Upconversion Visible Light Emission in Yb/Pr Co-Doped Yttria-Stabilized Zirconia (YSZ) Single Crystals
Abstract
:1. Introduction
2. Materials and Methods
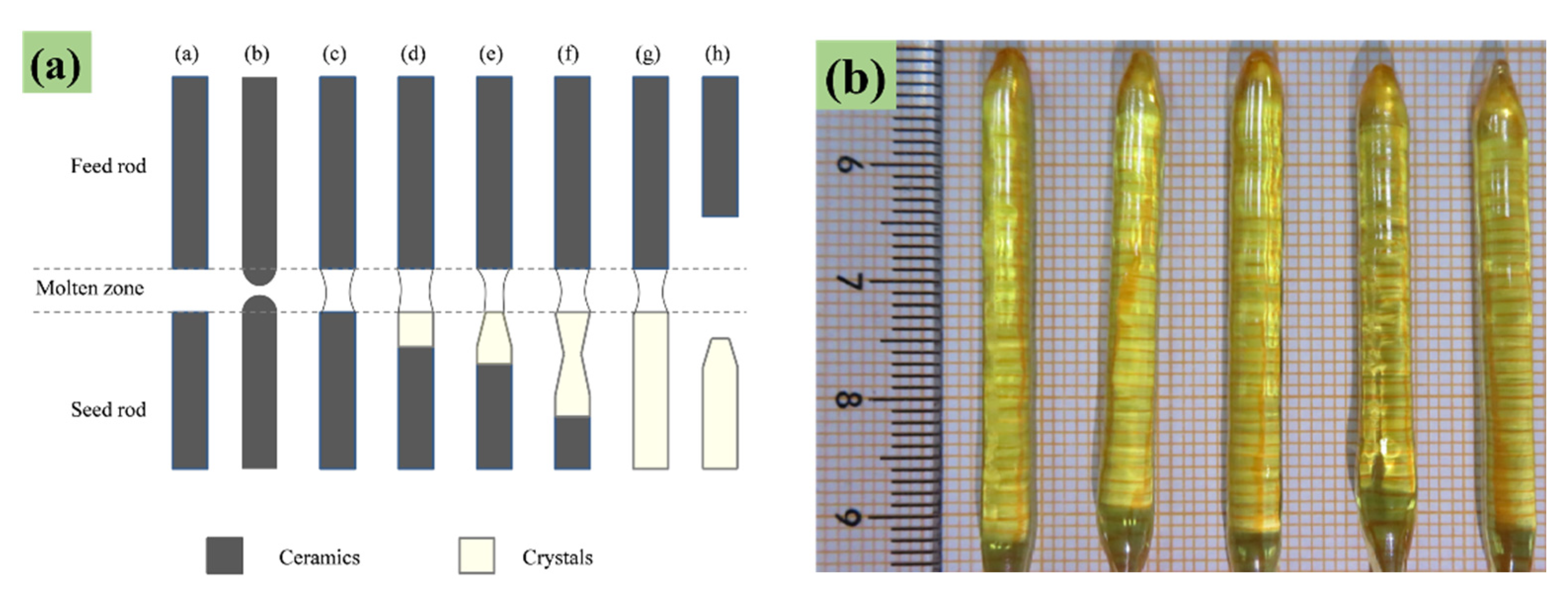
2.1. Sample Preparation
2.2. Sample Characterization
3. Results and Discussion
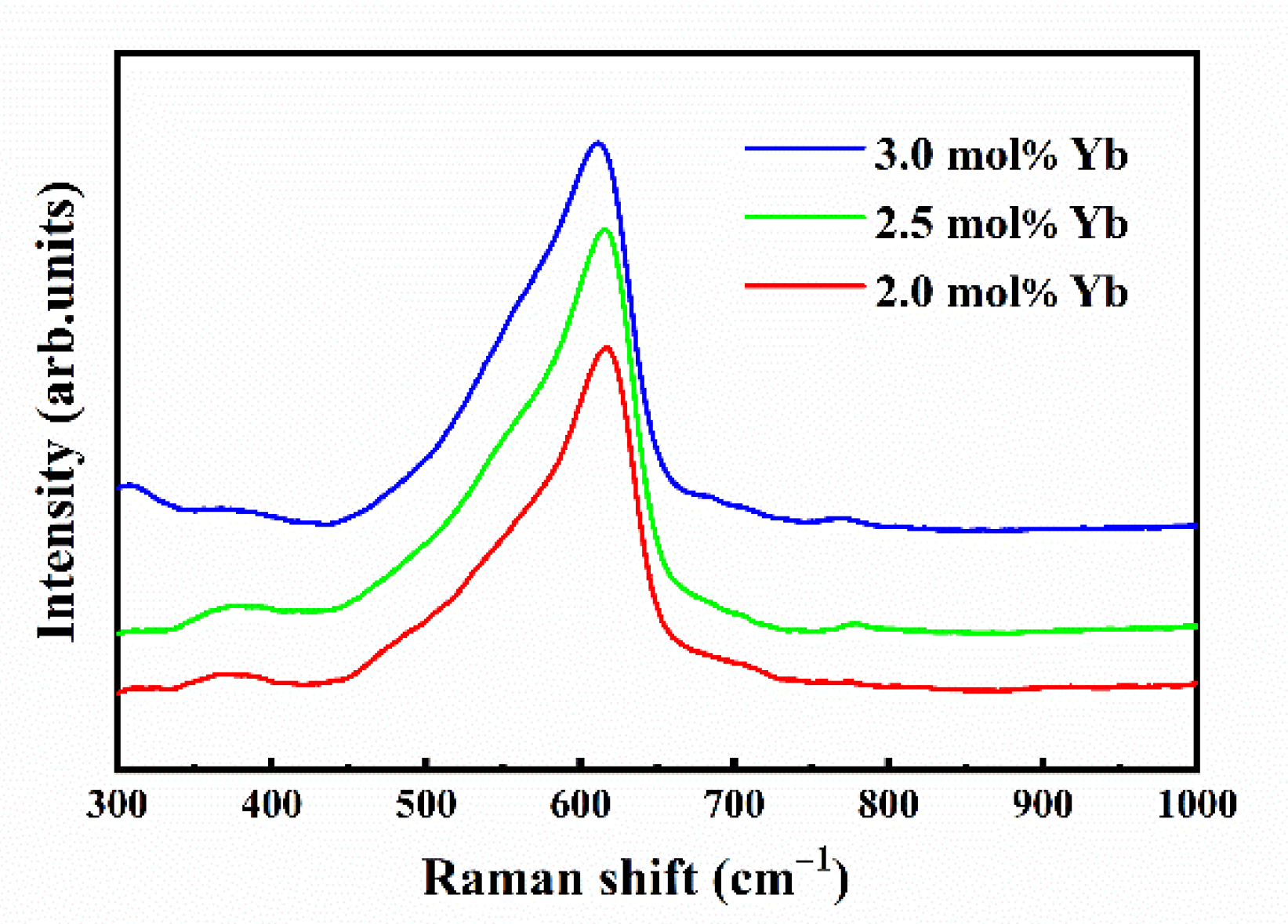
3.1. Raman Spectroscopic Analysis
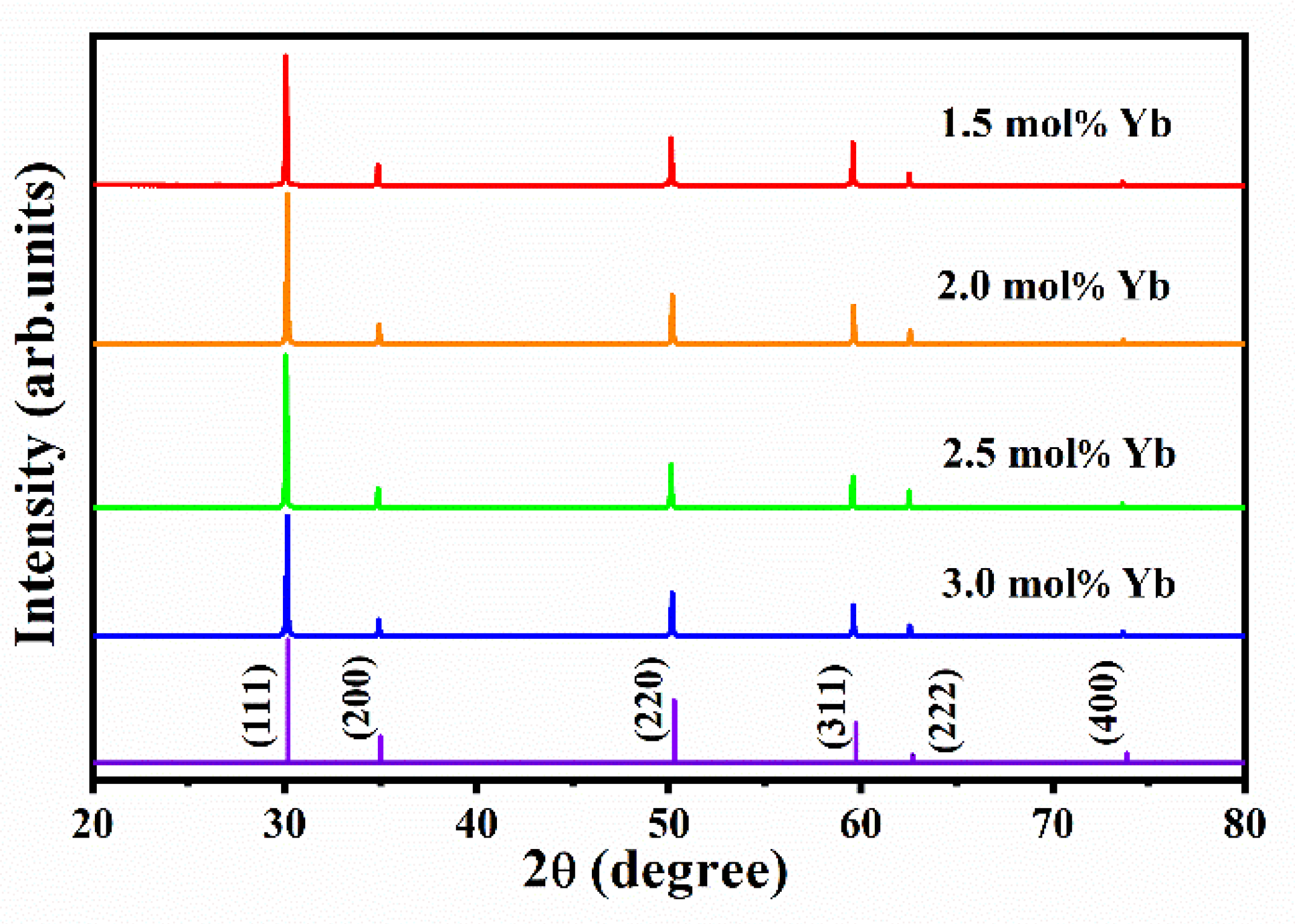
3.2. X-ray Diffraction (XRD)
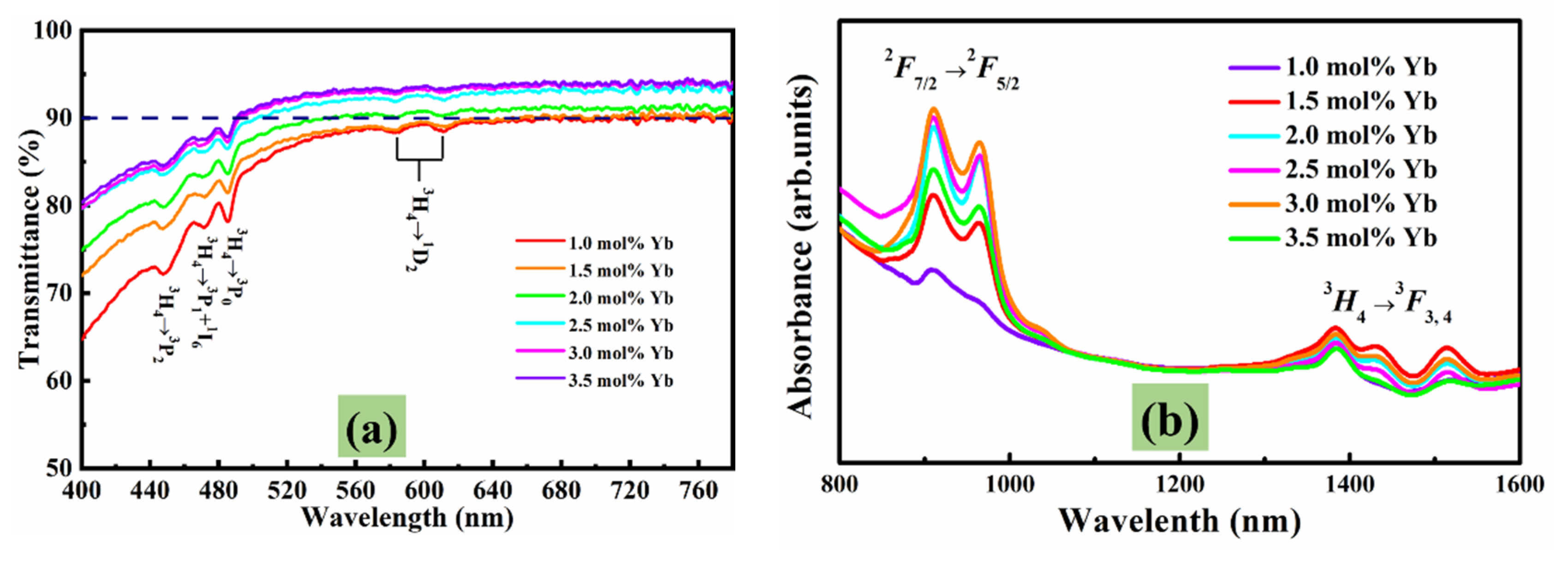
3.3. Transmission Spectra and Absorption Spectra
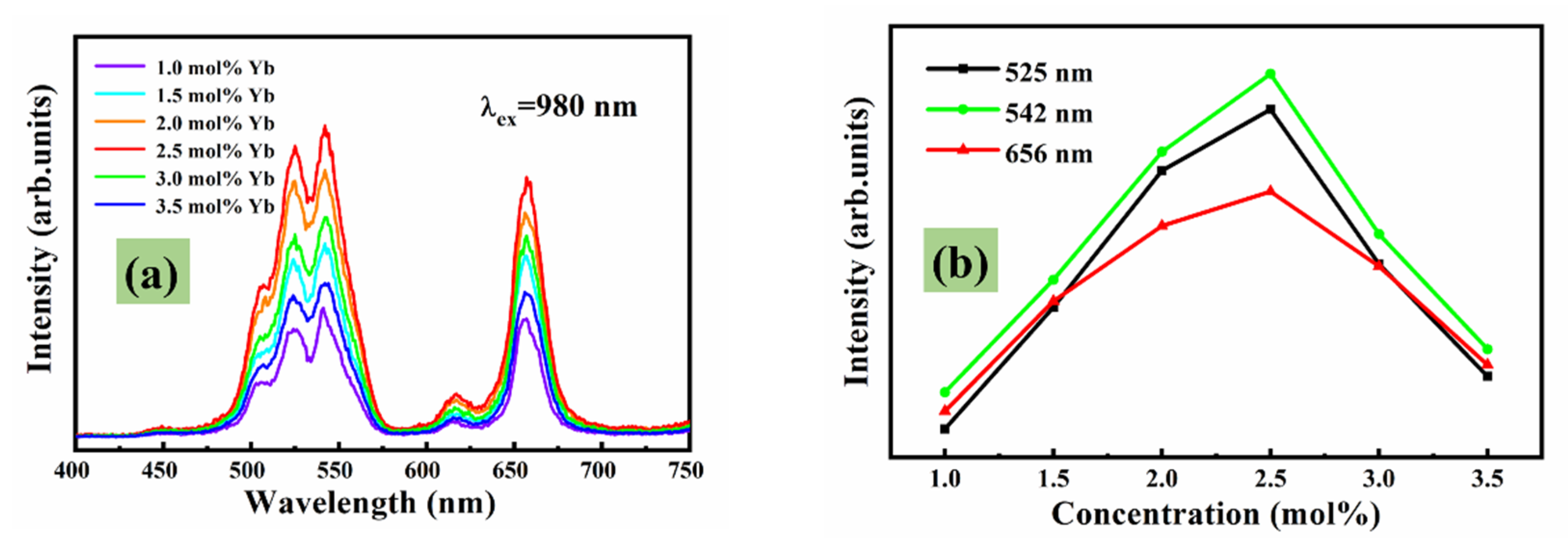
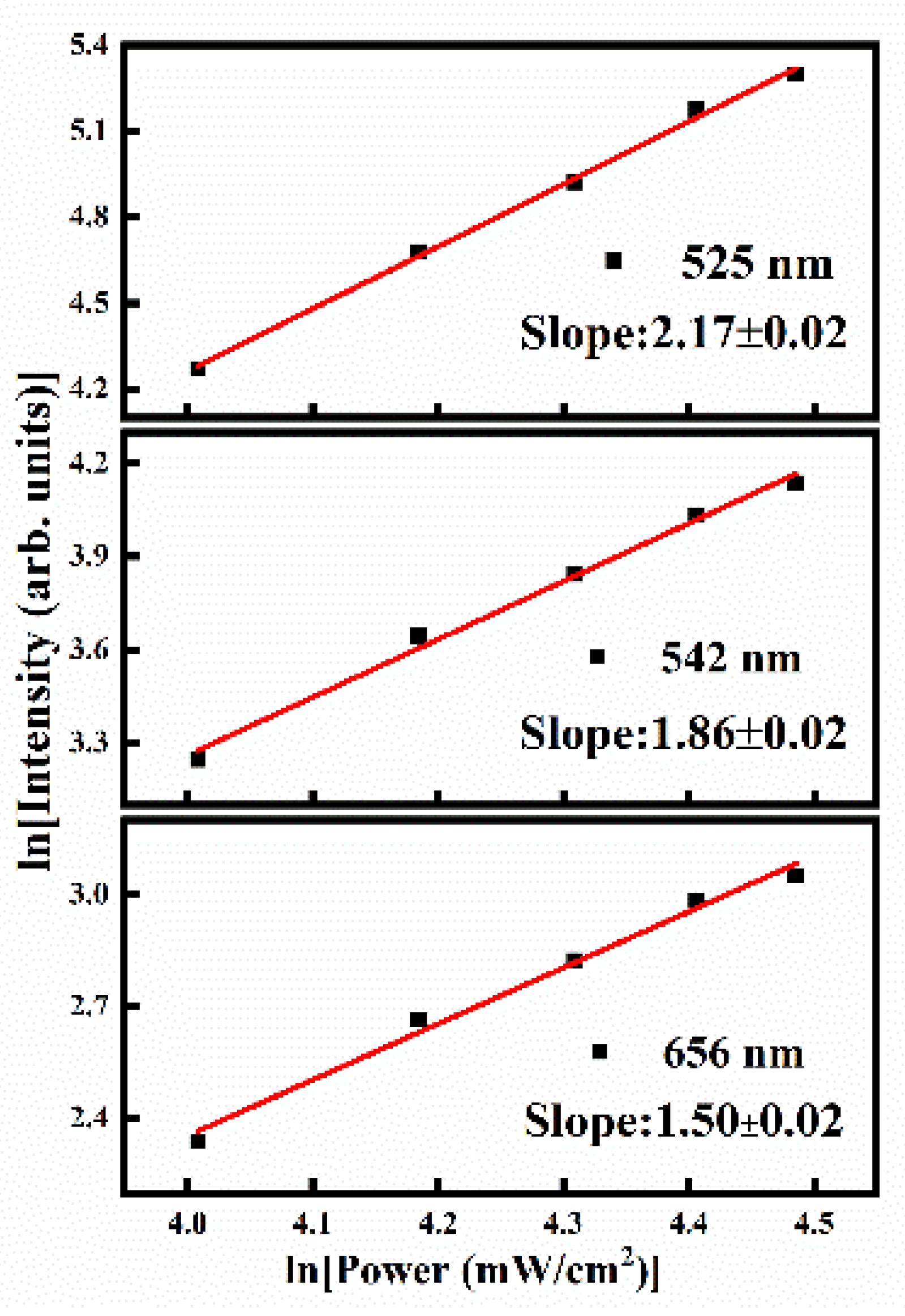
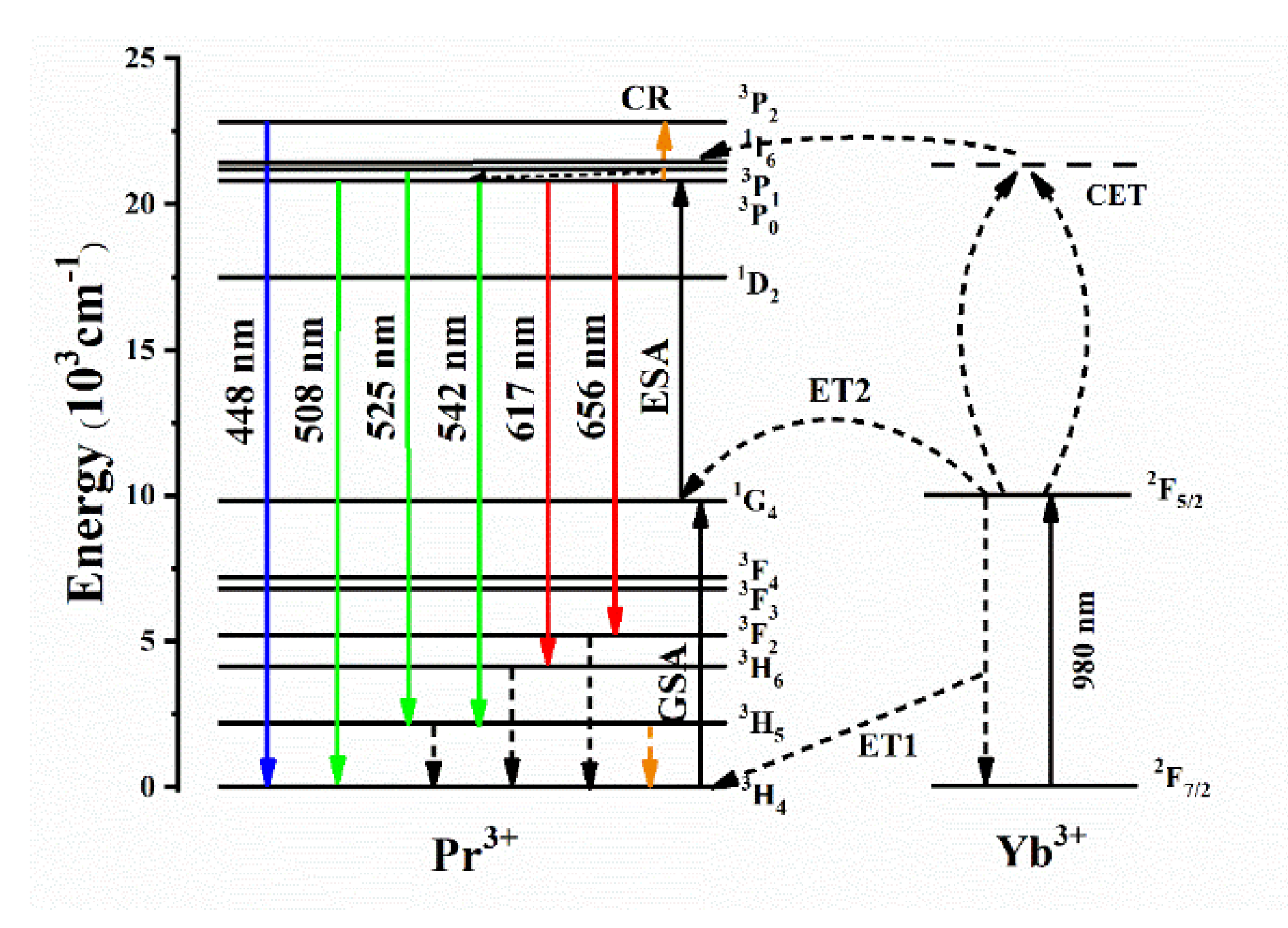
3.4. Luminescence Properties
3.5. Chromaticity Coordinates
4. General Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Fu, X.; Niu, S.; Xin, Q. Blue emission of ZrO2: Tm nanocrystals with different crystal structure under UV excitation. J. Non-Cryst. Solids 2008, 354, 1559–1563. [Google Scholar] [CrossRef]
- Romero, V.H.; De la Rosa, E.; López-Luke, T.; Salas, P.; Angeles-Chavez, C. Brilliant blue, green and orange-red emission band on Tm3+-, Tb3+- and Eu3+-doped ZrO2 nanocrystals. J. Phys. D 2010, 43, 465105. [Google Scholar] [CrossRef]
- Barabás, R.; Fort, C.I.; Turdean, G.L.; Bizo, L. Influence of HAP on the Morpho-Structural Properties and Corrosion Resistance of ZrO2-Based Composites for Biomedical Applications. Crystals 2021, 11, 202. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Rafique, M.; Usman, M.; Ain, Q.u.; Ghaffar, A. Evaluation of densification effects on the properties of 8 mol % yttria stabilized zirconia electrolyte synthesized by cost effective coprecipitation route. Ceram. Int. 2021, 47, 2857–2863. [Google Scholar] [CrossRef]
- Borik, M.A.; Bublik, V.T.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Osiko, V.V.; Seryakov, S.V.; Tabachkova, N.Y. Change in the phase composition, structure and mechanical properties of directed melt crystallised partially stabilised zirconia crystals depending on the concentration of Y2O3. J. Eur. Ceram. Soc. 2015, 35, 1889–1894. [Google Scholar] [CrossRef]
- Feinberg, A.; Perry, C.H. Structural disorder and phase transitions in ZrO2-Y2O3 system. J. Phy. Chem. Solids 1981, 42, 513–518. [Google Scholar] [CrossRef]
- Ishigame, M.; Yoshida, E. Study of the defect-induced Raman spectra in cubic zirconia. Solid State Ionics 1987, 23, 211–218. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Xu, B.; Cao, L. Concentration-dependent up-converted luminescence of Ho3+-Yb3+ co-doped yttrium-stabilized zirconia phosphors. J. Lumin. 2015, 161, 288–292. [Google Scholar] [CrossRef]
- Costa, G.C.C.; Zhu, D.; Kulis, M.J.; Acosta, W.A.; Ghoshal, A. Reactivity between rare-earth oxides based thermal barrier coatings and a silicate melt. J. Am. Ceram. Soc. 2018, 101, 3674–3693. [Google Scholar] [CrossRef]
- Hwang, K.J.; Shin, M.; Lee, M.H.; Lee, H.; Oh, M.Y.; Shin, T.H. Investigation on the phase stability of yttria-stabilized zirconia electrolytes for high-temperature electrochemical application. Ceram. Int. 2019, 45, 9462–9467. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, J.Z. Synthesis of composite La1.67Sr0.33NiO4-YSZ for a potentiometric NOx sensor by microwave-assisted complex-gel auto-combustion. Ceram. Int. 2013, 39, 9599–9603. [Google Scholar] [CrossRef]
- Yang, L.W.; Han, H.L.; Zhang, Y.Y.; Zhong, J.X. White Emission by Frequency Up-Conversion in Yb3+-Ho3+-Tm3+ Triply Doped Hexagonal NaYF4 Nanorods. J. Phys. Chem. C 2009, 113, 18995–18999. [Google Scholar] [CrossRef]
- Hong, E.; Liu, L.; Bai, L.; Xia, C.; Gao, L.; Zhang, L.; Wang, B. Control synthesis, subtle surface modification of rare-earth-doped upconversion nanoparticles and their applications in cancer diagnosis and treatment. Mater. Sci. Eng. C. 2019, 105, 110097. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Ovsyankin, V.V.; Feofilov, P.P. Cooperative Processes in Luminescent Systems. In Luminescence of Crystals, Molecules, and Solutions; Williams, F., Baron, B., Martens, M., Varma, S.P., Eds.; Plenum Press: New York, NY, USA, 1973; pp. 64–77. [Google Scholar]
- Serrano, D.; Braud, A.; Doualan, J.L.; Camy, P.; Moncorge, R. Pr3+ cluster management in CaF2 by codoping with Lu3+ or Yb3+ for visible lasers and quantum down-converters. J. Opt. Soc. Am. B 2012, 29, 1854–1862. [Google Scholar] [CrossRef]
- Mishra, K.; Dwivedi, Y.; Rai, A.; Rai, S.B. Spectral characteristics of intense red luminescence in Pr:Y2O3 nanophosphor on UV excitation. Appl. Phys. B 2012, 109, 663–669. [Google Scholar] [CrossRef]
- Wang, D.N.; Xu, S.L.; Wang, X.Y.; Li, S.Y.; Hong, X.; Goodman, B.A.A.; Deng, W. Crystal growth, structure and optical properties of Pr3+-doped yttria-stabilized zirconia single crystals. Chin. Phys. B 2021, 30, 078103. [Google Scholar] [CrossRef]
- Sung Lim, C.; Aleksandrovsky, A.; Atuchin, V.; Molokeev, M.; Oreshonkov, A. Microwave-Employed Sol-Gel Synthesis of Scheelite-Type Microcrystalline AgGd(MoO4)2:Yb3+/Ho3+ Upconversion Yellow Phosphors and Their Spectroscopic Properties. Crystals 2020, 10, 1000. [Google Scholar] [CrossRef]
- Seshadri, M.; Bell, M.J.V.; Anjos, V.; Messaddeq, Y. Spectroscopic investigations on Yb3+ doped and Pr3+/Yb3+ codoped tellurite glasses for photonic applications. J. Rare Earth. 2021, 39, 33–42. [Google Scholar] [CrossRef]
- Zhu, Q.; Miao, J.; Wen, Y.; Rao, W. Hydrothermal preparation and white up-conversion luminescence of NaGd(WO4)2:Yb3+/Pr3+ microcrystals. J. Mater. Sci. Mater. Electron. 2016, 28, 3048–3051. [Google Scholar] [CrossRef]
- Zheng, C.B.; Xia, Y.Q.; Qin, F.; Yu, Y.; Miao, J.P.; Zhang, Z.G.; Cao, W.W. Femtosecond pulsed laser induced synthesis of ultrafine Y2O3: Pr, Yb nanoparticles with improved upconversion efficiency. Chem. Phys. Lett. 2010, 496, 316–320. [Google Scholar] [CrossRef]
- Hölsä, J.; Laamanen, T.; Laihinen, T.; Lastusaari, M.; Pihlgren, L.; Rodrigues, L.C.V. White up-conversion luminescence of NaYF4:Yb3+,Pr3+,Er3+. Opt. Mater. 2014, 36, 1627–1630. [Google Scholar] [CrossRef]
- Negita, K.; Takao, H. Condensations of phonons at the tetragonal to monoclinic phase-transition in ZrO2. J. Phys. Chem. Solids 1989, 50, 325–331. [Google Scholar] [CrossRef]
- Soares, M.R.N.; Nico, C.; Rodrigues, J.; Peres, M.; Soares, M.J.; Fernandes, A.J.S.; Costa, F.M.; Monteiro, T. Red and infrared luminescence from tetragonal YSZ:Pr3+ single crystal fibres grown by LFZ. Opt. Mater. 2011, 34, 27–29. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, T.; Wang, H.J.; Wang, F.; Pan, X.Y. Synthesis of acetyl salicylic acid over WO3/ZrO2 solid superacid catalyst. Chem. Eng. J. 2011, 174, 236–241. [Google Scholar] [CrossRef]
- Popov, V.V.; Menushenkov, A.P.; Yastrebtsev, A.A.; Tsarenko, N.A.; Arzhatkina, L.A.; Shchetinin, I.V.; Zheleznyi, M.V.; Ponkratov, K.V. Regularities of formation of complex oxides with the fluorite structure in the ZrO2-Y2O3 system. Russ. J. Inorg. Chem. 2017, 62, 1147–1154. [Google Scholar] [CrossRef]
- Li, P.; Chen, I.W.; Penner-Hahn, J.E. Effect of Dopants on Zirconia Stabilization-An X-ray Absorption Study: I, Trivalent Dopants. J. Am. Ceram. Soc. 1994, 77, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Borrero-González, L.J.; Galleani, G.; Manzani, D.; Nunes, L.A.O.; Ribeiro, S.J.L. Visible to infrared energy conversion in Pr3+-Yb3+ co-doped fluoroindate glasses. Opt. Mater. 2013, 35, 2085–2089. [Google Scholar] [CrossRef]
- Fawad, U.; Kim, H.J.; Gul, I.; Khan, M.; Tahir, S.; Jamal, T.; Muhammad, W. Proton, UV, and X-ray Induced Luminescence in Tb3+ Doped LuGd2Ga2Al3O12 Phosphors. Crystals 2020, 10, 844. [Google Scholar] [CrossRef]
- Maurya, A.; Dwivedi, A.; Bahadur, A.; Rai, S.B. Enhanced upconversion and downshifting emissions from Tm3+, Yb3+ co-doped CaZrO3 phosphor in the presence of alkali ions (Li+, Na+ and K+). J. Alloy. Compd. 2019, 786, 457–467. [Google Scholar] [CrossRef]
- Yadav, R.S.; Rai, S.B. Frequency upconversion and downshifting emissions in solution combustion derived Yb3+, Pr3+ co-doped strontium aluminate nano-phosphor: A multi-modal phosphor. J. Lumin. 2017, 190, 171–178. [Google Scholar] [CrossRef]
- Ding, D.; Gao, J.; Zhang, S.; Duo, L. The photoluminescence properties of Pr3+-Yb3+ co-doped gallo-germanate glasses and glass ceramics as energy converter. J. Lumin. 2020, 226, 117512. [Google Scholar] [CrossRef]


| Sample Number | Composition (mol%) | ||
|---|---|---|---|
| 8YSZ | Pr6O11 | Yb2O3 | |
| 1 | 98.85% | 0.15 | 1.00 |
| 2 | 98.35% | 0.15 | 1.50 |
| 3 | 97.85% | 0.15 | 2.00 |
| 4 | 97.35% | 0.15 | 2.50 |
| 5 | 96.85% | 0.15 | 3.00 |
| 6 | 96.35% | 0.15 | 3.50 |
| Yb2O3 (mol%) | Lattice Parameters | |
|---|---|---|
| a = b = c (Å) | Volume (Å3) | |
| 1.00 | 5.140 | 135.76 |
| 1.50 | 5.140 | 135.81 |
| 2.00 | 5.141 | 135.85 |
| 2.50 | 5.144 | 136.11 |
| 3.00 | 5.140 | 135.76 |
| 3.50 | 5.135 | 135.39 |
| Yb2O3 (mol%) | CIE x | CIE y |
|---|---|---|
| 1.0 | 0.2901 | 0.6118 |
| 1.5 | 0.2893 | 0.6171 |
| 2.0 | 0.2884 | 0.6253 |
| 2.5 | 0.2878 | 0.6222 |
| 3.0 | 0.2907 | 0.6173 |
| 3.5 | 0.2896 | 0.6178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wu, W.; Tan, X.; Goodman, B.A.; Xu, S.; Deng, W. Upconversion Visible Light Emission in Yb/Pr Co-Doped Yttria-Stabilized Zirconia (YSZ) Single Crystals. Crystals 2021, 11, 1328. https://doi.org/10.3390/cryst11111328
Wang D, Wu W, Tan X, Goodman BA, Xu S, Deng W. Upconversion Visible Light Emission in Yb/Pr Co-Doped Yttria-Stabilized Zirconia (YSZ) Single Crystals. Crystals. 2021; 11(11):1328. https://doi.org/10.3390/cryst11111328
Chicago/Turabian StyleWang, Daini, Wenxia Wu, Xiaojun Tan, Bernard A. Goodman, Shoulei Xu, and Wen Deng. 2021. "Upconversion Visible Light Emission in Yb/Pr Co-Doped Yttria-Stabilized Zirconia (YSZ) Single Crystals" Crystals 11, no. 11: 1328. https://doi.org/10.3390/cryst11111328
APA StyleWang, D., Wu, W., Tan, X., Goodman, B. A., Xu, S., & Deng, W. (2021). Upconversion Visible Light Emission in Yb/Pr Co-Doped Yttria-Stabilized Zirconia (YSZ) Single Crystals. Crystals, 11(11), 1328. https://doi.org/10.3390/cryst11111328







