A Novel Phosphorescent Iridium(III) Complex Bearing Formamide for Quantitative Fluorine Anion Detection
Abstract
:1. Introduction
2. Materials and Methods
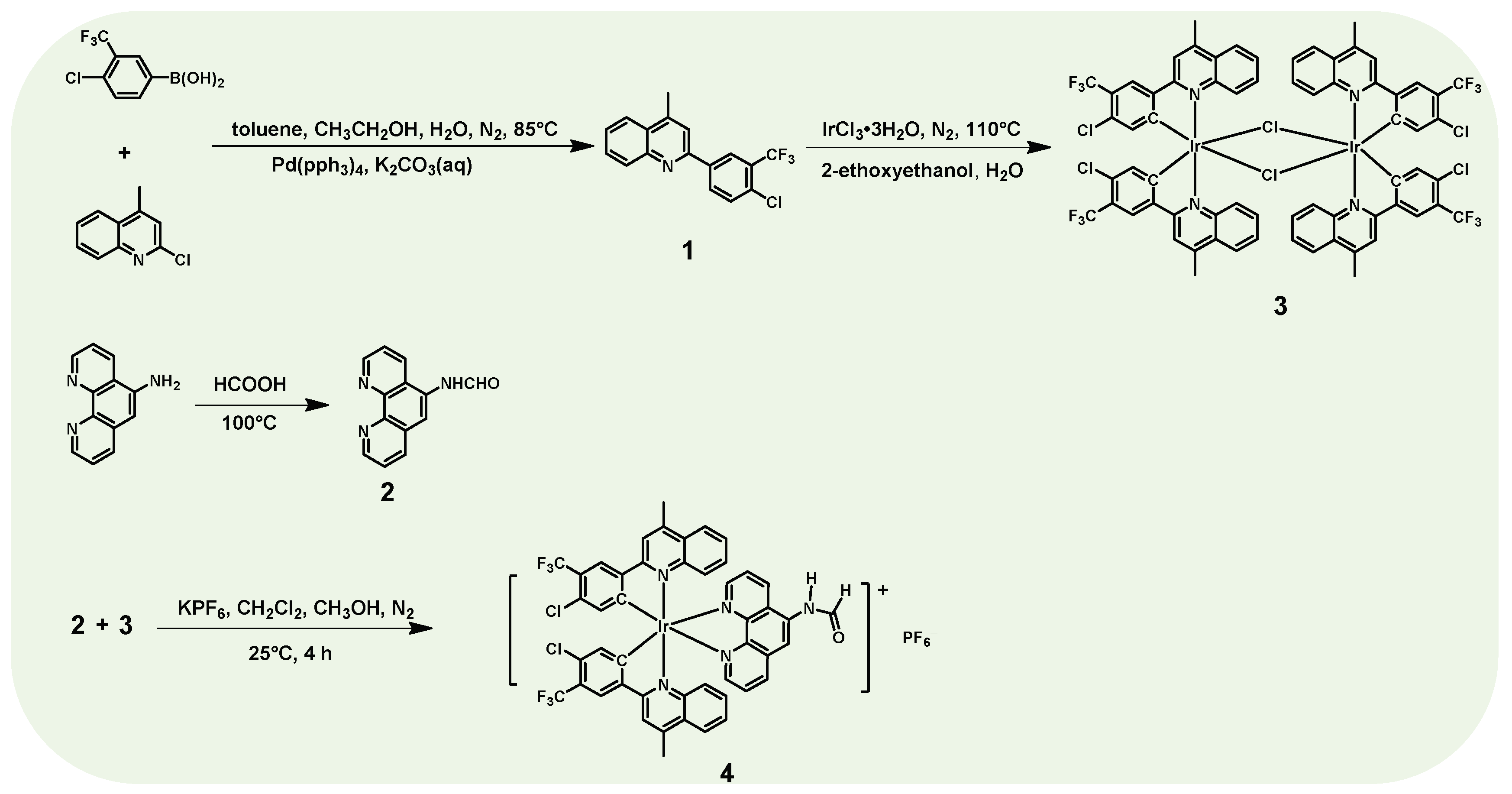
2.1. Synthesis
2.1.1. Synthesis of Main Ligand 1
2.1.2. Synthesis of Auxiliary Ligand 2
2.1.3. Synthesis of μ-Dichloro Bridged Ir(III) Dimer Complex 3
2.1.4. Synthesis of Ir(III) Complex 4
3. Results
3.1. Structural Characterization
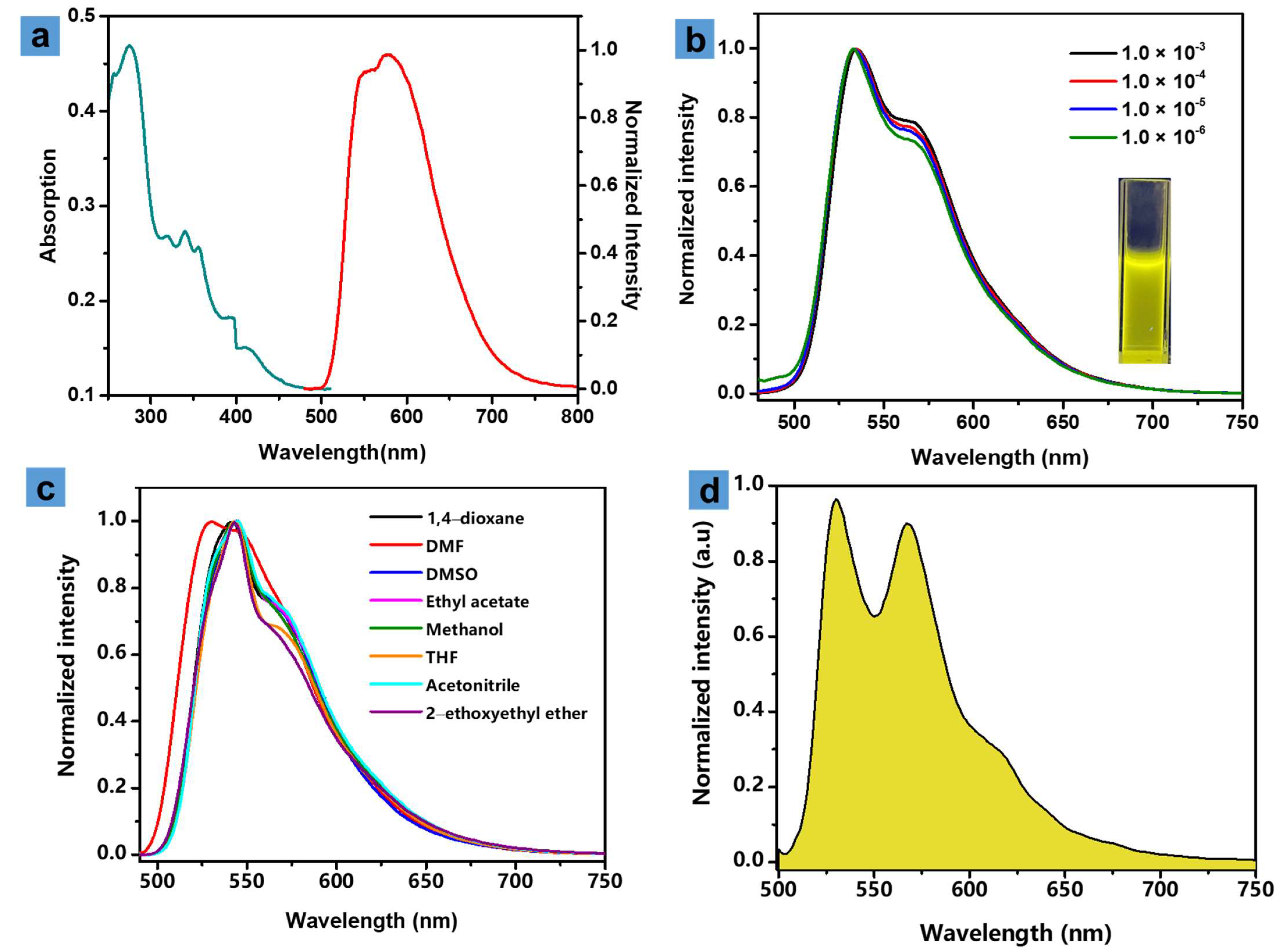
3.2. Photophysical Properties
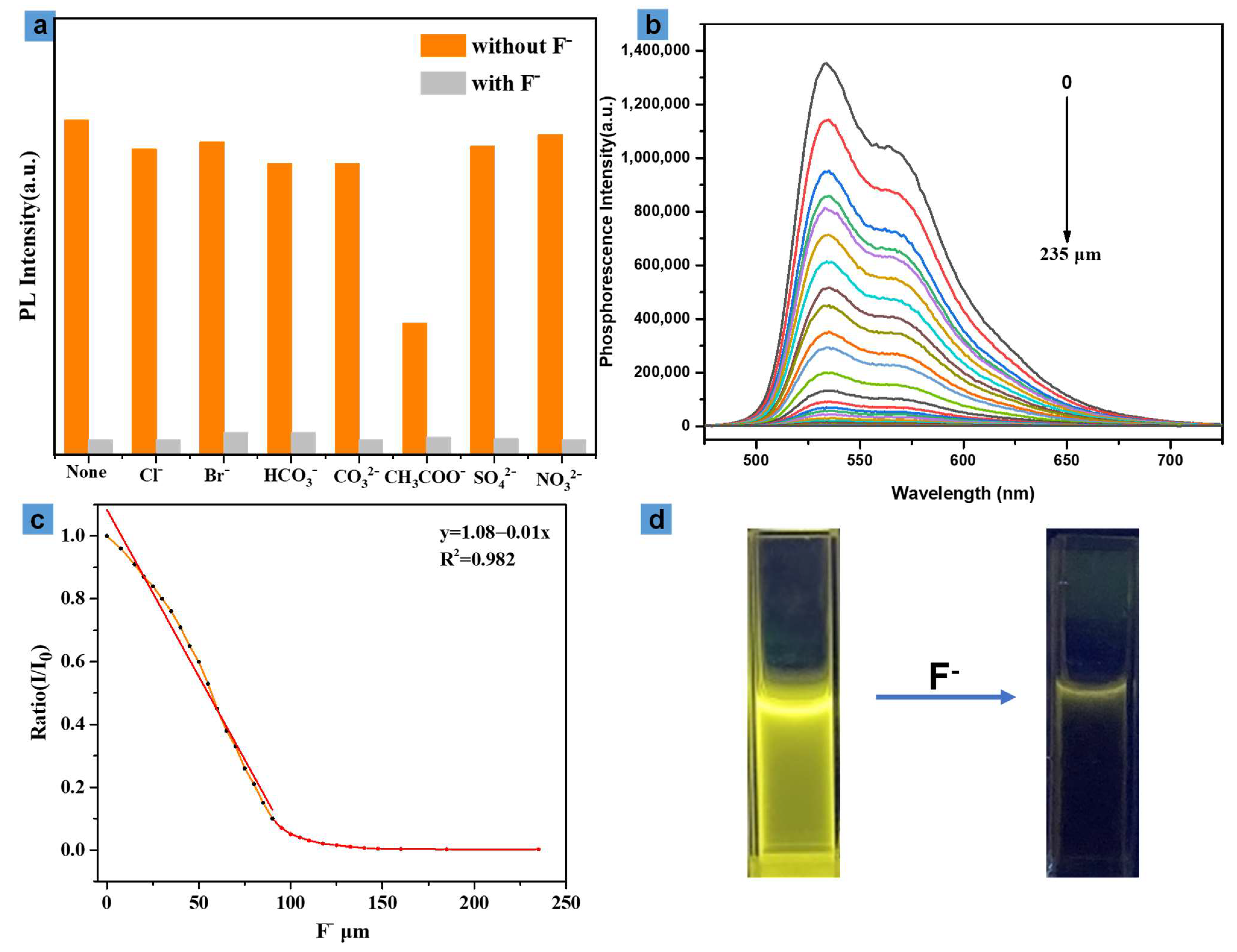
3.3. Fluoride Anion Detection
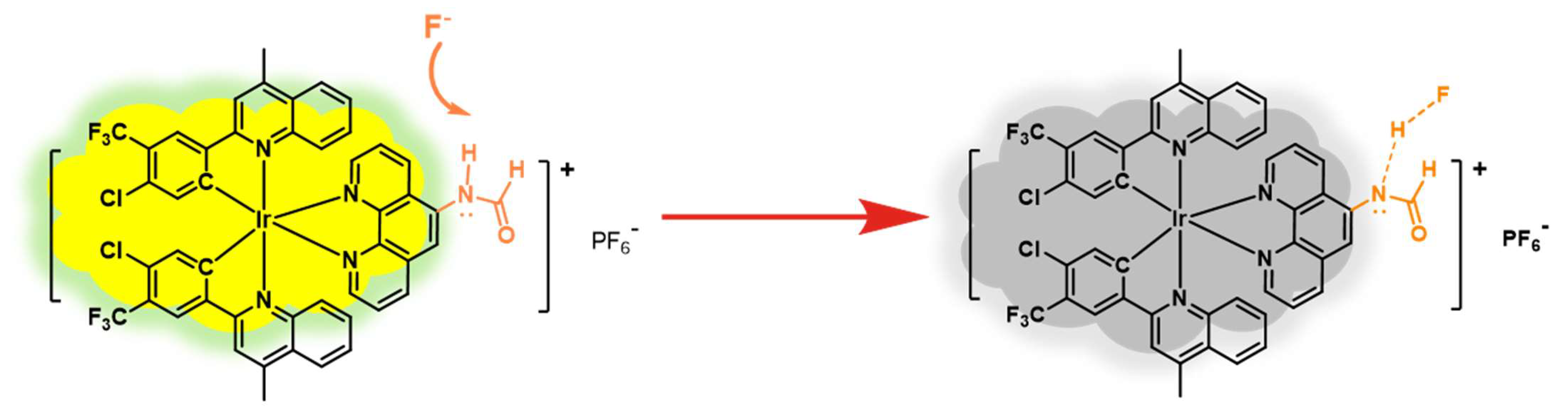
3.4. Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baykov, A.A.; Fabrichniy, L.P.; Pohjanjoki, P.; Zyryanov, A.B.; Lahti, R. Fluoride effects along the reaction pathway of pyrophosphatase: evidence for a second enzyme·pyrophosphate intermediate. Biochemistry 2000, 39, 11939–11947. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Munno, K.; Box, C.; Cummins, A.; Zhu, X.; Sutton, R. Think global, act local: Local knowledge is critical to inform positive change when it comes to microplastics. Environ. Sci. Technol. 2021, 55, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Máñez, R.M.; Sancenón, F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar] [CrossRef] [PubMed]
- Cametti, M.; Rissanen, K. Recognition and sensing of fluoride anion. Chem. Commun. 2009, 2809–2829. [Google Scholar] [CrossRef]
- Qiu, H.; Ye, M.C.; Zhang, M.D.; Zhang, X.L.; Zhao, Y.; Yu, J.H. Nano-hydroxyapatite encapsulated inside an anion exchanger for efficient defluoridation of neutral and weakly alkaline water. ACS ES&T Eng. 2021, 1, 46–54. [Google Scholar]
- Hättig, C.; Klopper, W.; Köhn, A.; Tew, D.P. Explicitly correlated electrons in molecules. Chem. Rev. 2012, 112, 4–74. [Google Scholar] [CrossRef]
- Dhillon, A.; Nair, M.; Kumar, D. Analytical methods for determination and sensing of fluoride in biotic and abiotic sources: A review. Anal. Methods 2016, 8, 5338–5352. [Google Scholar] [CrossRef]
- D’Ulivo, A.; Pagliano, E.; Onor, M.; Pitzalis, E.; Zamboni, R. Vapor Generation of Inorganic anionic species after aqueous phase alkylation with trialkyloxonium tetrafluoroborates. Anal. Chem. 2009, 81, 6399–6406. [Google Scholar] [CrossRef]
- Cao, Z.B.; Cao, Y.; Kubota, R.; Sasaki, Y.; Asano, K.; Lyu, X.J.; Zhang, Z.J.; Zhou, Q.; Zhao, X.L.; Xu, X.; et al. Fluorescence anion chemosensor array based on pyrenylboronic acid. Front. Chem. 2020, 8, 414. [Google Scholar] [CrossRef]
- Chen, Z.J.; Yan, P.; Zou, L.; Zhao, M.L.; Jiang, J.Y.; Liu, S.J.; Zhang, K.Y.; Huang, W.; Zhao, Q. Using ultrafast responsive phosphorescent nanoprobe to visualize elevated peroxynitrite in vitro and in vivo via ratiometric and time-resolved photoluminescence imaging. Adv. Healthc. Mater. 2018, 7, 1800309. [Google Scholar] [CrossRef]
- Chen, Z.J.; Meng, X.C.; Xie, M.J.; Shi, Y.X.; Zou, L.; Guo, S.; Jiang, J.Y.; Liu, S.J.; Zhao, Q. A self-calibrating phosphorescent polymeric probe for measuring PH fluctuations in subcellular organelles and the zebrafish digestive tract. J. Mater. Chem. C 2020, 8, 2265–2271. [Google Scholar] [CrossRef]
- Guo, S.; Han, M.P.; Chen, R.Z.; Zhuang, Y.L.; Zou, L.; Liu, S.J.; Huang, W.; Zhao, Q. Mitochondria-localized iridium(III) complexes with anthraquinone groups as effective photosensitizers for photodynamic therapy under hypoxia. Sci. China Chem. 2019, 62, 1639–1648. [Google Scholar] [CrossRef]
- Tao, P.; Li, W.L.; Zhang, J.; Guo, S.; Zhao, Q.; Wang, H.; Wei, B.; Liu, S.J.; Zhou, X.H.; Yu, Q.; et al. Facile synthesis of highly efficient lepidine-based phosphorescent iridium(III) complexes for yellow and white organic light-emitting diodes. Adv. Funct. Mater. 2016, 26, 881–894. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Tao, P.; Zou, L.; Gao, P.L.; Liu, Y.; Liu, X.; Wang, H.; Liu, S.J.; Dong, Q.C.; Li, J.; et al. Hyperbranched phosphorescent conjugated polymer dots with iridium(III) complex as the core for hypoxia imaging and photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 28319–28330. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Zhang, C.Q.; Lin, W.P.; Liu, Y.H.; Liu, S.J.; Xu, Y.J.; Zhao, Q.; Huang, W. Long-lived phosphorescent iridium(III) complexes conjugated with cationic polyfluorenes for heparin sensing and cellular imaging. Macromol. Rapid Commun. 2015, 36, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Gao, P.L.; Sun, G.L.; Zhang, T.W.; Li, X.L.; Liu, S.J.; Zhao, Q.; Lo, K.K.W.; Huang, W. Dual-phosphorescent iridium(iii) complexes extending oxygen sensing from hypoxia to hyperoxia. J. Am. Chem. Soc. 2018, 140, 7827–7834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Zhang, T.W.; Wei, H.J.; Wu, Q.; Liu, S.J.; Zhao, Q.; Huang, W. Phosphorescent iridium(iii) complexes capable of imaging and distinguishing between exogenous and endogenous analytes in living cells. Chem. Sci. 2018, 9, 7236–7240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Zhang, C.Q.; Liu, S.J.; Liu, Y.H.; Zhang, K.Y.; Zhou, X.B.; Jiang, J.Y.; Xu, W.J.; Yang, T.S.; Huang, W. Dual-emissive polymer dots for rapid detection of fluoride in pure water and biological systems with improved reliability and accuracy. Sci. Rep. 2015, 5, 16420. [Google Scholar] [CrossRef]
- Chen, Z.J.; Jiang, J.Y.; Zhao, W.L.; Hu, X.M.; Xie, M.J.; Li, F.Y.; Liu, S.J.; Zhao, Q. An aggregation-induced phosphorescent emission-active iridium(iii) complex for fluoride anion imaging in living cells. J. Organomet. Chem. 2021, 932, 121644. [Google Scholar] [CrossRef]
- Guo, S.; Ma, Y.; Liu, S.J.; Yu, Q.; Xu, A.Q.; Han, J.M.; Wei, L.W.; Zhao, Q.; Huang, W. A phosphorescent ir(iii) complex with formamide for the luminescence determination of low-level water content in organic solvents. J. Mater. Chem. C 2016, 4, 6110–6116. [Google Scholar] [CrossRef]
- Tao, P.; Zheng, X.K.; Wei, X.Z.; Lau, M.T.; Lee, Y.K.; Li, Z.K.; Guo, Z.L.; Zhao, F.Q.; Liu, X.; Liu, S.J.; et al. Chlorinated yellow phosphorescent cyclometalated neutral iridophosphors featuring broad emission bandwidths for white electroluminescence. Mater. Today Energy 2021, 21, 100773. [Google Scholar] [CrossRef]
- Zheng, X.K.; Zhao, F.Q.; Yin, M.N.; Qian, C.; Bi, S.H.; Tao, P.; Miao, Y.Q.; Liu, S.J.; Zhao, Q. New trifluoromethyl modified iridium(iii) complex for high-efficiency sky-blue phosphorescent organic light-emitting diode. Tetrahedron Lett. 2021, 75, 153181. [Google Scholar] [CrossRef]
- Tao, P.; Zheng, X.K.; Lee, Y.K.; Wang, G.L.; Li, F.Y.; Li, Z.K.; Zhao, Q.; Miao, Y.Q.; Wong, W.Y. Vacuum-sublimable ionic yellow phosphorescent iridium(iii) complexes with broad emission for white electroluminescence. Adv. Photonics Res. 2021, 2, 2100115. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Matsuo, N. Benzo[h]quinolin-10-yl-n iridium(iii) complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767–768. [Google Scholar]
- Sun, H.B.; Liu, S.J.; Lin, W.P.; Zhang, K.Y.; Lv, W.; Huang, X.; Huo, F.W.; Yang, H.R.; Jenkins, G.; Zhao, Q.; et al. Smart responsive phosphorescent materials for data recording and security protection. Nat. Commun. 2014, 5, 3601. [Google Scholar] [CrossRef] [Green Version]
- Han, J.M.; Guo, S.; Wang, J.; Wei, L.W.; Zhuang, Y.L.; Liu, S.J.; Zhao, Q.; Zhang, X.W.; Huang, W. Circularly polarized phosphorescent electroluminescence from chiral cationic iridium(iii) isocyanide complexes. Adv. Opt. Mater. 2017, 5, 1700359. [Google Scholar] [CrossRef]
- Wang, R.B.; Cai, K.M.; Wang, H.; Yin, C.; Cheng, J.J. A caged metabolic precursor for dt-diaphorase-responsive cell labeling. Chem. Commun. 2018, 54, 4878–4881. [Google Scholar] [CrossRef]
- Tao, P.; Miao, Y.Q.; Wang, H.; Xu, B.S.; Zhao, Q. High-performance organic electroluminescence: Design from organic light-emitting materials to devices. Chem. Rec. 2019, 19, 1531–1561. [Google Scholar] [CrossRef]

| Complex 4 | Absorption | Emission | ||||||
|---|---|---|---|---|---|---|---|---|
| λabs (nm) | λem (nm) (77k) | λem (nm) (RT) | τ (μs) | ΦPL | Eonsetox (eV) | Eg a (eV) | T1 b (eV) | |
| 278, 325–350, 375–450 | 530/567 | 535/566 | 2.09 | 0.15 | 1.75 | 2.32 | 2.34 |
| Complex | State | λ/nm | Configuration | Character |
|---|---|---|---|---|
| 4 4 + F− | T1 T1 | 495.60 643.74 | H-1 -> L + 2 21.5%, H -> L 19.4%, H -> L + 2 65.2%, H -> L + 3 20.0%, H -> L 10.4%, | MLCT/LLCT MLCT/LLCT LLCT LLCT LLCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Guo, C.; Lu, Z.; Du, L.; Gao, M.; Liu, S.; Liu, Y.; Zhao, Q. A Novel Phosphorescent Iridium(III) Complex Bearing Formamide for Quantitative Fluorine Anion Detection. Crystals 2021, 11, 1190. https://doi.org/10.3390/cryst11101190
Guo S, Guo C, Lu Z, Du L, Gao M, Liu S, Liu Y, Zhao Q. A Novel Phosphorescent Iridium(III) Complex Bearing Formamide for Quantitative Fluorine Anion Detection. Crystals. 2021; 11(10):1190. https://doi.org/10.3390/cryst11101190
Chicago/Turabian StyleGuo, Song, Chaoxiong Guo, Zhao Lu, Linlin Du, Man Gao, Shujuan Liu, Yuanli Liu, and Qiang Zhao. 2021. "A Novel Phosphorescent Iridium(III) Complex Bearing Formamide for Quantitative Fluorine Anion Detection" Crystals 11, no. 10: 1190. https://doi.org/10.3390/cryst11101190
APA StyleGuo, S., Guo, C., Lu, Z., Du, L., Gao, M., Liu, S., Liu, Y., & Zhao, Q. (2021). A Novel Phosphorescent Iridium(III) Complex Bearing Formamide for Quantitative Fluorine Anion Detection. Crystals, 11(10), 1190. https://doi.org/10.3390/cryst11101190






