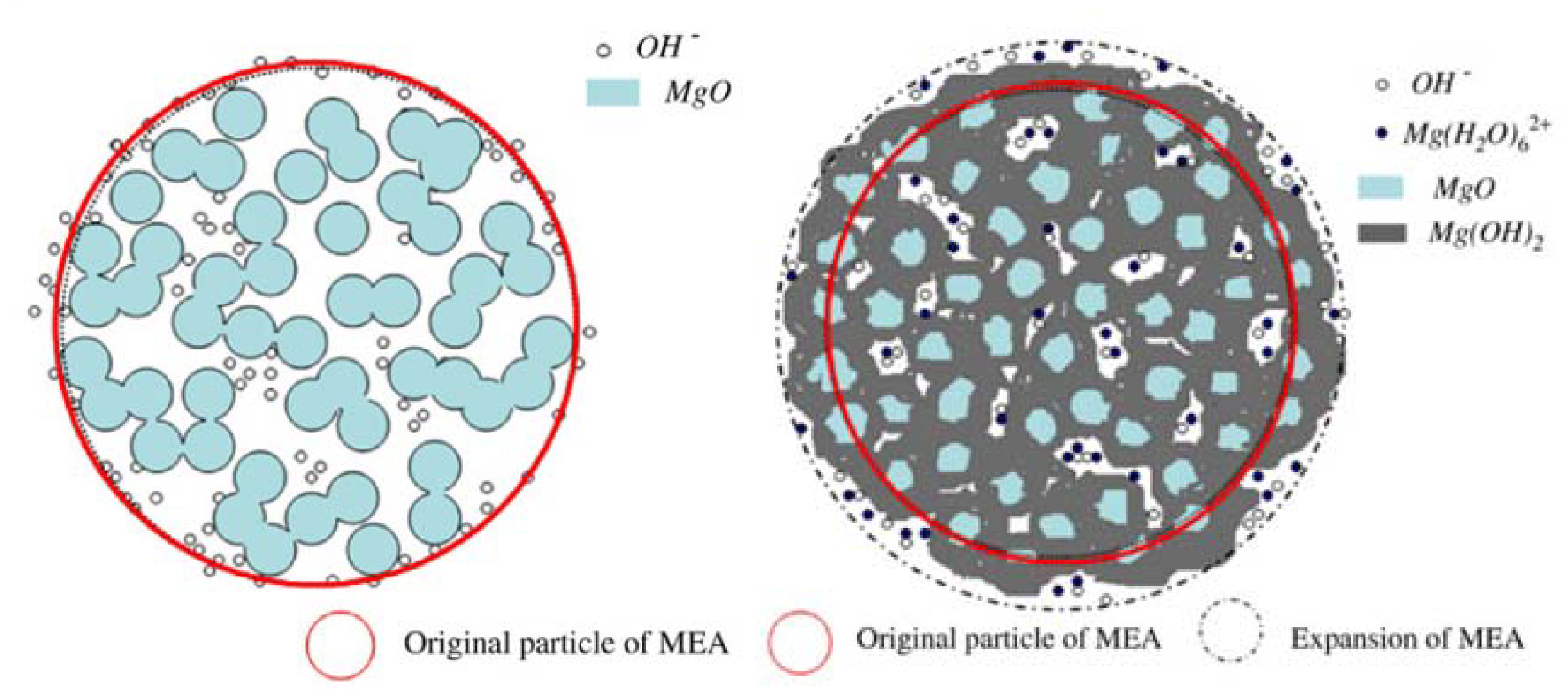
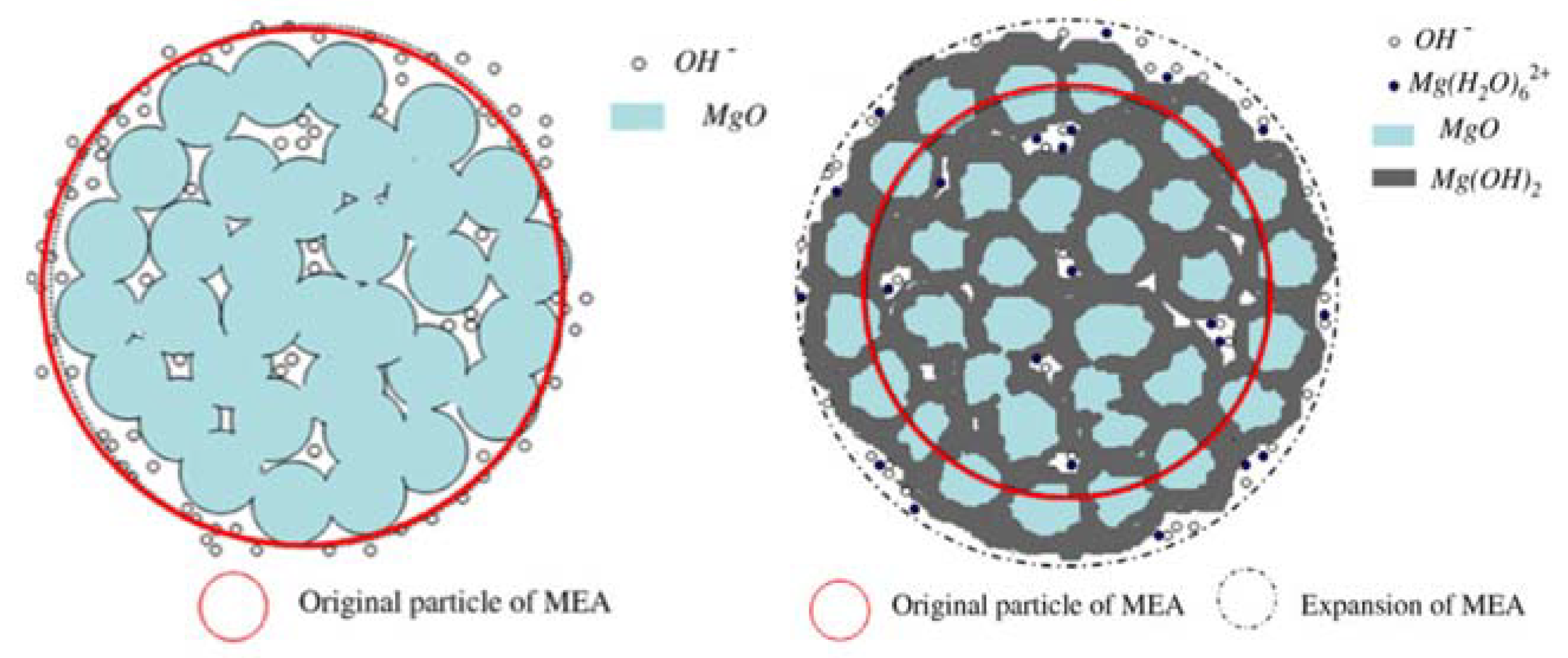
3.4. Discussion According to the Aforementioned Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lea, F.M. The Chemistry of Cement and Concrete; Chemical Publishing Company: New York, NY, USA, 1971. [Google Scholar]
- Mehta, P. History and Status of Performance Tests for Evaluation of Soundness of Cements; ASTM International: West Conshohocken, PA, USA, 1978. [Google Scholar]
- Mo, L.W. Microstructure and Property of MgO-Type Expansive Agent. Ph.D. Thesis, Nanjing Tech University, Nanjing, China, 2008. [Google Scholar]
- Mehta, P.; Pirtz, D. Magnesium oxide additive for producing selfstress in mass concrete. In Proceedings of the 7th International Congress on the Chemistry of Cement, Paris, France, 30 June–4 July 1980. [Google Scholar]
- Li, C. Review of quick damming technology of MgO concrete. Adv. Sci. Technol. Water Resour. 2013, 33, 82–87. [Google Scholar] [CrossRef]
- Gao, P.W.; Wu, S.X.; Lu, X.L.; Deng, M.; Lin, P.H.; Wu, Z.R.; Tang, M.S. Soundness evaluation of concrete with MgO. Constr. Build. Mater. 2007, 21, 132–138. [Google Scholar] [CrossRef]
- Zhu, B. On construction of dams by concrete with gentle volume expansion. J. Hydroelectr. Eng. 2000, 1–13. [Google Scholar] [CrossRef]
- Ye, Q.; Chen, H.X.; Wang, Y.Q. Effect of MgO and gypsum content on long-term expansion of low heat Portland slag cement with slight expansion. Cem. Concr. Compos. 2004, 26, 331–337. [Google Scholar] [CrossRef]
- Gao, P.; Lu, X.; Geng, F.; Li, X.; Hou, J.; Lin, H.; Shi, N. Production of MgO-type expansive agent in dam concrete by use of industrial by-products. Build. Environ. 2008, 43, 453–457. [Google Scholar] [CrossRef]
- Xu, L.L.; Deng, M. Dolomite used as raw material to produce MgO-based expansive agent. Cem. Concr. Res. 2005, 35, 1480–1485. [Google Scholar] [CrossRef]
- Xu, L.L.; Deng, M.; Wang, X.; Zhao, X. Studies on preparation of a new expansive agent based on MgO. J. Mater. Sci. Eng. 2004, 22, 249–253. [Google Scholar] [CrossRef]
- Xu, L.L.; Deng, M.; Zhao, X. Compensating shrinkage of cement paste by new MgO-based expansive material. J. Build. Mater. 2005, 8, 67–70. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, M. Preparation and Performance of MgO-type Expansive Agent. J. East China Inst. Technol. 2005, 28, 71–75. [Google Scholar] [CrossRef]
- Cao, F.; Liu, Y.; Yan, P. Properties and mechanism of the compound MgO expansive agent (CMEA) produced by calcining the mixture of dolomite and serpentine tailings. Constr. Build. Mater. 2021, 277, 122331. [Google Scholar] [CrossRef]
- Liu, P.C.; Deng, M. Regulating the Expansion Characteristics of Cementitious Materials Using Blended MgO-Type Expansive Agent. Materials 2019, 12, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Kendelewicz, T.; Brown, G.E.; Parks, G.A. Reaction of water with MgO (100) surfaces. Part I: Synchrotron X-ray photoemission studies of low-defect surfaces. Surf. Sci. 1998, 412, 287–314. [Google Scholar] [CrossRef]
- Liu, P.; Kendelewicz, T.; Brown, G.E.; Parks, G.A. Reaction of water with MgO (100) surfaces. Part II: Synchrotron photoemission studies of defective surfaces. Surf. Sci. 1998, 412, 315–332. [Google Scholar] [CrossRef]
- Mejias, J.A.; Berry, A.J.; Refson, K.; Fraser, D.G. The kinetics and mechanism of MgO dissolution. Chem. Phys. Lett. 1999, 314, 558–563. [Google Scholar] [CrossRef]
- Xu, Y.M.; Xu, L.L.; Li, W.W. Research Progress of the Periclase and Quantitative Method in Cement Clinker. Mater. Rep. 2013, 27, 355–358. [Google Scholar]
- Cao, F.; Miao, M.; Yan, P. Effects of reactivity of MgO expansive agent on its performance in cement-based materials and an improvement of the evaluating method of MEA reactivity. Constr. Build. Mater. 2018, 187, 257–266. [Google Scholar] [CrossRef]
- Zheng, H.W. Magnesium Oxide in Cement. Fujian Build. Mater. 2000, 3, 9–11. [Google Scholar]
- Qian, H.Y.; Li, S.Y.; Deng, M. Hydrochemical Kinetics of Light-Burned Magnesia. Ind. Miner. Process. 2007, 12, 1–4. [Google Scholar]
- Liu, J.P.; Wang, Y.J.; Tian, Q.; Zhang, S.Z. Temperature sensitivity of light calcined magnesia expansion agent and its mechanism analysis. J. Southeast Univ. 2011, 41, 359–364. [Google Scholar]
- Yu, L.Q.; Deng, M.; Mo, L.W. Effects of Lightly Burnt MgO Expansive Agent on the Deformation and Microstructure of Reinforced Concrete Wall. Adv. Mater. Sci. Eng. 2019, 4, 1948123. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Tu, Y.J.; Liu, J.P.; Miao, C.W. Temperature Sensitivity Analysis on Expansive Property of MgO Composite Expansion Agent. Water Power 2010, 36, 49–51, 86. [Google Scholar] [CrossRef]
- Ye, Q.; Yu, K.; Zhang, Z. Expansion of ordinary Portland cement paste varied with nano-MgO. Constr. Build. Mater. 2015, 78, 189–193. [Google Scholar] [CrossRef]
- Chatterji, S. Mechanism of expansion of concrete due to the presence of dead-burnt CaO and MgO. Cem. Concr. Res. 1995, 25, 51–56. [Google Scholar] [CrossRef]
- Kitamura, A.; Oniduka, K.; Tanaka, K. Hydration characteristics of magnesia. Taikabutsu Overseas 1996, 16, 3–11. [Google Scholar]
- Cao, F.; Yan, P. The influence of the hydration procedure of MgO expansive agent on the expansive behavior of shrinkage-compensating mortar. Constr. Build. Mater. 2019, 202, 162–168. [Google Scholar] [CrossRef]
- Cao, F.; Miao, M.; Yan, P. Hydration characteristics and expansive mechanism of MgO expansive agents. Constr. Build. Mater. 2018, 183, 234–242. [Google Scholar] [CrossRef]
- Salomao, R.; Bittencourt, L.; Pandolfelli, V. A novel approach for magnesia hydration assessment in refractory castables. Ceram. Int. 2007, 33, 803–810. [Google Scholar] [CrossRef]
- Jennings, H.M.; Bullard, J.W.; Thomas, J.J.; Andrade, J.E.; Chen, J.J.; Scherer, G.W. Characterization and modeling of pores and surfaces in cement paste. J. Adv. Concr. Technol. 2008, 6, 5–29. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.J.; Jennings, H.M. A colloidal interpretation of chemical aging of the C-S-H gel and its effects on the properties of cement paste. Cem. Concr. Res. 2006, 36, 30–38. [Google Scholar] [CrossRef]
- Mo, L.W.; Deng, M.; Tang, M.S. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cem. Concr. Res. 2010, 40, 437–446. [Google Scholar] [CrossRef]


| Material | Loss | SiO2 | Fe2O3 | Al2O3 | SO3 | CaO | K2O | Na2O | MgO | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| DM1# | 1.16 | 20.51 | 4.47 | 3.89 | 0.38 | 65.53 | 0.54 | 0.23 | 1.81 | 98.52 |
| Material | C3S | C2S | C4AF | C3A | f-CaO | f-MgO | Total |
|---|---|---|---|---|---|---|---|
| DM1# | 60.00 | 22.18 | 13.19 | 3.38 | 0.56 | 1.25 | 100.46 |
| Samples | Active Reaction Time (s) | Density (kg/m3) | Specific Surface Area (m2/g) |
|---|---|---|---|
| A1 | 65 | 3790 | 17.99 |
| A2 | 110 | 3500 | 12.25 |
| A3 | 143 | 3430 | 13.13 |
| A4 | 190 | 3730 | 11.69 |
| Material | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | Na2O | SO3 | Loss | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 2.76 | 0.26 | 0.42 | 3.18 | 88.52 | 0.03 | 0.06 | 0.61 | 3.62 | 99.46 |
| A2 | 2.46 | 0.29 | 0.43 | 3.15 | 90.02 | 0.02 | 0.05 | 0.68 | 2.54 | 99.64 |
| A3 | 4.28 | 0.32 | 0.45 | 3.22 | 87.82 | 0.02 | 0.05 | 0.18 | 2.16 | 99.1 |
| A4 | 3.98 | 0.3 | 0.4 | 3.01 | 89.32 | 0.01 | 0.04 | 0.63 | 2.04 | 99.73 |
| Curing Temperature/°C | Curing Age/Day | Loss/% | Content of Brucite /% | Content of Periclase/% | Hydration Degree/% |
|---|---|---|---|---|---|
| 20 | 3 | 16.82 | 6.23 | 4.65 | 45.88 |
| 28 | 23.47 | 6.48 | 3.95 | 50.03 | |
| 90 | 23.93 | 7.18 | 3.25 | 68.64 | |
| 30 | 3 | 17.93 | 7.16 | 3.76 | 55.64 |
| 28 | 22.24 | 7.13 | 3.45 | 57.05 | |
| 90 | 23.29 | 9.35 | 1.41 | 82.20 | |
| 38 | 3 | 18.91 | 8.50 | 2.51 | 70.01 |
| 28 | 22.6 | 8.83 | 1.85 | 77.06 | |
| 90 | 24.04 | 9.62 | 0.95 | 87.89 | |
| 60 | 3 | 18.97 | 7.52 | 3.37 | 59.73 |
| 28 | 21.93 | 9.82 | 1.01 | 90.48 | |
| 90 | 23.97 | 10.52 | 0.38 | 95.16 | |
| 80 | 3 | 18.78 | 9.44 | 1.70 | 79.73 |
| 28 | 20.46 | 10.62 | 0.68 | 91.73 | |
| 90 | 20.37 | 10.77 | 0.34 | 96.27 |
| Curing Temperature/°C | Curing Age/Day | Loss/% | Content of Brucite /% | Content of Periclase/% | Hydration Degree/% |
|---|---|---|---|---|---|
| 20 | 3 | 18.28 | 4.94 | 5.68 | 30.28 |
| 28 | 18.30 | 6.68 | 4.16 | 32.59 | |
| 90 | 23.23 | 6.38 | 4.06 | 58.92 | |
| 30 | 3 | 14.36 | 5.59 | 5.34 | 41.94 |
| 28 | 20.50 | 6.04 | 4.57 | 46.47 | |
| 90 | 22.15 | 7.47 | 3.14 | 65.24 | |
| 38 | 3 | 14.07 | 6.61 | 4.95 | 43.34 |
| 28 | 16.52 | 6.67 | 4.29 | 48.15 | |
| 90 | 23.59 | 8.48 | 2.07 | 71.77 | |
| 60 | 3 | 16.85 | 6.44 | 4.47 | 47.06 |
| 28 | 17.01 | 11.39 | 0.19 | 83.92 | |
| 90 | 23.18 | 9.93 | 0.76 | 87.78 | |
| 80 | 3 | 14.30 | 11.42 | 0.48 | 84.78 |
| 28 | 14.49 | 11.43 | 0.45 | 85.10 | |
| 90 | 22.40 | 10.45 | 0.38 | 89.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, C.; Xu, L. Effect of Mixing Light-Burned MgO with Different Activity on the Expansion of Cement Paste. Crystals 2021, 11, 1360. https://doi.org/10.3390/cryst11111360
Wang Y, Zhang C, Xu L. Effect of Mixing Light-Burned MgO with Different Activity on the Expansion of Cement Paste. Crystals. 2021; 11(11):1360. https://doi.org/10.3390/cryst11111360
Chicago/Turabian StyleWang, Yang, Caoning Zhang, and Lingling Xu. 2021. "Effect of Mixing Light-Burned MgO with Different Activity on the Expansion of Cement Paste" Crystals 11, no. 11: 1360. https://doi.org/10.3390/cryst11111360
APA StyleWang, Y., Zhang, C., & Xu, L. (2021). Effect of Mixing Light-Burned MgO with Different Activity on the Expansion of Cement Paste. Crystals, 11(11), 1360. https://doi.org/10.3390/cryst11111360





