Penetration of Chitosan into the Single Walled Armchair Carbon Nanotubes: Atomic Scale Insight
Abstract
:1. Introduction
2. Materials and Methods
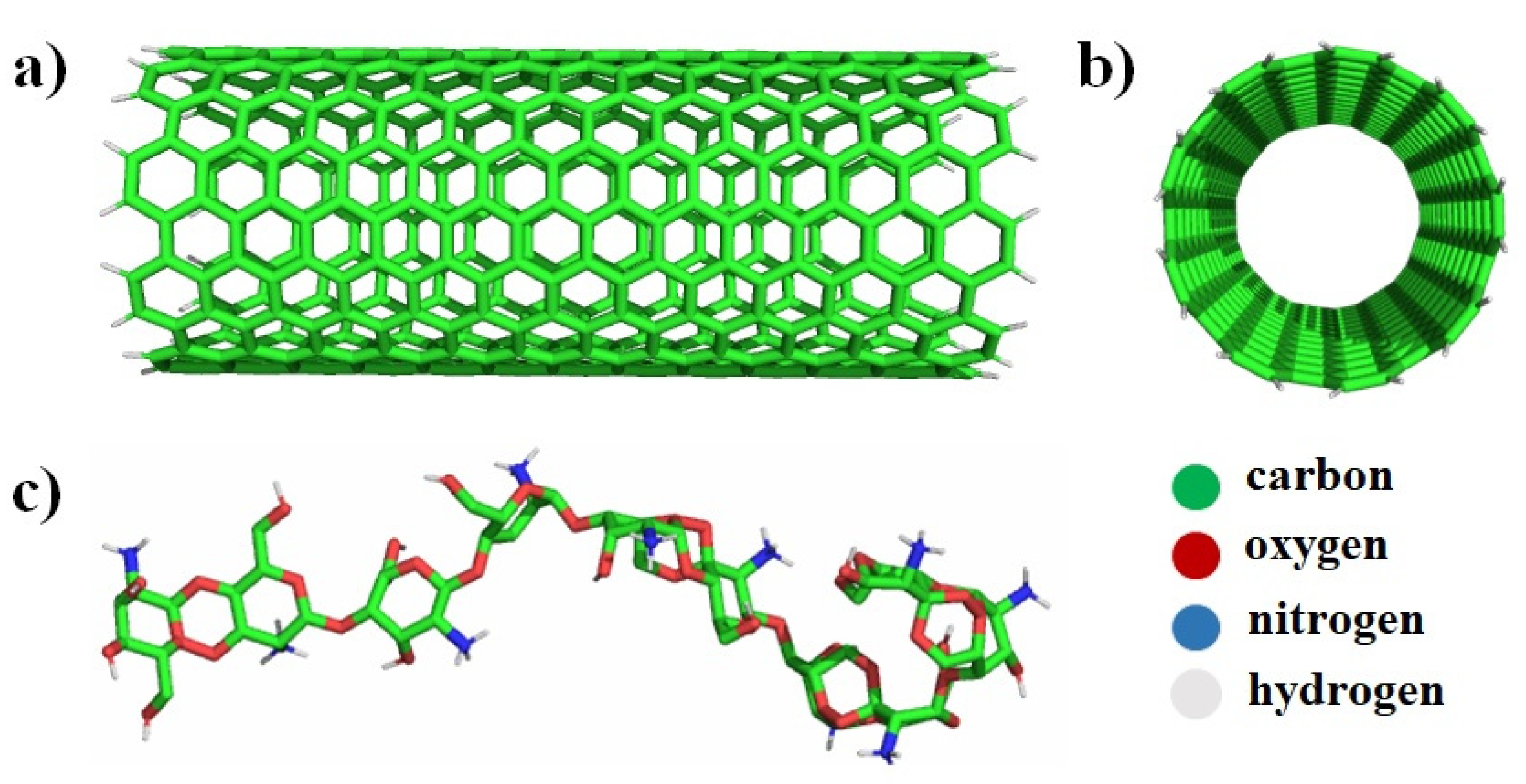
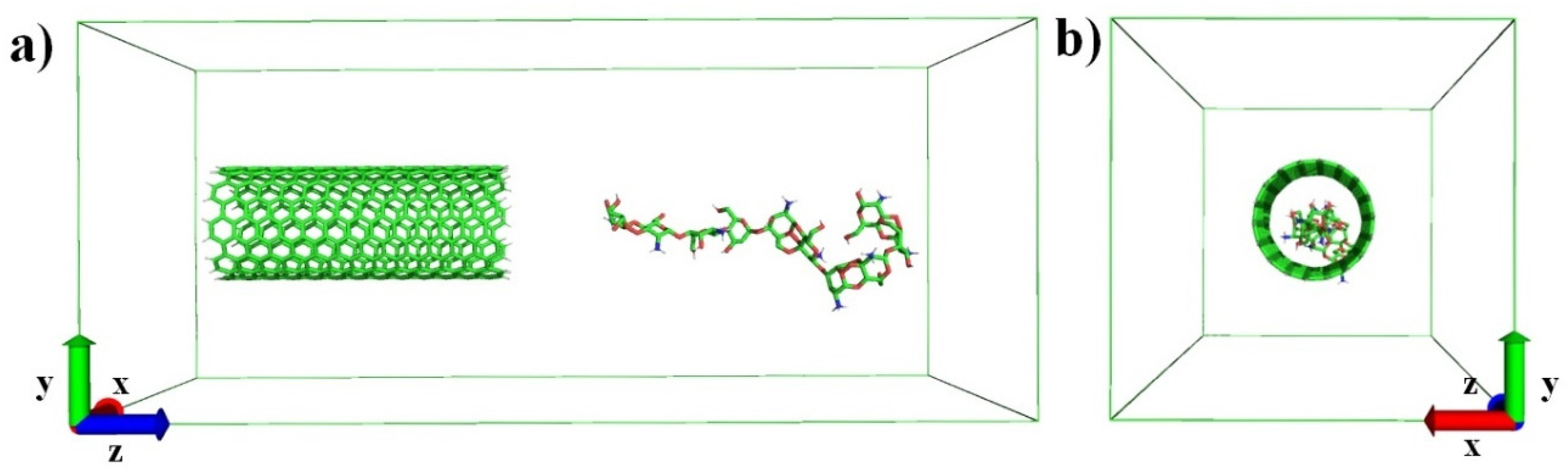
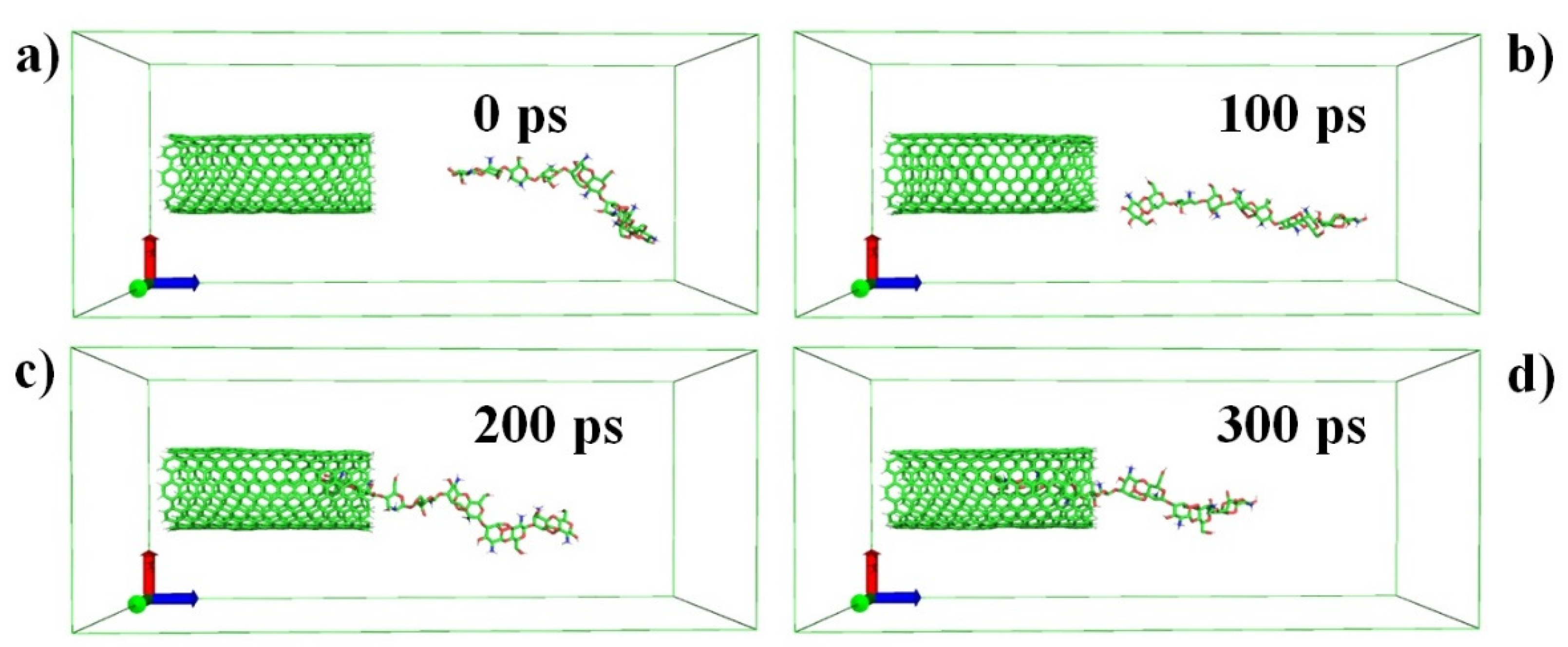
2.1. Molecular Dynamics Simulations
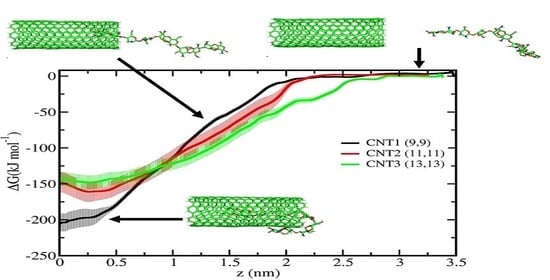
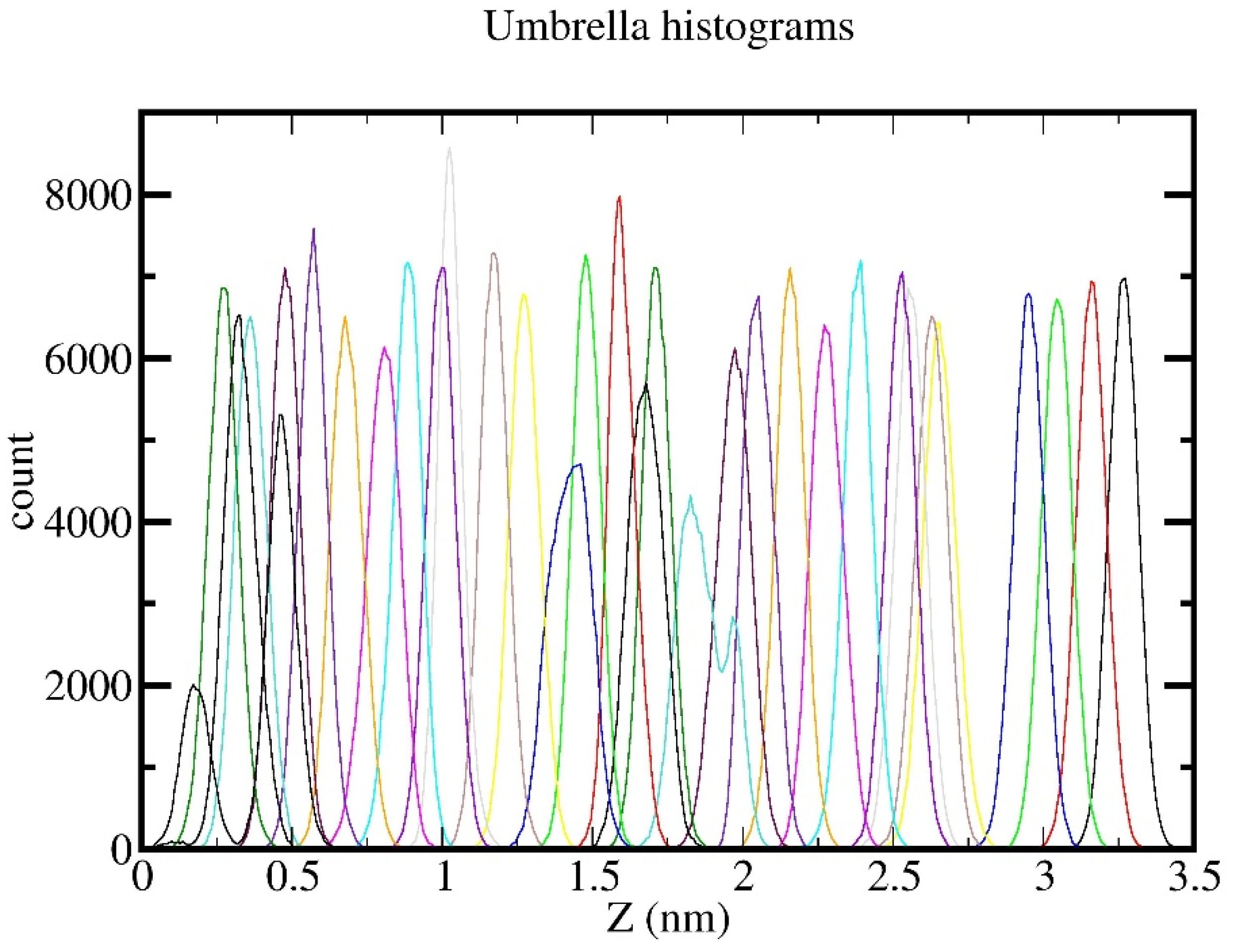
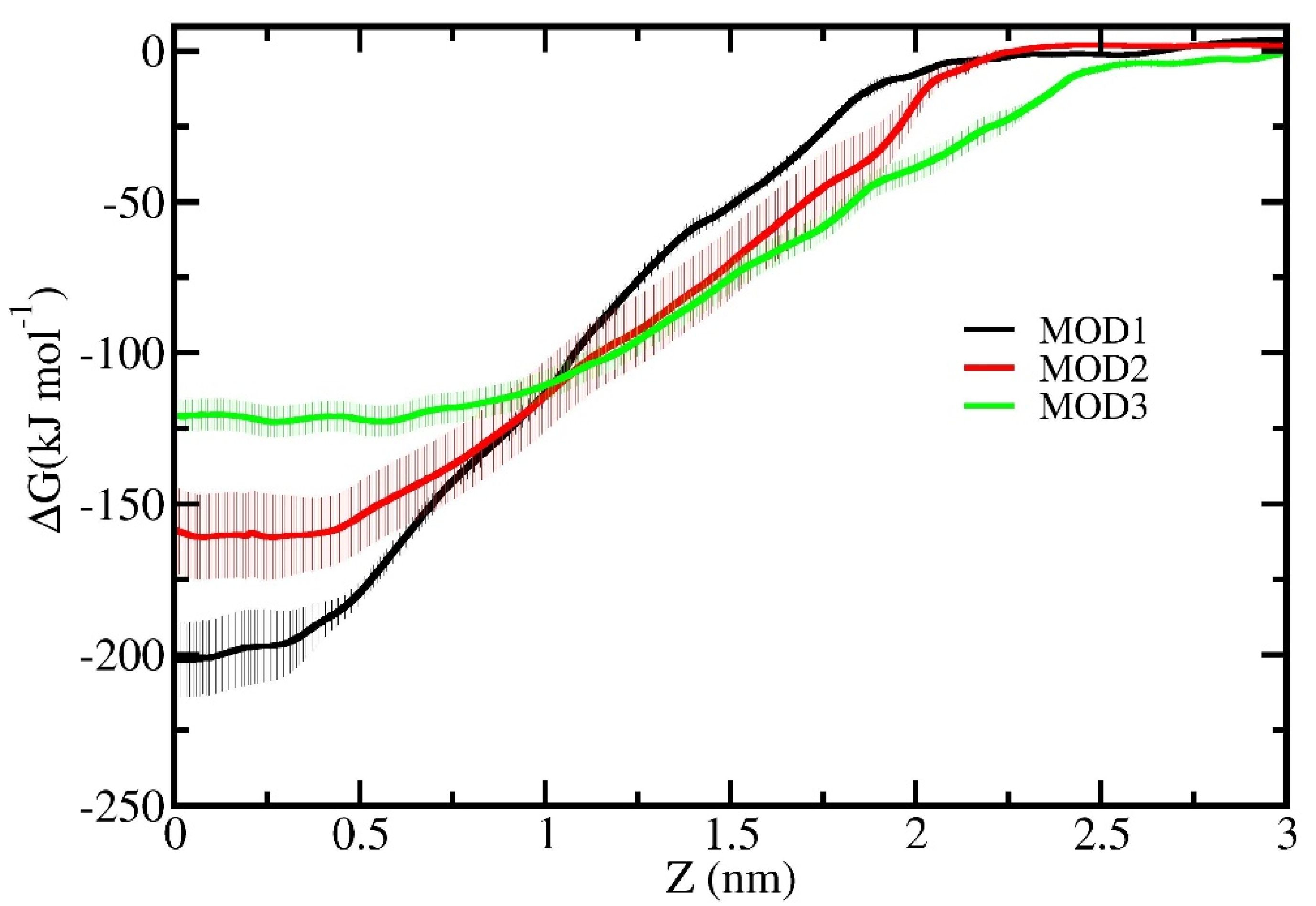
2.2. Umbrella Sampling Simulations
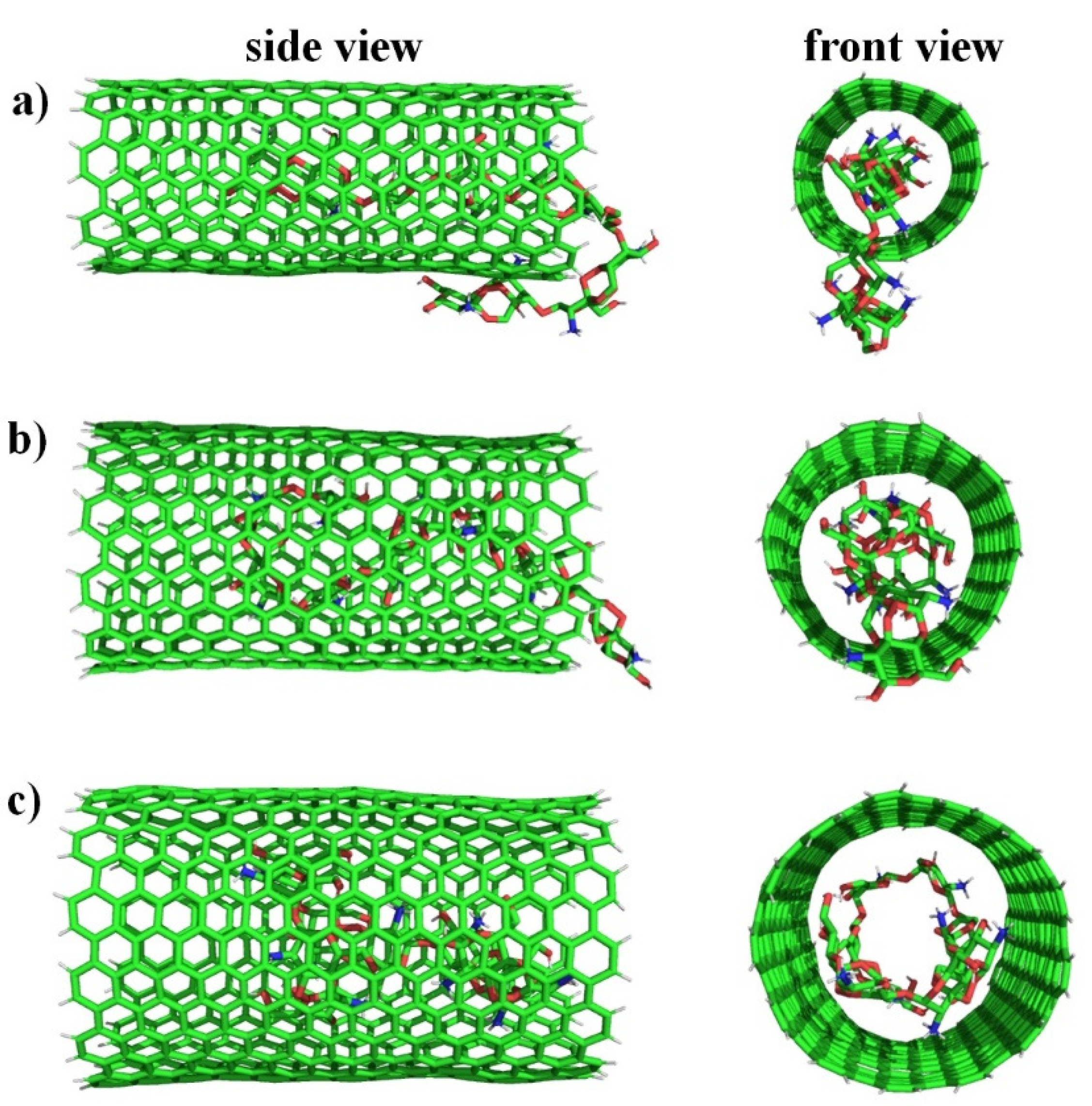
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model System | SASA (nm2) | Rg (Å) |
|---|---|---|
| MOD1 | 19.32 + 0.36 | 9.60 ± 0.17 |
| MOD2 | 16.99 + 0.43 | 9.58 ± 0.22 |
| MOD3 | 16.43 + 0.77 | 7.13 ± 0.32 |
References
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C.; Rao, A.M. Carbon nanotubes. In The Physics of Fullerene-Based and Fullerene-Related Materials; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Khan, F.S.A.; Mubarak, N.; Khalid, M.; Khan, M.M.; Tan, Y.H.; Walvekar, R.; Abdullah, E.; Karri, R.R.; Rahman, M.E. Comprehensive review on carbon nanotubes embedded in different metal and polymer matrix: Fabrications and applications. Crit. Rev. Solid State Mater. Sci. 2021, 1–28. [Google Scholar] [CrossRef]
- Peng, H.; Li, Q.; Chen, T. Industrial Applications of Carbon Nanotubes; William Andrew, Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan/carbon nanotube hybrids: Recent progress and achievements for industrial applications. New J. Chem. 2021, 45, 3756–3777. [Google Scholar] [CrossRef]
- Kuralay, F.; Vural, T.; Bayram, C.; Denkbas, E.B.; Abaci, S.J.C.; Biointerfaces, S.B. Carbon nanotube–chitosan modified disposable pencil graphite electrode for Vitamin B12 analysis. Colloids Surf. B Biointerfaces 2011, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Polizu, S.; Savadogo, O.; Poulin, P.; Yahia, L.H. Applications of carbon nanotubes-based biomaterials in biomedical nanotechnology. J. Nanosci. Nanotechnol. 2006, 6, 1883–1904. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A. Electrochemical carbon based nanosensors: A promising tool in pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2018, 147, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Mohandas, A.; Bhatnagar, I.; Kim, S.-K. Antimicrobial activity of chitosan-carbon nanotube hydrogels. Materials 2014, 7, 3946–3955. [Google Scholar] [CrossRef] [Green Version]
- Pyman, H.; Roshanfekr, H.; Ansari, S. DNA-based electrochemical biosensor using chitosan–carbon nanotubes composite film for biodetection of Pirazon. Eurasian Chem. Commun. 2020, 2, 213–225. [Google Scholar]
- Parvaiz, M.S.; Shah, K.A.; Alrobei, H.; Dar, G.; Khanday, F.A.; Andrabi, S.M.A.; Hamid, R. Modeling and simulation of carbon nanotube amino-acid sensor: A first-principles study. Comput. Theor. Chem. 2021, 1204, 113402. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, A.; Gorski, W. Carbon nanotube–chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal. Chem. 2004, 76, 5045–5050. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-F.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.-S. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem. Res. Toxicol. 2010, 23, 1131–1147. [Google Scholar] [CrossRef]
- Arora, S.; Kaur, H.; Kumar, R.; Kaur, R.; Rana, D.; Rayat, C.S.; Kaur, I.; Arora, S.K.; Bubber, P.; Bharadwaj, L.M. In vitro cytotoxicity of multiwalled and single-walled carbon nanotubes on human cell lines. Nanotub. Carbon Nanostruct. 2015, 23, 377–382. [Google Scholar] [CrossRef]
- Adorinni, S.; Cringoli, M.C.; Perathoner, S.; Fornasiero, P.; Marchesan, S. Green Approaches to Carbon Nanostructure-Based Biomaterials. Appl. Sci. 2021, 11, 2490. [Google Scholar] [CrossRef]
- Aoki, K.; Ogihara, N.; Tanaka, M.; Haniu, H.; Saito, N. Carbon nanotube-based biomaterials for orthopaedic applications. J. Mater. Chem. B 2020, 8, 9227–9238. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.E.; Pauliukaite, R.; Fatibello-Filho, O.; Brett, C.M. Application of functionalised carbon nanotubes immobilised into chitosan films in amperometric enzyme biosensors. Sens. Actuators B Chem. 2009, 142, 308–315. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Chen, X.; Xin, J.H. Decoration of carbon nanotubes with chitosan. Carbon 2005, 43, 3178–3180. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Moztarzadeh, F.; Haghighipour, N.; Ghazizadeh, L.; Baghbani, F.; Shokrgozar, M.A.; Allahyari, Z. Preparation and characterization of novel functionalized multiwalled carbon nanotubes/chitosan/β-Glycerophosphate scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 365–372. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surf. B Biointerfaces 2017, 154, 253–262. [Google Scholar] [CrossRef]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Multimodal, pH sensitive, and magnetically assisted carrier of doxorubicin designed and analyzed by means of computer simulations. Langmuir 2018, 34, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Wolski, P.; Nieszporek, K.; Panczyk, T. Cytosine-Rich DNA Fragments Covalently Bound to Carbon Nanotube as Factors Triggering Doxorubicin Release at Acidic pH. A Molecular Dynamics Study. Int. J. Mol. Sci. 2021, 22, 8466. [Google Scholar] [CrossRef]
- Mejri, A.; Tangour, B.; Herlem, G.; Picaud, F. Confinement of the antitumoral drug cisplatin inside edge-functionalized carbon nanotubes and its release near lipid membrane. Eur. Phys. J. D 2021, 75, 1–10. [Google Scholar] [CrossRef]
- Aztatzi-Pluma, D.; Castrejón-González, E.O.; Almendarez-Camarillo, A.; Alvarado, J.F.; Duran-Morales, Y. Study of the molecular interactions between functionalized carbon nanotubes and chitosan. J. Phys. Chem. C 2016, 120, 2371–2378. [Google Scholar] [CrossRef]
- Azimov, J.; Mamatkulov, S.; Turaeva, N.; Oxengendler, B.; Rashidova, S.S. Computer modeling of chitosan adsorption on a carbon nanotube. J. Struct. Chem. 2012, 53, 829–834. [Google Scholar] [CrossRef]
- Rungnim, C.; Rungrotmongkol, T.; Hannongbua, S.; Okumura, H. Modelling. Replica exchange molecular dynamics simulation of chitosan for drug delivery system based on carbon nanotube. J. Mol. Graph. Model. 2013, 39, 183–192. [Google Scholar] [CrossRef]
- Yu, R.; Ran, M.; Wen, J.; Sun, W.; Chu, W.; Jiang, C.; He, Z. The effect of hydroxylation on CNT to form Chitosan-CNT composites: A DFT study. Appl. Surf. Sci. 2015, 359, 643–650. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Ghafoori-Tabrizi, K.; Rafii-Tabar, H. Multi-scale computational modelling of the mechanical behaviour of the chitosan biological polymer embedded with graphene and carbon nanotube. Comput. Mater. Sci. 2012, 53, 347–353. [Google Scholar] [CrossRef]
- Kästner, J. Umbrella sampling. Comput. Mol. Sci. 2011, 1, 932–942. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Herraez, A. Biomolecules in the computer: Jmol to the rescue. Biochem. Mol. Biol. Educ. 2006, 34, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, L.; Yuan, Q.; Zhu, L.; Ding, F. Transition-metal-catalyzed unzipping of single-walled carbon nanotubes into narrow graphene nanoribbons at low temperature. Angew. Chem. 2011, 123, 8191–8195. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Lu, W.; Sinitskii, A.; Pera, G.; Sun, Z.; Tour, J.M. Highly conductive graphene nanoribbons by longitudinal splitting of carbon nanotubes using potassium vapor. ACS Nano 2011, 5, 968–974. [Google Scholar] [CrossRef]
- Yang, F.H.; Lachawiec, A.J.; Yang, R.T. Adsorption of spillover hydrogen atoms on single-wall carbon nanotubes. J. Phys. Chem. B 2006, 110, 6236–6244. [Google Scholar] [CrossRef] [PubMed]
- Khalilov, U.; Bogaerts, A.; Xu, B.; Kato, T.; Kaneko, T.; Neyts, E. How the alignment of adsorbed ortho H pairs determines the onset of selective carbon nanotube etching. Nanoscale 2017, 9, 1653–1661. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Wu, Y.; Tepper, H.L.; Voth, G.A. Flexible simple point-charge water model with improved liquid-state properties. J. Chem. Phys. 2006, 124, 024503. [Google Scholar] [CrossRef]
- Fliege, J.; Svaiter, B.F. Steepest descent methods for multicriteria optimization. Math. Methods Oper. Res. 2000, 51, 479–494. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free—Energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Rungnim, C.; Rungrotmongkol, T.; Poo-Arporn, R.P. pH-controlled doxorubicin anticancer loading and release from carbon nanotube noncovalently modified by chitosan: MD simulations. J. Mol. Graph. Model. 2016, 70, 70–76. [Google Scholar] [CrossRef]
- Mohammadi, Z.A.; Aghamiri, S.F.; Zarrabi, A.; Talaie, M.R. A comparative study on non-covalent functionalization of carbon nanotubes by chitosan and its derivatives for delivery of doxorubicin. Chem. Phys. Lett. 2015, 642, 22–28. [Google Scholar] [CrossRef]
- Alsuhybani, M.; Alshahrani, A.; Haidyrah, A.S. Synthesis, Characterization, and Evaluation of Evaporated Casting MWCNT/Chitosan Composite Membranes for Water Desalination. J. Chem. 2020, 2020, 5207680. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Huang, S.-C.; Chou, P.-H.; Den, W.; Hou, C.-H. Application of a multiwalled carbon nanotube-chitosan composite as an electrode in the electrosorption process for water purification. Chemosphere 2016, 146, 113–120. [Google Scholar] [CrossRef]
| Model System | ECS_CNT (kJ/mol) | ECS_water (kJ/mol) |
|---|---|---|
| MOD1 | −432.42 ± 12.51 | −177.08 ± 12.02 |
| MOD2 | −452.64 ± 16.44 | −104.05 ± 13.97 |
| MOD3 | −484.05 ± 12.11 | −68.94 ± 12.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzokov, J.; Marimuthu, P.; Saidov, K.; Ruzimuradov, O.; Mamatkulov, S. Penetration of Chitosan into the Single Walled Armchair Carbon Nanotubes: Atomic Scale Insight. Crystals 2021, 11, 1174. https://doi.org/10.3390/cryst11101174
Razzokov J, Marimuthu P, Saidov K, Ruzimuradov O, Mamatkulov S. Penetration of Chitosan into the Single Walled Armchair Carbon Nanotubes: Atomic Scale Insight. Crystals. 2021; 11(10):1174. https://doi.org/10.3390/cryst11101174
Chicago/Turabian StyleRazzokov, Jamoliddin, Parthiban Marimuthu, Kamoladdin Saidov, Olim Ruzimuradov, and Shavkat Mamatkulov. 2021. "Penetration of Chitosan into the Single Walled Armchair Carbon Nanotubes: Atomic Scale Insight" Crystals 11, no. 10: 1174. https://doi.org/10.3390/cryst11101174
APA StyleRazzokov, J., Marimuthu, P., Saidov, K., Ruzimuradov, O., & Mamatkulov, S. (2021). Penetration of Chitosan into the Single Walled Armchair Carbon Nanotubes: Atomic Scale Insight. Crystals, 11(10), 1174. https://doi.org/10.3390/cryst11101174