The Development of Graphene/Silica Hybrid Composites: A Review for Their Applications and Challenges
Abstract
:1. Introductions
2. Synthesis Methods
2.1. Synthesis Method of Silica
2.1.1. Sol-Gel Method
2.1.2. Hydrothermal
2.1.3. Leaching
2.1.4. Pyrolysis
2.2. Synthesis Method of Graphene
2.2.1. Top-Down Approaches
Exfoliation Method
Chemical Reduction of Graphene Oxide/Organic Treatment
Electrochemical Method
Ball Milling
2.2.2. Bottom-Up Approaches
Chemical Vapor Deposition (CVD)
Arc Discharge
CNT Unzipping
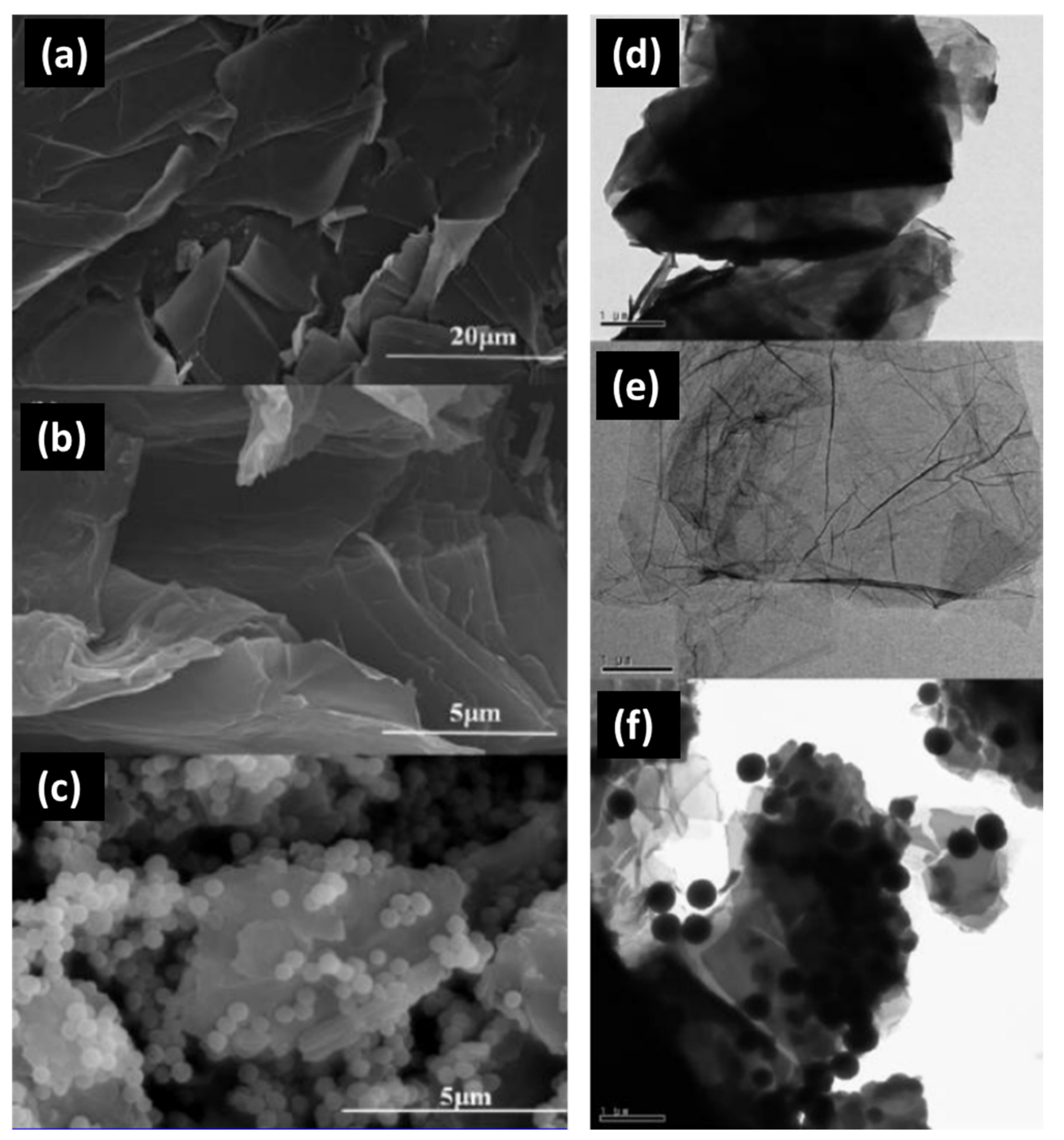
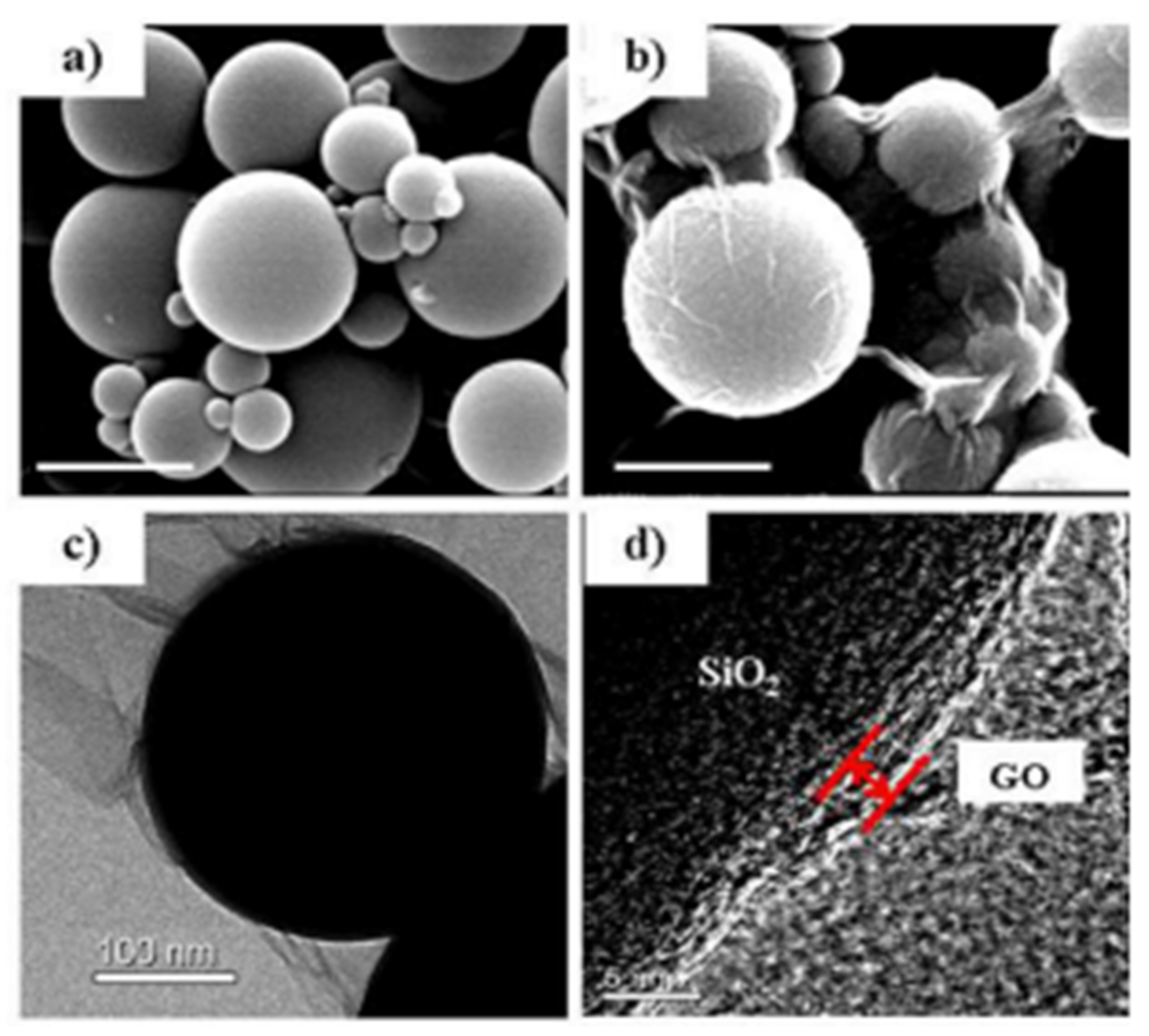
2.3. Synthesis Method of Silica–Graphene Hybrid Composites
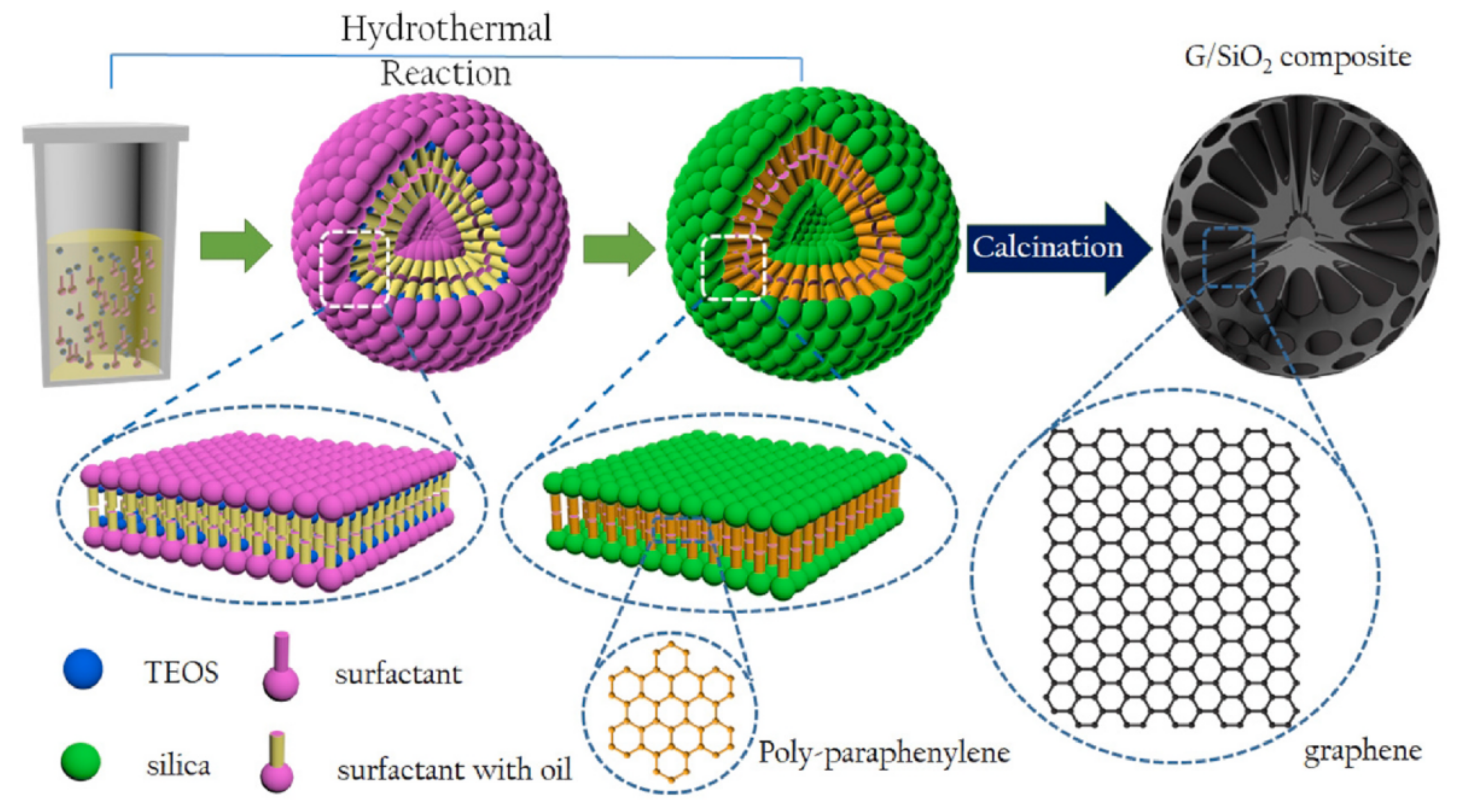
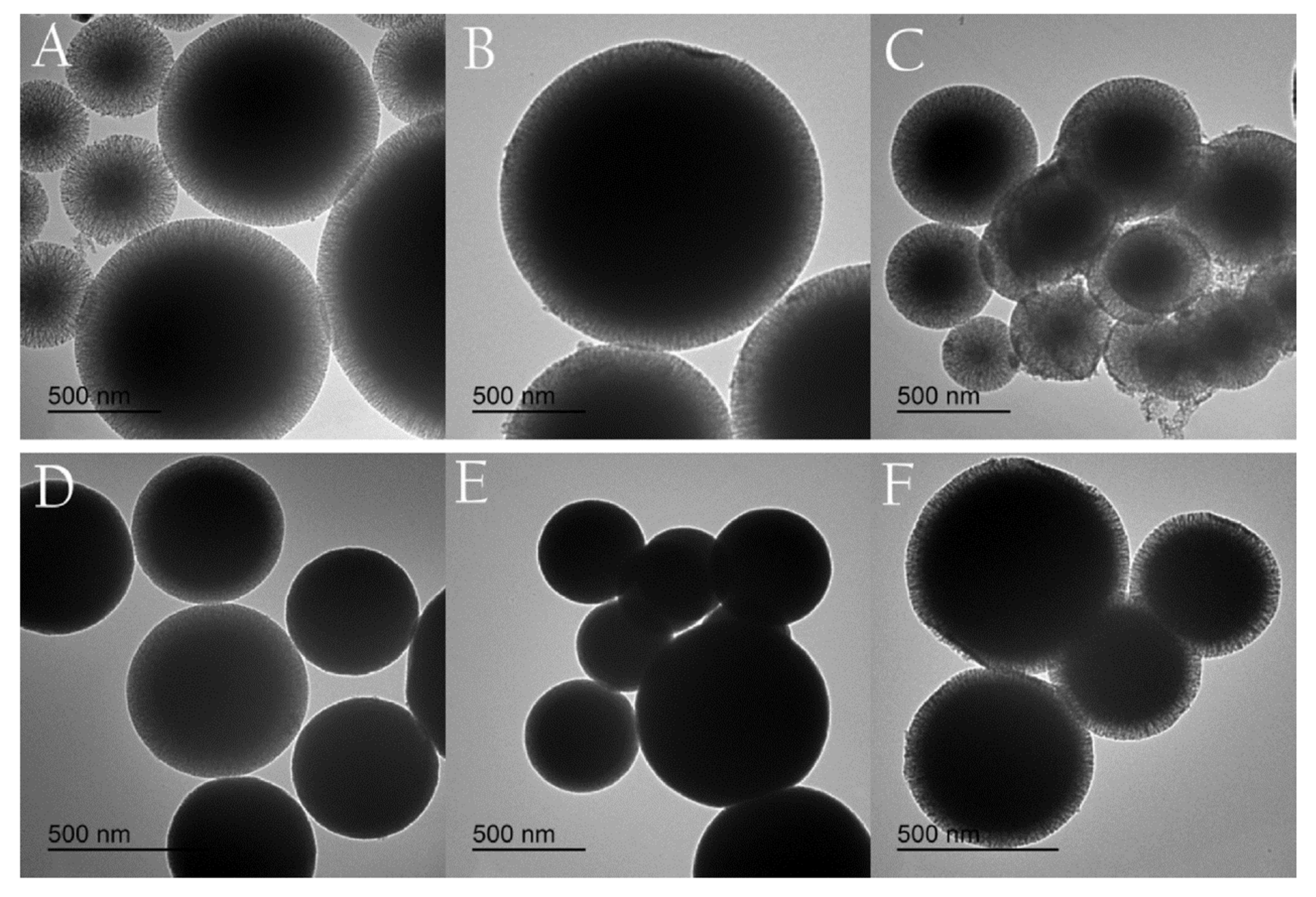
2.3.1. Hydrothermal
2.3.2. Sol-Gel
2.3.3. Hydrolysis
2.3.4. Encapsulation
3. Applications of Silica-Graphene-Based Hybrid Composites
3.1. Adsorbent
3.2. Energy Storage
3.3. Biomedical Fields
3.4. Catalysis
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Komarneni, S. Nanocornposites. J. Mater. Chem. 1992, 2, 1219–1230. [Google Scholar] [CrossRef]
- Thostenson, E.; Li, C.; Chou, T. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Bogue, R. Nanocomposites: A review of technology and applications. Assem. Autom. 2011, 31, 106–112. [Google Scholar] [CrossRef]
- Sonawane, G.H.; Patil, S.P.; Sonawane, S.H. Nanocomposites and Its Applications. In Applications of Nanomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nanosci. Technol A Collect. Rev. Nat. J. 2009, 11–19. [Google Scholar]
- Handayani, M.; Sulistiyono, E.; Rokhmanto, F.; Darsono, N.; Fransisca, P.L.; Erryani, A.; Wardono, J.T. Fabrication of Graphene Oxide/Calcium Carbonate/Chitosan Nanocomposite Film with Enhanced Mechanical Properties. IOP Conf. Series: Mater. Sci. Eng. 2019, 578, 12073. [Google Scholar] [CrossRef]
- Alvial-Palavicino, C.; Konrad, K. The rise of graphene expectations: Anticipatory practices in emergent nanotechnologies. Futures 2019, 109, 192–202. [Google Scholar] [CrossRef]
- Latiff, N.M.; Fu, X.; Mohamed, D.K.; Veksha, A.; Handayani, M.; Lisak, G. Carbon based copper(II) phthalocyanine catalysts for electrochemical CO2 reduction: Effect of carbon support on electrocatalytic activity. Carbon 2020, 168, 245–253. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Abdullah, I.; Prabawanta, I.B.G.; Handayani, M. In-situ hydrothermal synthesis of nickel nanoparticle/reduced graphene oxides as catalyst on CO2 methanation. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2242, p. 40046. [Google Scholar] [CrossRef]
- Abergel, D.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T. Properties of graphene: A theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar] [CrossRef] [Green Version]
- Tsang, A.C.H.; Huang, H.; Xuan, J.; Wang, H.; Leung, D. Graphene materials in green energy applications: Recent development and future perspective. Renew. Sustain. Energy Rev. 2020, 120, 109656. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene-based Materials: Graphene and Graphene Oxide: Synthesis, Properties, and Applications (Adv. Mater. 35/2010). Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef] [Green Version]
- Zainal, N.S.; Mohamad, Z.; Mustapa, M.S.; Badarulzaman, N.A.; Zulkifli, A.Z.; Bhd, S.A.N.S.S. The Ability of Crystalline and Amorphous Silica from Rice Husk Ash to Perform Quality Hardness for Ceramic Water Filtration Membrane. Int. J. Integr. Eng. 2019, 11, 229–235. [Google Scholar] [CrossRef]
- Mulyati, S.; Muchtar, S.; Yusuf, M.; Arahman, N.; Sofyana, S.; Rosnelly, C.M.; Fathanah, U.; Takagi, R.; Matsuyama, H.; Shamsuddin, N.; et al. Production of High Flux Poly(Ether Sulfone) Membrane Using Silica Additive Extracted from Natural Resource. Membranes 2020, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Husain, S.W. Synthesis and characterization of silica nanoparticles from clay. J. Asian Ceram. Soc. 2016, 4, 91–96. [Google Scholar] [CrossRef]
- Chen, J.-J.; Li, H.-J.; Zhou, X.-H.; Li, E.-Z.; Wang, Y.; Guo, Y.-L.; Feng, Z.-S. Efficient synthesis of hollow silica microspheres useful for porous silica ceramics. Ceram. Int. 2017, 43, 13907–13912. [Google Scholar] [CrossRef]
- Abbas, N.; Khalid, H.R.; Ban, G.; Kim, H.T.; Lee, H. Silica aerogel derived from rice husk: An aggregate replacer for lightweight and thermally insulating cement-based composites. Constr. Build. Mater. 2019, 195, 312–322. [Google Scholar] [CrossRef]
- Sdiri, A.; Higashi, T.; Bouaziz, S.; Benzina, M. Synthesis and characterization of silica gel from siliceous sands of southern Tunisia. Arab. J. Chem. 2014, 7, 486–493. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.; Saputri, L.N.M.Z.; Dwiatmoko, A.A.; Susanto, B.H.; Nasikin, M. A facile approach to synthesis of silica nanoparticles from silica sand and their application as superhydrophobic material. J. Asian Ceram. Soc. 2021, 9, 665–672. [Google Scholar] [CrossRef]
- Qian, H.; Li, W.; Wang, X.; Xie, F.; Li, W.; Qu, Q. Simultaneous growth of graphene/mesoporous silica composites using liquid precursor for HPLC separations. Appl. Surf. Sci. 2021, 537, 148101. [Google Scholar] [CrossRef]
- Ma, M.; Li, H.; Xiong, Y.; Dong, F. Rational design, synthesis, and application of silica/graphene-based nanocomposite: A review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Azat, S.; Sartova, Z.; Bekseitova, K.; Askaruly, K. Extraction of high-purity silica from rice husk via hydrochloric acid leaching treatment. Turk. J. Chem. 2019, 43, 1258–1269. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Y.; Li, Z.; Zhang, J.; Shen, T.; Chen, Y.; Yang, D.-Q.; Sacher, E.; Jifan, Z. Synthesis of amorphous SiO2 nanowires by one-step low temperature hydrothermal process. Mater. Res. Express 2019, 6, 115202. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Mercado-Silva, A.; García-Cerda, L.A.; Castruita, G.; Perera-Mercado, Y.A. Hydrothermal Synthesis of Mesoporous Silica MCM-41 Using Commercial Sodium Silicate. J. Mex. Chem. Soc. 2017, 57, 73–79. [Google Scholar] [CrossRef]
- Park, J.; Gu, Y.; Park, S.; Hwang, E.; Sang, B.-I.; Chun, J.; Lee, J. Two-Stage Continuous Process for the Extraction of Silica from Rice Husk Using Attrition Ball Milling and Alkaline Leaching Methods. Sustainability 2021, 13, 7350. [Google Scholar] [CrossRef]
- Sulistiyono, E.; Handayani, M.; Prasetyo, A.B.; Irawan, J.; Febriana, E.; Firdiyono, F.; Yustanti, E.; Sembiring, S.N.; Nugroho, F.; Muslih, E.Y. Implementation of sulfuric acid leaching for aluminum and iron removal for improvement of low-grade silica from quartz sand of Sukabumi, Indonesia. East. Eur. J. Enterp. Technol. 2021, 3, 32–40. [Google Scholar] [CrossRef]
- Febriana, E.; Manurung, U.A.B.; Prasetyo, A.B.; Handayani, M.; Muslih, E.Y.; Nugroho, F.; Sulistiyono, E.; Firdiyono, F. Dissolution of quartz sand in sodium hydroxide solution for producing amorphous precipitated silica. IOP Conf. Series: Mater. Sci. Eng. 2020, 858, 012047. [Google Scholar] [CrossRef]
- Gao, F.; Peng, Z.; Fu, X. One-Step Synthesis and Characterization of Silica Nano-/Submicron Spheres by Catalyst-Assisted Pyrolysis of a Preceramic Polymer. J. Nanomater. 2013, 2013, 5. [Google Scholar] [CrossRef]
- Cho, K.; Chang, H.; Kil, D.S.; Park, J.; Jang, H.D.; Sohn, H.Y. Mechanisms of the Formation of Silica Particles from Precursors with Different Volatilities by Flame Spray Pyrolysis. Aerosol Sci. Technol. 2009, 43, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Fraser, A.; Ye, S.; Merle, G.; Barralet, J.E. Top-down bottom-up graphene synthesis. Nano Futur. 2019, 3, 042003. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymers 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E.; et al. Experimental Review of Graphene. ISRN Condens. Matter Phys. 2012, 2012, 501686. [Google Scholar] [CrossRef] [Green Version]
- Eswaraiah, V.; Aravind, S.S.J.; Ramaprabhu, S. Top down method for synthesis of highly conducting graphene by exfoliation of graphite oxide using focused solar radiation. J. Mater. Chem. 2011, 21, 6800–6803. [Google Scholar] [CrossRef]
- Handayani, M.; Kepakisan, K.A.A.; Anshori, I.; Darsono, N.; Thaha, Y.N. Graphene oxide based nanocomposite modified screen printed carbon electrode for qualitative cefixime detection. Proc. Int. Semin. Metall. Mater. Accel. Res. Innov. Metall. Mater. Incl. Sustain. Ind. 2021, 2382, 40005. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; MacOsko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Febriana, E.; Handayani, M.; Susilo, D.N.A.; Yahya, M.S.; Ganta, M.; Sunnardianto, G.K. A simple approach of synthesis of graphene oxide from pure graphite: Time stirring duration variation. Proc. Int. Semin. Metall. Mater. Accel. Res. Innov. Metall. Mater. Incl. Sustain. Ind. 2021, 2382, 40006. [Google Scholar] [CrossRef]
- Handayani, M.; Ganta, M.; Susilo, D.N.A.; Yahya, M.S.; Sunnardianto, G.K.; Darsono, N.; Sulistiyono, E.; Setiawan, I.; Lestari, F.P.; Erryani, A. Synthesis of graphene oxide from used electrode graphite with controlled oxidation process. IOP Conf. Ser. Mater. Sci. Eng. 2019, 541, 12032. [Google Scholar] [CrossRef]
- Abbas, A.; Eng, X.E.; Ee, N.; Saleem, F.; Wu, D.; Chen, W.; Handayani, M.; Tabish, T.A.; Wai, N.; Lim, T.M. Development of reduced graphene oxide from biowaste as an electrode material for vanadium redox flow battery. J. Energy Storage 2021, 41, 102848. [Google Scholar] [CrossRef]
- Chen, H.; He, G.; Zhu, J.; Bei, F.; Sun, X.; Wang, X. Synthesis and characterization of graphene paper with controllable properties via chemical reduction. J. Mater. Chem. 2011, 21, 14631–14638. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Li, H.; Lu, J.; Fu, Q.; Chu, Y. Graphene nanosheets synthesis via chemical reduction of graphene oxide using sodium acetate trihydrate solution. Synth. Met. 2014, 193, 132–138. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Zhen, S.G.; Poh, C.K.; Chaurasia, A.; Luo, J.; Wu, X.; Yeow, E.K.L.; Sahoo, N.G.; Lin, J.; et al. A Green Approach to the Synthesis of High-Quality Graphene Oxide Flakes via Electrochemical Exfoliation of Pencil Core. RSC Adv. 2013, 207890, 8669–8679. [Google Scholar] [CrossRef]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Mullen, K. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhao, W.; Hu, H.; Chen, G. One-step in situ ball milling synthesis of polymer-functionalized graphene nanocomposites. J. Mater. Chem. 2011, 21, 8626–8632. [Google Scholar] [CrossRef]
- Mondal, O.; Mitra, S.; Pal, M.; Datta, A.; Dhara, S.; Chakravorty, D. Reduced graphene oxide synthesis by high energy ball milling. Mater. Chem. Phys. 2015, 161, 123–129. [Google Scholar] [CrossRef]
- Lin, C.; Yang, L.; Ouyang, L.; Liu, J.; Wang, H.; Zhu, M. A new method for few-layer graphene preparation via plasma-assisted ball milling. J. Alloys Compd. 2017, 728, 578–584. [Google Scholar] [CrossRef]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-Area Graphene Single Crystals Grown by Low-Pressure Chemical Vapor Deposition of Methane on Copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef]
- Li, X.; Magnuson, C.W.; Venugopal, A.; An, J.; Suk, J.W.; Han, B.; Borysiak, M.; Cai, W.; Velamakanni, A.; Zhu, Y.; et al. Graphene Films with Large Domain Size by a Two-Step Chemical Vapor Deposition Process. Nano Lett. 2010, 10, 4328–4334. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, Y. Controlled Chemical Synthesis in CVD Graphene. Phys. Sci. Rev. 2017, 2, 1–28. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Ma, Y.; Huang, Y.; Li, N.; Zhang, F.; Chen, Y. Efficient and large-scale synthesis of few-layered graphene using an arc-discharge method and conductivity studies of the resulting films. Nano Res. 2010, 3, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Song, Y.; Wright, J.; Heller, M.J. Graphene bi- and trilayers produced by a novel aqueous arc discharge process. Carbon 2016, 102, 339–345. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Gao, L.; Zhao, J.; Chen, Z.; Liu, B.; Tang, D.; Yu, B.; Jiang, C.; Cheng, H.-M. Synthesis of Graphene Sheets with High Electrical Conductivity and Good Thermal Stability by Hydrogen Arc Discharge Exfoliation. ACS Nano 2009, 3, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Xing, B.; Zeng, H.; Wang, X.; Zhang, C.; Cao, J.; Chen, L. One-Step Synthesis of Hierarchical Micro-Mesoporous SiO2/Reduced Graphene Oxide Nanocomposites for Adsorption of Aqueous Cr(VI). J. Nanomater. 2017, 2017, 6286549. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.; Oh, J.-S.; Lee, E.-J.; Han, C.-M. Synthesis of uniformly dispersed silica/graphene oxide composite hydrogel using acid/base combinatorial catalysts system. Mater. Today Commun. 2021, 26, 101841. [Google Scholar] [CrossRef]
- Haeri, S.; Asghari, M.; Ramezanzadeh, B. Enhancement of the mechanical properties of an epoxy composite through inclusion of graphene oxide nanosheets functionalized with silica nanoparticles through one and two steps sol-gel routes. Prog. Org. Coatings 2017, 111, 1–12. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.; Li, B.; Zhang, W.; Zheng, H.; Wu, G.; Huang, Y.; Song, G. One-step generation of silica particles onto graphene oxide sheets for superior mechanical properties of epoxy composite and scale application. Compos. Commun. 2020, 22, 100514. [Google Scholar] [CrossRef]
- Zhang, W.L.; Choi, H.J. Silica-Graphene Oxide Hybrid Composite Particles and Their Electroresponsive Characteristics. Langmuir 2012, 28, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chai, S.; Liu, K.; Ning, N.; Gao, J.; Liu, Q.; Chen, F.; Fu, Q. Enhanced Epoxy/Silica Composites Mechanical Properties by Introducing Graphene Oxide to the Interface. ACS Appl. Mater. Interfaces 2012, 4, 4398–4404. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, L.; Li, C. Facile fabrication of silica–polymer–graphene collaborative nanostructure-based hybrid materials with high conductivity and robust mechanical performance. RSC Adv. 2015, 5, 25450–25456. [Google Scholar] [CrossRef]
- Liu, P.; Liang, Q.; Luo, H.; Fang, W.; Geng, J. Synthesis of nano-scale zero-valent iron-reduced graphene oxide-silica nano-composites for the efficient removal of arsenic from aqueous solutions. Environ. Sci. Pollut. Res. 2019, 26, 33507–33516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, Y.; Lu, M.; Lu, X.; Du, X. Highly efficient adsorption of heavy metals from wastewaters by graphene oxide-ordered mesoporous silica materials. J. Mater. Sci. 2015, 50, 2113–2121. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Lu, M.; Teng, R.; Du, X. Facile synthesis of phenyl-modified magnetic graphene/mesoporous silica with hierarchical bridge-pore structure for efficient adsorption of pesticides. Mater. Chem. Phys. 2017, 198, 393–400. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Z.; Wang, D.; Yu, J.; Jiang, X.; Chen, X. Magnetic porous silica–graphene oxide hybrid composite as a potential adsorbent for aqueous removal of p -nitrophenol. Colloids Surfaces A: Physicochem. Eng. Asp. 2016, 490, 207–214. [Google Scholar] [CrossRef]
- Wang, W.; Motuzas, J.; Zhao, X.S.; da Costa, J.C.D. 2D/3D amine functionalised sorbents containing graphene silica aerogel and mesoporous silica with improved CO2 sorption. Sep. Purif. Technol. 2019, 222, 381–389. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, T.; Zhou, X.; Liu, X.; Li, X.; Zhou, K.; Dong, L.; Wei, D. Three-dimensional graphene composite containing graphene-SiO2 nanoballs and its potential application in stress sensors. Nanomaterials 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.L.; Zhou, R.; Zhao, X.S. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010, 20, 5983–5992. [Google Scholar] [CrossRef]
- Ghosh, A.; Miah, M.; Majumder, C.; Bag, S.; Chakravorty, D.; Saha, S.K. Synthesis of multilayered structure of nano-dimensional silica glass/reduced graphene oxide for advanced electrochemical applications. Nanoscale 2018, 10, 5539–5549. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.K. Ultraviolet-enhanced photodetection in a graphene/SiO2/Si capacitor structure with a vacuum channel. J. Appl. Phys. 2015, 118, 104504. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; Xiang, Y.; Zhang, Q. Enhanced electrochemical capacitance and oil-absorbability of N-doped graphene aerogel by using amino-functionalized silica as template and doping agent. J. Power Sources 2018, 379, 240–248. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Li, H.; Lu, C. Preparation of three-dimensional graphene networks for use as anode of lithium ion batteries. Funct. Mater. Lett. 2013, 6, 2–5. [Google Scholar] [CrossRef]
- Yin, L.-H.; Wu, M.; Li, Y.-P.; Wu, G.-L.; Wang, Y.-K.; Wang, Y. Synthesis of SiO2 @carbon-graphene hybrids as anode materials of lithium-ion batteries. New Carbon Mater. 2017, 32, 311–318. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, Z.-Q.; Jia, R.; Yang, Y.; Zhu, F.-Y.; Yang, Z.-L.; Huang, Y.; Li, X.; Xu, W. Facile electrostatic self-assembly of silicon/reduced graphene oxide porous composite by silica assist as high performance anode for Li-ion battery. Appl. Surf. Sci. 2018, 456, 379–389. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Ryu, J.H.; Yoon, S. One pot synthesis of ordered mesoporous carbon–silica–titania with parallel alignment against graphene as advanced anode material in lithium ion batteries. J. Ind. Eng. Chem. 2019, 71, 93–98. [Google Scholar] [CrossRef]
- Kim, K.H.; Jun, Y.-S.; Gerbec, J.A.; See, K.A.; Stucky, G.D.; Jung, H.-T. Sulfur infiltrated mesoporous graphene–silica composite as a polysulfide retaining cathode material for lithium–sulfur batteries. Carbon 2014, 69, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zheng, L.; Hu, W.; Zheng, H. Synergistic effect of silane and graphene oxide for enhancing the photoelectrochemical water oxidation performance of WO3NS arrays. Electrochim. Acta 2018, 292, 322–330. [Google Scholar] [CrossRef]
- He, D.; Li, X.; He, X.; Wang, K.; Tang, J.; Yang, X.; He, X.; Yang, X.; Zou, Z. Noncovalent assembly of reduced graphene oxide and alkyl-grafted mesoporous silica: An effective drug carrier for near-infrared light-responsive controlled drug release. J. Mater. Chem. B 2015, 3, 5588–5594. [Google Scholar] [CrossRef]
- Gui, W.; Zhang, J.; Chen, X.; Yu, D.; Ma, Q. N-Doped graphene quantum dot@mesoporous silica nanoparticles modified with hyaluronic acid for fluorescent imaging of tumor cells and drug delivery. Microchim. Acta 2018, 185, 66. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Y.; Li, C.; Yang, X.-Q.; An, J.; Cheng, K.; Xuan, Y.; Shi, X.-M.; Gao, M.-J.; Song, X.-L.; Zhao, Y.-D.; et al. Graphene oxide coating core–shell silver sulfide@mesoporous silica for active targeted dual-mode imaging and chemo-photothermal synergistic therapy against tumors. J. Mater. Chem. B 2018, 6, 4808–4820. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhang, R.; Lu, J.; Zhao, C.; Deng, X.; Wu, Y. Mesoporous Silica Coated Polydopamine Functionalized Reduced Graphene Oxide for Synergistic Targeted Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, B.; Dong, S.; Tang, Y.; Hou, L.; Chen, Z.; Li, Z.; Yang, W.; Xu, C.; Wang, M.; et al. Silica nanosphere supported palladium nanoparticles encapsulated with graphene: High-performance electrocatalysts for methanol oxidation reaction. Appl. Surf. Sci. 2018, 452, 11–18. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Woo, J.-H.; Cho, K.Y.; Jung, C.-H.; Oh, W.-C. Highly efficient visible light driven photocatalytic activities of the LaCuS2-graphene composite-decorated ordered mesoporous silica. Sep. Purif. Technol. 2018, 205, 11–21. [Google Scholar] [CrossRef]
- Oh, W.-C.; Nguyen, D.C.T.; Areerob, Y. Novel cadmium oxide-graphene nanocomposite grown on mesoporous silica for simultaneous photocatalytic H2-evolution. Chemosphere 2020, 239, 124825. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Bian, T.; Zhang, B.; Zhang, D.; Wu, L.-Z.; Tung, C.-H.; Yin, Y.; Zhang, T. Graphene-Supported Ultrafine Metal Nanoparticles Encapsulated by Mesoporous Silica: Robust Catalysts for Oxidation and Reduction Reactions. Angew Chem. 2014, 126, 254–258. [Google Scholar] [CrossRef]
- Sarkar, C.; Pendem, S.; Shrotri, A.; Dao, D.Q.; Mai, P.P.T.; Ngoc, T.N.; Chandaka, D.R.; Rao, T.V.; Trinh, Q.T.; Sherburne, M.P.; et al. Interface Engineering of Graphene-Supported Cu Nanoparticles Encapsulated by Mesoporous Silica for Size-Dependent Catalytic Oxidative Coupling of Aromatic Amines. ACS Appl. Mater. Interfaces 2019, 11, 11722–11735. [Google Scholar] [CrossRef]

| Paper | Source of Silica | Method | Purity (%) | Particle Size (nm) | Product | Surface Area (m2/g) |
|---|---|---|---|---|---|---|
| Abbas, et al. (2019) [20] | Rice husk | Sol-gel | - | - | Mesoporous | 760 |
| Ismail, et al. (2021) [22] | Silica sand | Sol-gel | - | 170.3 ± 14.3 | Nanoparticle | - |
| Sdiri, et al. (2014) [21] | Siliceous sand | Sol-gel | 88.8–97.5 | - | Micro and mesoporous | 340 |
| Zhu Y, et al. (2019) [26] | Silicate glass | Hydrothermal | - | 20–100 | Amorphous nanowires | - |
| Ortiz, et al. (2013) [27] | Sodium silicate | Hydrothermal | - | 200–500 | Mesoporous | 860–1028 |
| Azat, et al. (2019) [25] | Rice husk | Leaching | 98.2–99.7 | - | Amorphous | 120–980 |
| Park, et al. (2021) [28] | Rice husk | Leaching | 98,5 | - | Amorphous silica | 1973 |
| Zainal, et al. (2019) [15] | Rice husk | Leaching | 98 | - | Amorphous and crystalline | - |
| Gao, et al. (2013) [31] | Polylazane preceramic powder | Pyrolysis | - | 600–800 | Nano-/submicron spheres | - |
| Cho, et al. (2009) [32] | TEOS and silicate acid | Pyrolysis | - | 9.6–33.5 | Particle | 81,4 |
| No | Composites | Processing Method | Strength | Weakness | Ref. |
|---|---|---|---|---|---|
| 1 | Graphene/mesoporous silica (G/SiO2) | Hydrothermal | Graphene does not need to be prepared in advance, graphene and silica layer overlapped to form intercalation, uniformly distribution, organic solvents can be used as carbon sources, no toxic gas is generated during the reaction. It does not require the use of a catalyst | Graphene can only be made by adding TEOS as a precursor, if it is reacted in big open space then the rate of chemical reactions is too slow to produce graphene. | [23] |
| 2 | SiO2/RGO | Hydrothermal | Efficient method, easy-to-synthesize process, low cost, composites stability | Control sheet restacking and aggregation of SiO2 nanoparticles is required | [58] |
| 3 | Silica/graphene oxide hydrogel | Sol-gel | Mechanical properties of the composite hydrogel such as stiffness can be adjusted by adjusting the GO contents | Increasing the addition of GO can weaken and decrease the mechanical properties of the hydrogel | [59] |
| 4 | Silica-functionalized graphene oxide (GO) nanosheets (GONs) | Sol-gel | Using two different route methods which produce various results | - | [60] |
| 5 | Silica/graphene oxide sheets epoxy composites | Hydrolysis | Catalysts (DETA and NH4OH) improving mechanical properties of composites by functionalization GO and forming SiO2 from a promotion of the hydrolysis of TEOS on the GO surface | The mechanical properties and distribution of the resulting particles are highly dependent on the use of a catalyst | [61] |
| 6 | Silica-Graphene Oxide | Hydrolysis | Relatively simple, inexpensive, and fast method | - | [62] |
| 7 | Epoxy/silica composites by introducing graphene oxide | Encapsulation | Interfacial structures and properties can control by using GO as a novel coupling agent | - | [63] |
| 8 | SiO2@poly(methylmethacrylate)–reduced graphene oxide (SiO2@PMMA–rGO) | Encapsulation | Covalent molecular binding and strongly electrical interaction produce outstanding thermal stability, hardness, and electrical conductivity | The morphology of the composites are strongly influenced by the synthesis conditions | [64] |
| No | Applications of Graphene/Silica Composites | Method | Advantages | Challenge | Ref. |
|---|---|---|---|---|---|
| 1 | Adsorbent for As(III) and As(V) from aqueous solution | electrostatic attraction and complexation | Can be composited with other materials to increase absorption efficiency | Dependent on pH of the solution, unable to reach WHO drinking water standard | [65] |
| 2 | Adsorbent for heavy metal As, Cd, Cr, Hg, and Pb | Sol-gel | Low-cost, environmental friendly synthesis method, highly efficient adsorption | Complicated manufacturing process | [66] |
| 3 | Adsorbent for pesticides | One-step solvothermal and one-step method | Low cost and efficient adsorbents | low concentrations pesticides in complex wastewater. | [67] |
| 4 | Adsorbent for p-nitrophenol | Grafting and core-shell | High adsorption capacity, composites could be easily separated from solutions through an external magnetic force | The introduction of SiO2 and GO will reduce the magnetization so that an external magnetic field is needed, the rate of diffusion slows down in the first stage | [68] |
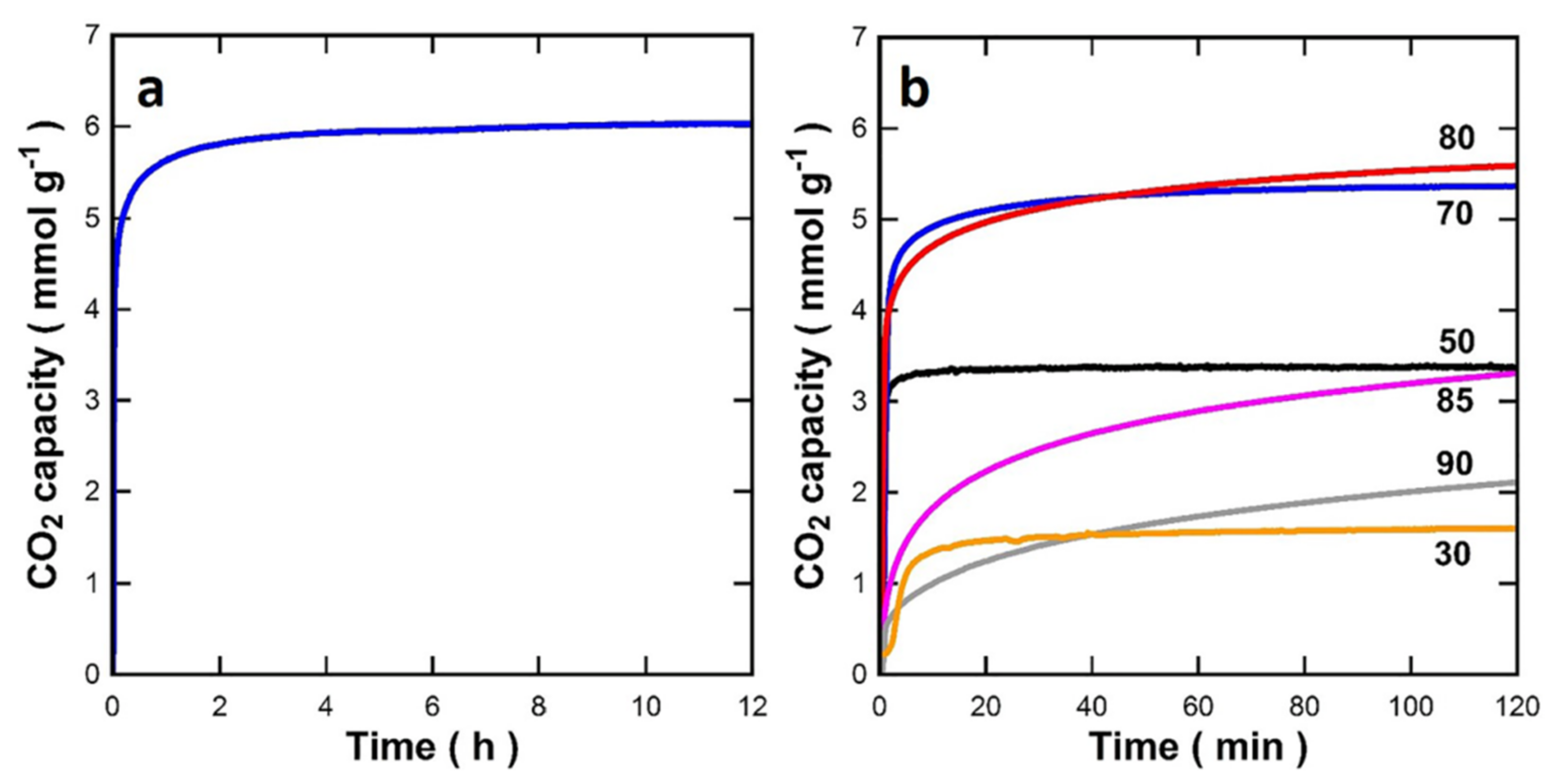
| 5 | Adsorbent for CO2 capture | Freeze-drying method | High CO2 sorption capacity, very stable under sorption | Morphological feature of the 2D/3D sorbent assembly is attributed to decreasing surface area and pore volume, very slow sorption kinetics | [69] |
| 6 | Energy storage: electrode material in supercapacitors | Sol-gel | specific capacitance of the composite is considerably higher than that of graphene and has good cyclic stability as electrode material for supercapacitor | The measurement of temperature dependent resistance for the composite in the temperature range from 5 K–300 K was performed under cycle cryostat and high vacuum condition | [72] |
| 7 | Energy storage: supercapacitor electrode | Hydrothermal method | Ultrahigh specific surface area, high capacitance and long lifetime | Need high temperature annealing process | [74] |
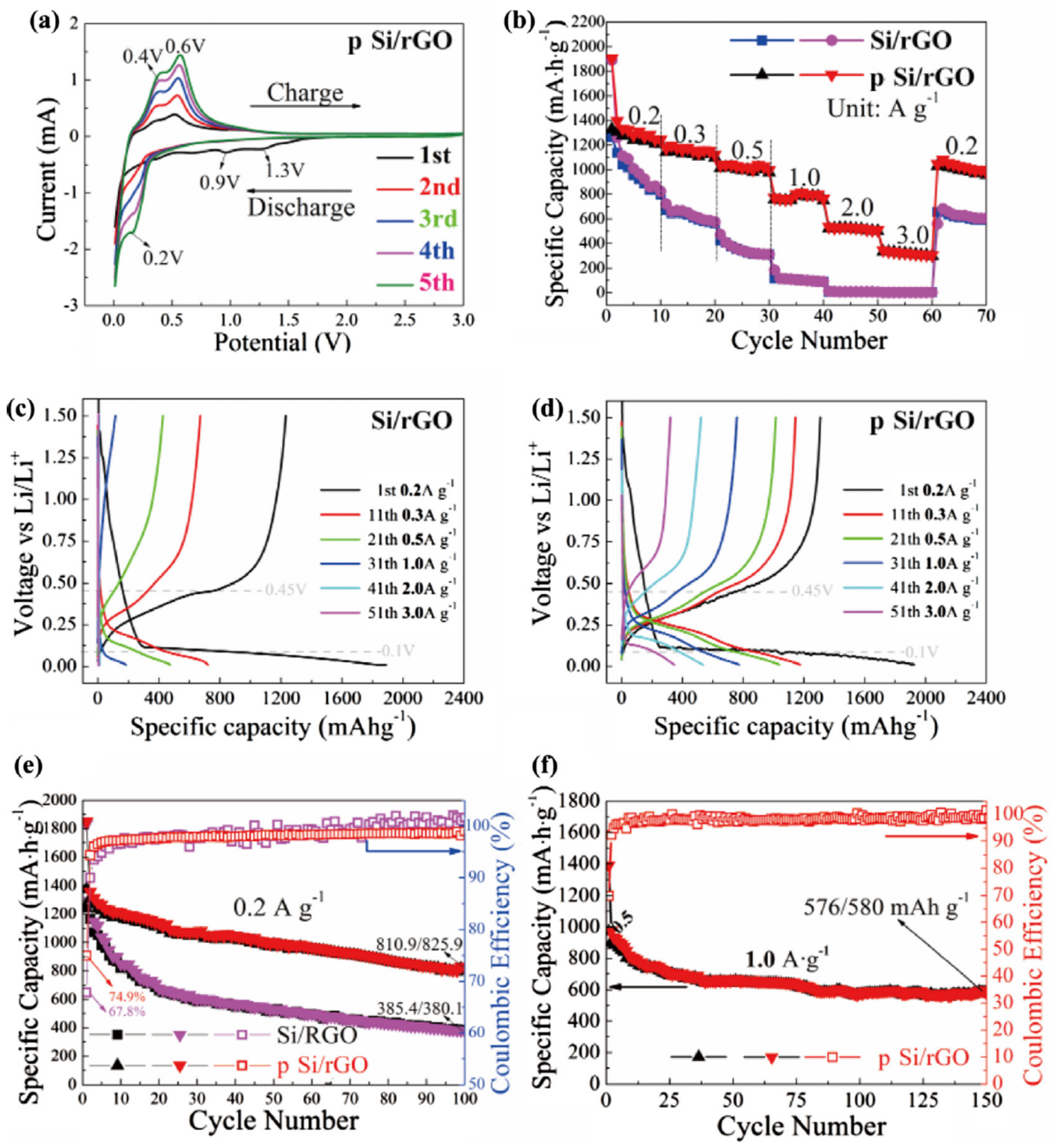
| 8 | Energy storage: as anode materials of lithium lithium-ion batteries | Hydrothermal method and heat treatments | enhance the electrical conductivity, and improve the electrochemical performance. | [77] | |
| 9 | Energy storage: Lithium battery electrode | electrostatic self-assembly method | Enhance the electronic conductivity, provide more transfer channels for Li+, excellent electrochemical performance | The pH value of process needs to be adjusted to help electrostatic self-assembly method | [78] |
| 10 | Biomedical field: drug carrier for near infraredlight-responsive controlled drug release | Capped noncovalent binding | Biocompatible, biofriendly, efficient killing efficacy towards cancer cells | NIR light is needed to control the drug release from mesopores to nucleus | [82] |
| 11 | Biomedical field: fluorescent imaging of tumor cells and drug delivery | Coating | enables simultaneous drug release, fluorescent monitoring | Metal ion can quench the intensity of the N-GQDs (N-Doped graphene quantum dot) | [83] |
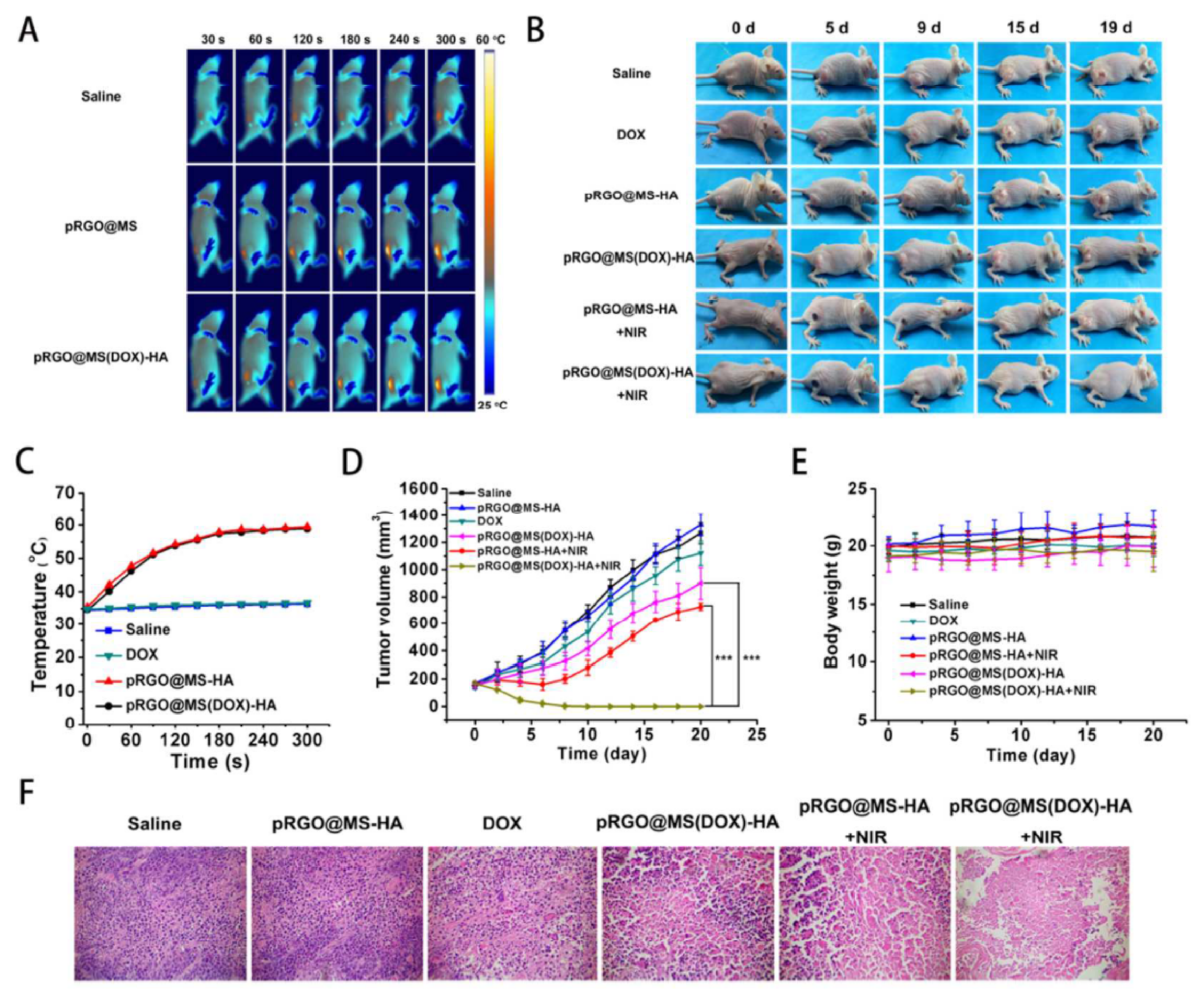
| 12 | Biomedical field: imaging and Chemo- Photothermal Synergistic Therapy Against Tumor | Coating Core-Shell | Biocompatibility, provide a basis for the early diagnosis and treatment of tumor | Laser radiation are needed to produce a more effective tumor killed | [84] |
| 13 | Biomedical field: Chemo- Photothermal Therapy | Coating | Good biocompatibility, dispersibility, excellent photothermalproperty, remarkable tumor cell killing efficiency, specificity to target tumor cells | Fluoroscopy results differ in certain body parts due to organ efficiency | [85] |
| 14 | Catalyst: electrocatalysts for methanol oxidation reaction | Hydrothermal method | Improve the electrocatalytic performance, long-time endurance and superior durability. | [86] | |
| 15 | Catalyst: photocatalytic of organic dyes, gallic acid | Hydrothermal method | Enhanced photocatalytic activity for organic dyes and gallic acid, improved the hydrogen evolution process | [88] | |
| 16 | Catalyst: for Oxidation and Reduction Reactions | Deposition–precipitation method. | High catalytic activity and excellent high-temperature stability | Nanosize catalyst can agglomerate and sinter very easily during high temperature calcination | [89] |
| No | Properties of Graphene | Properties of Silica | Properties of Graphene/Silica Composites | Potential Applications | Ref. |
|---|---|---|---|---|---|
| 1 | Graphene and its derivatives exhibit high specific surface area, however, graphene oxide will be easily agglomerated in the aqueous solution and re-stack between layers | SiO2 is a non-toxic and chemically stable material which not only easily overcomes the aggregation problem of GO but also improves the specific surface area and adsorption properties | The combination of graphene and silica nanoparticles enhance the specific surface area, prevent restacking of graphene sheet and produce an excellent adsorption capacity | Environment and adsorption material | [65,66,67,68] |
| 2 | Graphene-based materials have excellent chemical and physical stability and high electrical conductivity, however, graphene sheets are easy to restack | SiO2 particles could be inserted into the space between graphene sheets to produce a rigid support for flexible graphene sheets to prevent the π-π stacking of graphene sheets | Ultrahigh specific surface area, hierarchical porous structure, high capacitance and long lifetime | Energy storage | [74,77] |
| 3 | Graphene, especially graphene oxide (GO), has good water solubility, low toxicity, good biocompatibility, and easy surface modification | Silica has a high surface area, good biocompatibility, encapsulation capability in hydrophilic and hydrophobic molecules, tunable morphology, and scalable synthetic availability | The combination of graphene and silica nanoparticles exhibit excellent synergistic properties include high surface area, excellent biocompatibility, tunable morphology and low toxicity as biomedical composite materials | Biomedical application includes drug delivery system, imaging and therapy | [84,85] |
| 4 | Graphene shows strong catalytic activity in photocatalysis and electrocatalysis, owing to its large surface area, has excellent conductivity for electron capture and transport | Silica has large surface area, regular pore size, thermal and chemical stability, and variable chemical functional groups. Silica can prevent the agglomeration of graphene, and enhance the electrocatalytic performance of graphene | The combination of graphene and silica nanoparticles integrates the advantages of the two components and shows remarkable application prospects in improving the catalytic performance | Catalysis | [86,88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handayani, M.; Nafi’ah, N.; Nugroho, A.; Rasyida, A.; Prasetyo, A.B.; Febriana, E.; Sulistiyono, E.; Firdiyono, F. The Development of Graphene/Silica Hybrid Composites: A Review for Their Applications and Challenges. Crystals 2021, 11, 1337. https://doi.org/10.3390/cryst11111337
Handayani M, Nafi’ah N, Nugroho A, Rasyida A, Prasetyo AB, Febriana E, Sulistiyono E, Firdiyono F. The Development of Graphene/Silica Hybrid Composites: A Review for Their Applications and Challenges. Crystals. 2021; 11(11):1337. https://doi.org/10.3390/cryst11111337
Chicago/Turabian StyleHandayani, Murni, Nurin Nafi’ah, Adityo Nugroho, Amaliya Rasyida, Agus Budi Prasetyo, Eni Febriana, Eko Sulistiyono, and Florentinus Firdiyono. 2021. "The Development of Graphene/Silica Hybrid Composites: A Review for Their Applications and Challenges" Crystals 11, no. 11: 1337. https://doi.org/10.3390/cryst11111337
APA StyleHandayani, M., Nafi’ah, N., Nugroho, A., Rasyida, A., Prasetyo, A. B., Febriana, E., Sulistiyono, E., & Firdiyono, F. (2021). The Development of Graphene/Silica Hybrid Composites: A Review for Their Applications and Challenges. Crystals, 11(11), 1337. https://doi.org/10.3390/cryst11111337






