Donor-Type Nickel–Dithiolene Complexes Fused with Bulky Cycloalkane Substituents and Their Application in Molecular Conductors
Abstract
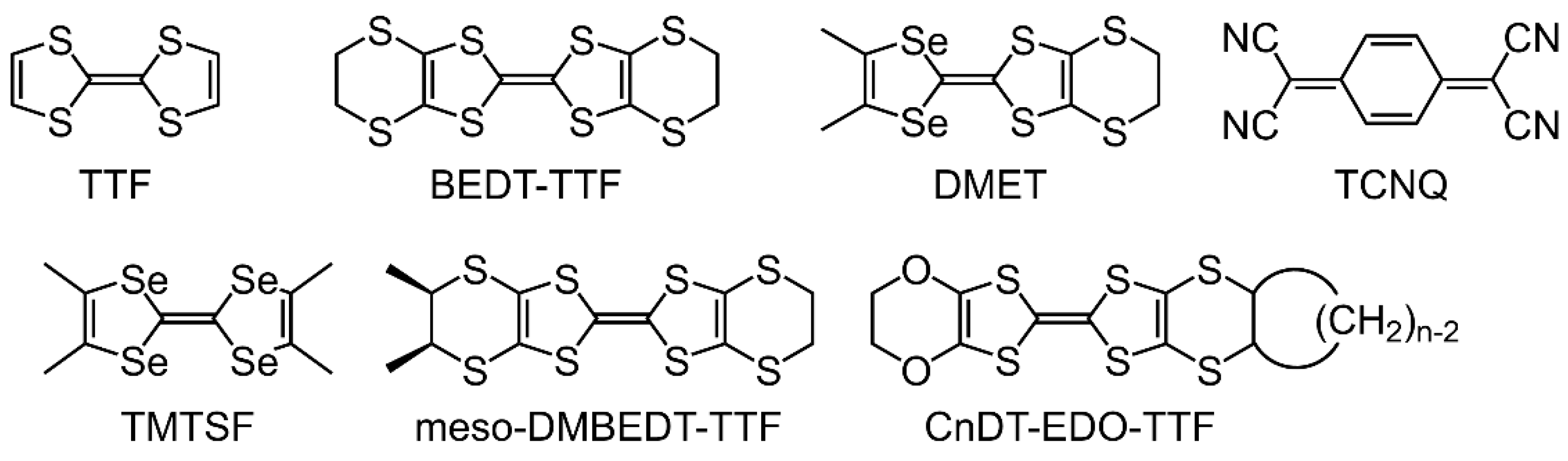
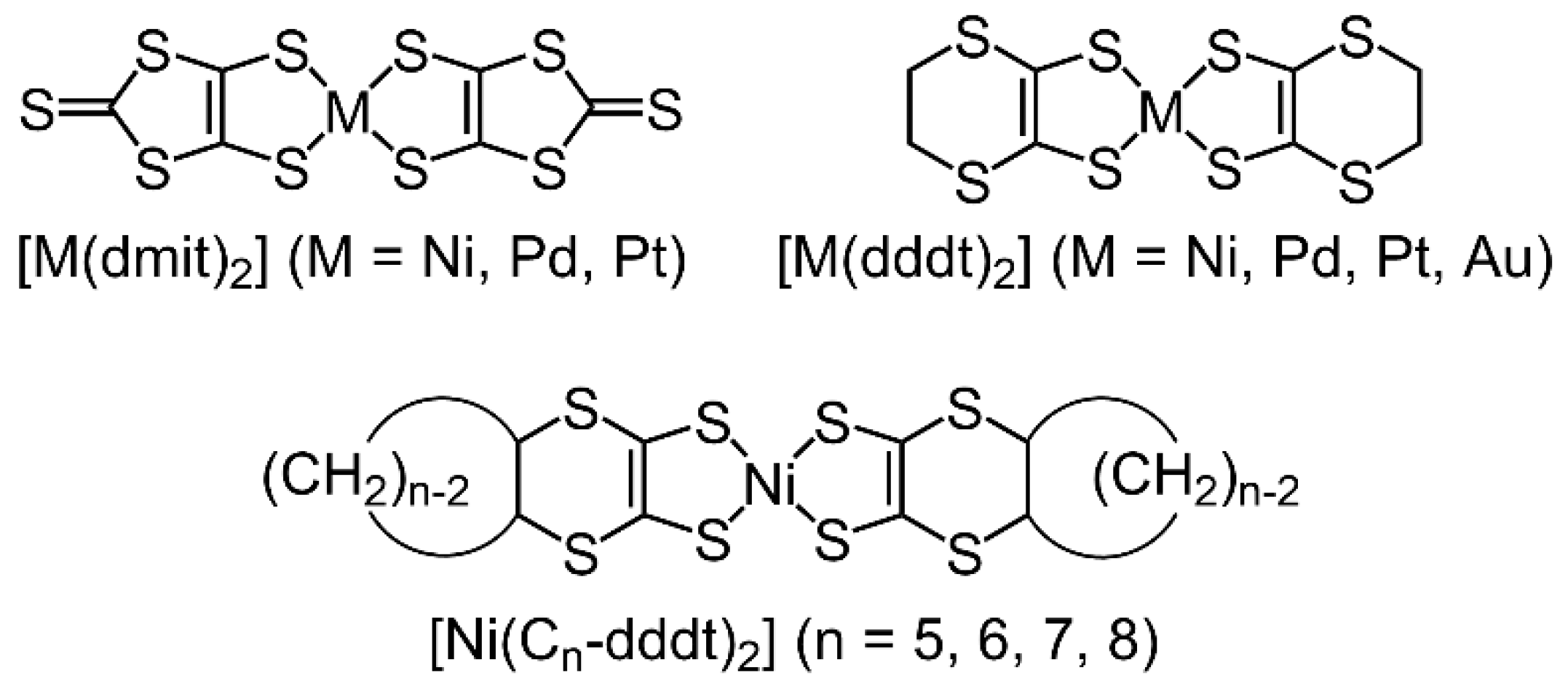
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
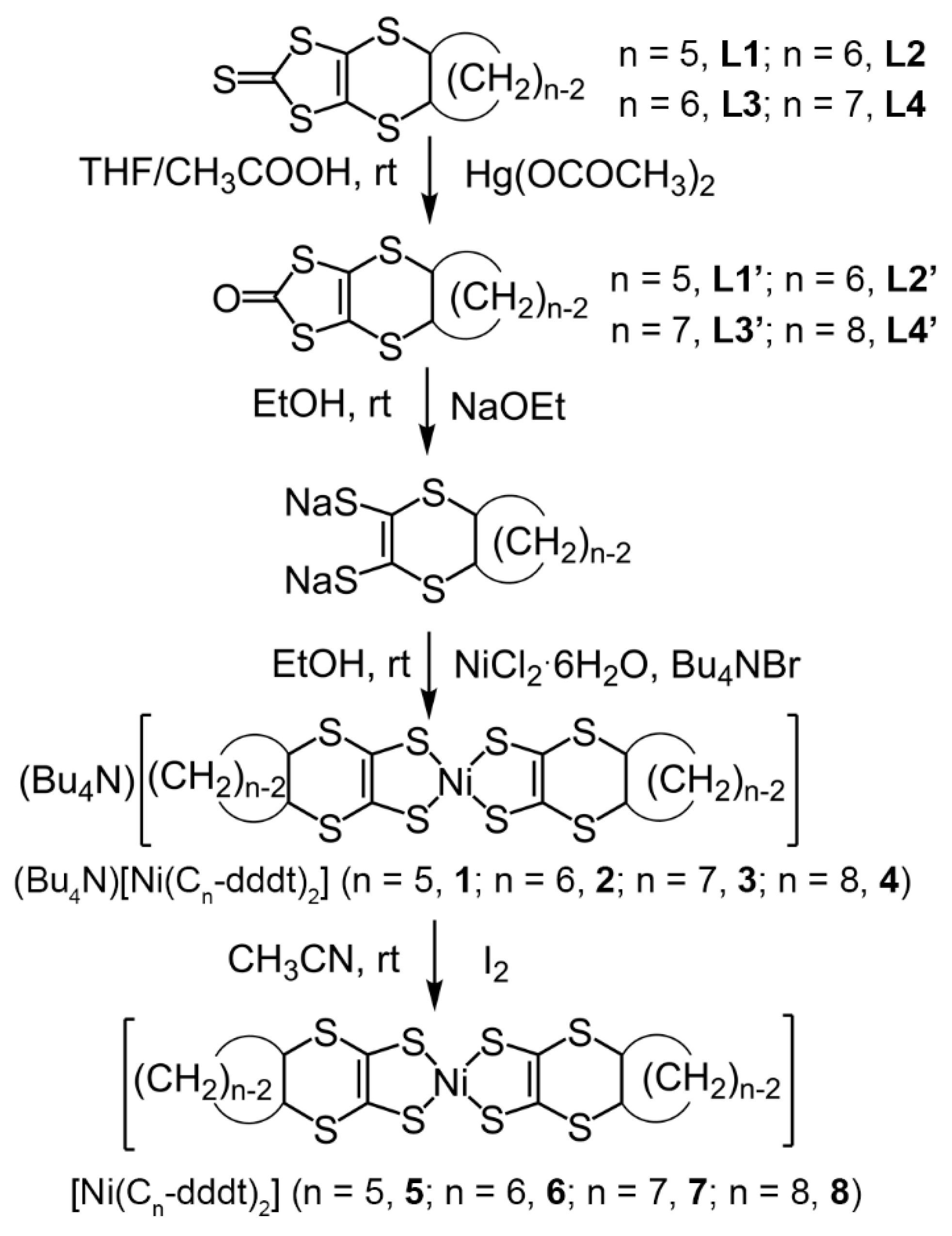
2.2.1. Synthesis of 4,5-Cis(cyclopentalenedithio)-1,3-dithiole-2-thione (L1), 4,5-Cis(cyclohexylenedithio)-1,3-dithiole-2-thione (L2), 4,5-Cis(cycloheptalenedithio)-1,3-dithiole-2-thione (L3), and 4,5-Cis(cyclooctalenedithio)-1,3-dithiole-2-thione (L4)
2.2.2. Synthesis of 4,5-Cis(cyclopentalenedithio)-1,3-dithiole-2-one (L1′) and 4,5-Cis(cyclohexylenedithio)-1,3-dithiole-2-one (L2′), 4,5-Cis(cycloheptalenedithio)-1,3-dithiole-2-one (L3′), and 4,5-Cis(cyclooctalenedithio)-1,3-dithiole-2-one (L4′)
2.2.3. Synthesis of Monoanionic Complexes (Bu4N)[Ni(Cn-dddt)2] (n = 5, 1; n = 6, 2; n = 7, 3; and n = 8, 4)
2.2.4. Synthesis of Neutral Complexes [Ni(Cn-dddt)2] (n = 5, 5; n = 6, 6; n = 7, 7; and n = 8, 8)
2.2.5. Synthesis of Radical Cation Crystals [Ni(C5-dddt)2]2[ClO4] (9) and [Ni(C5-dddt)2]2[PF6] (10)
2.3. Physical Measurements
2.3.1. Elemental Analyses
2.3.2. Electrochemical Measurements
2.3.3. Electronic Absorption Spectra
2.3.4. Electrical Resistivity
2.3.5. Magnetic Susceptibility
2.3.6. Band Calculation
2.3.7. Crystal Structure Determinations of L2–L4, Neutral Complexes 5–8, and Radical Cation Crystals 9 and 10
3. Results and Discussion
3.1. Synthesis of Complexes 1–8
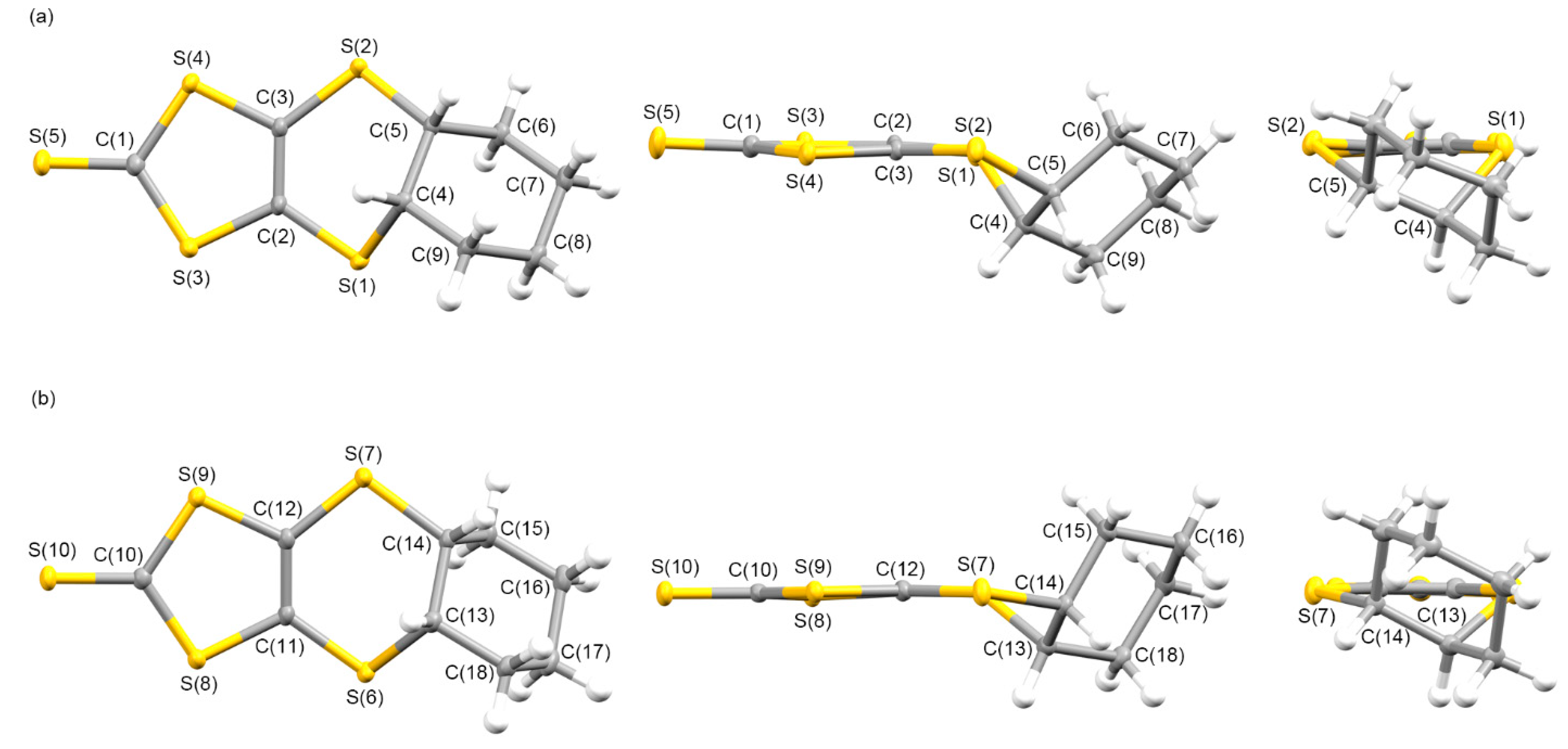
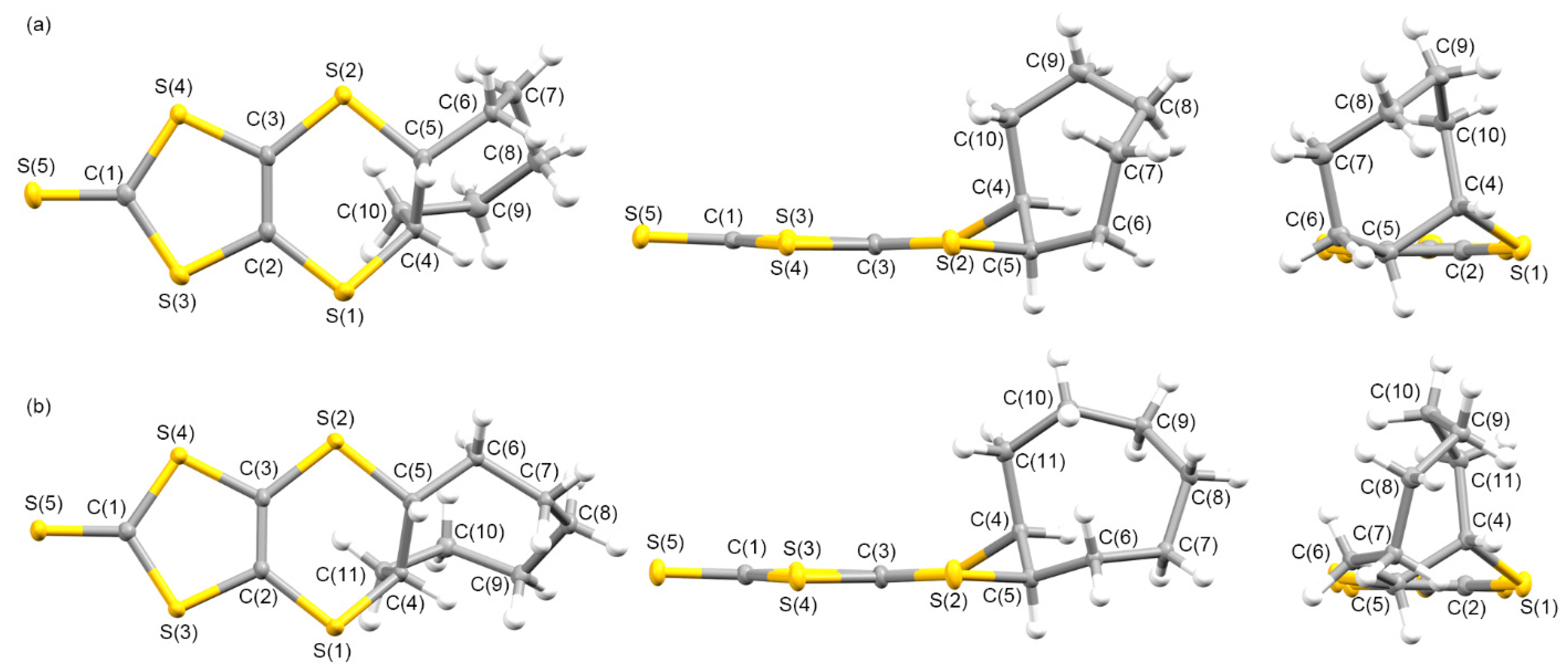
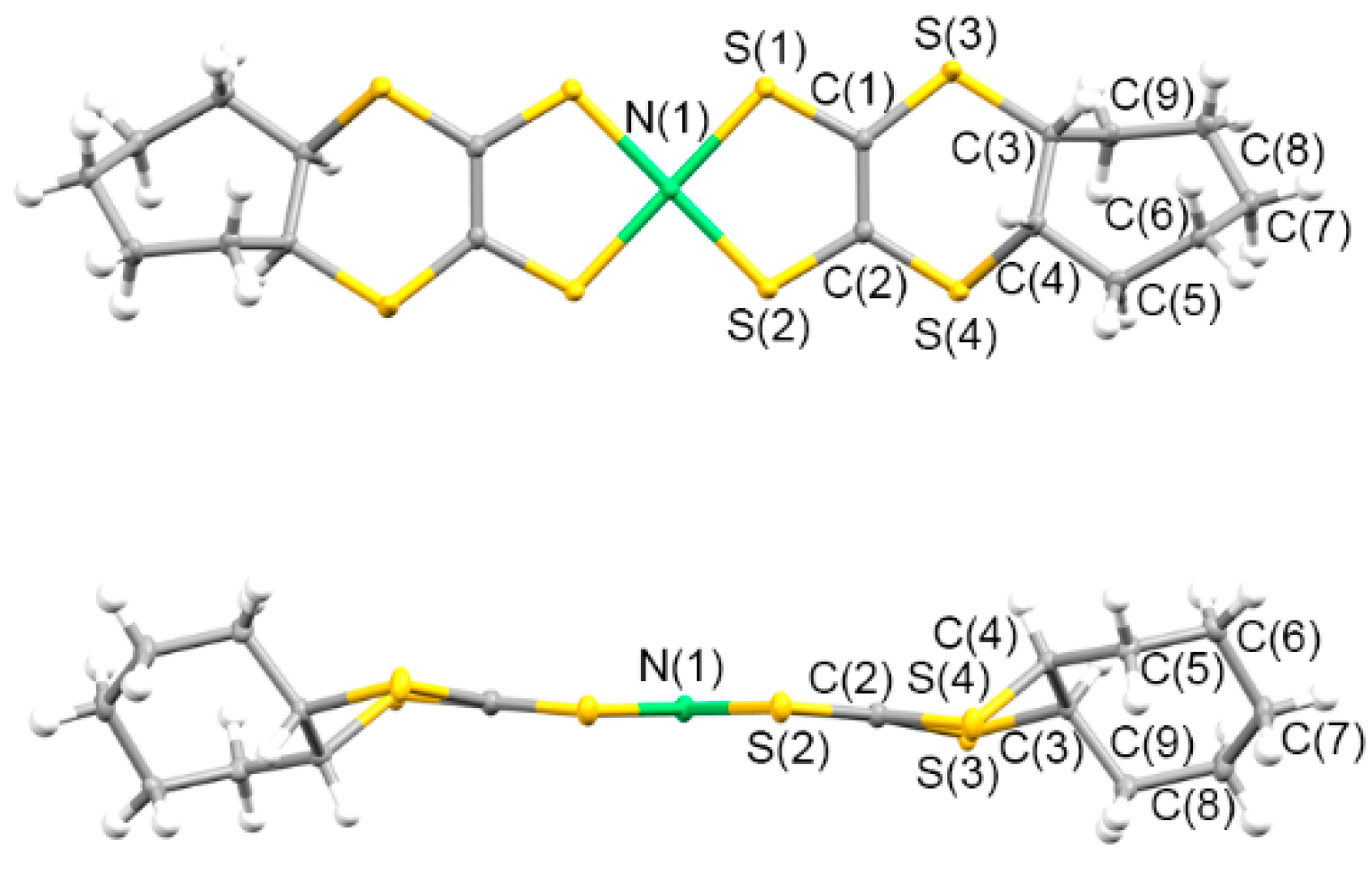
3.1.1. Syntheses and Molecular Structures of Precursors L1–L4
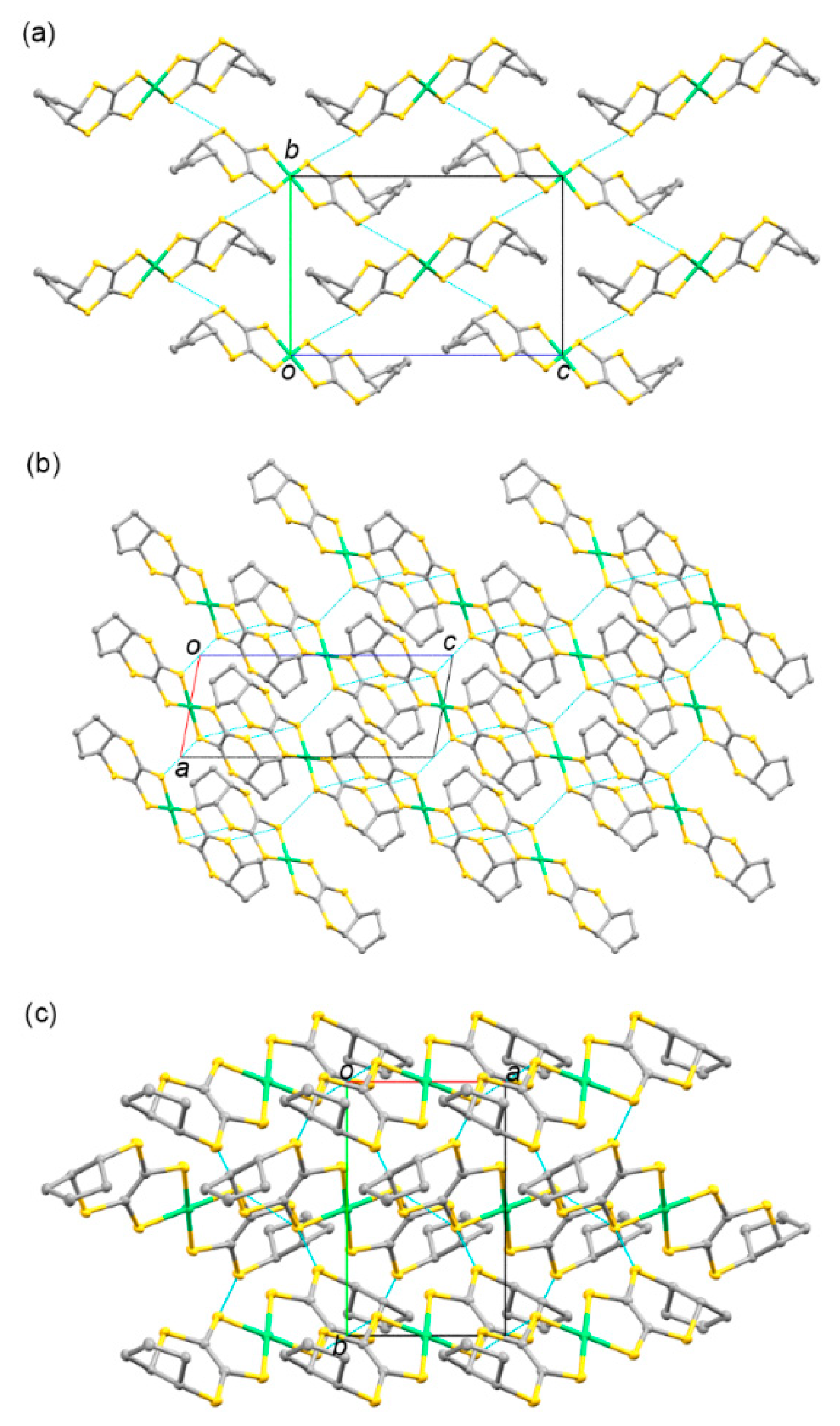
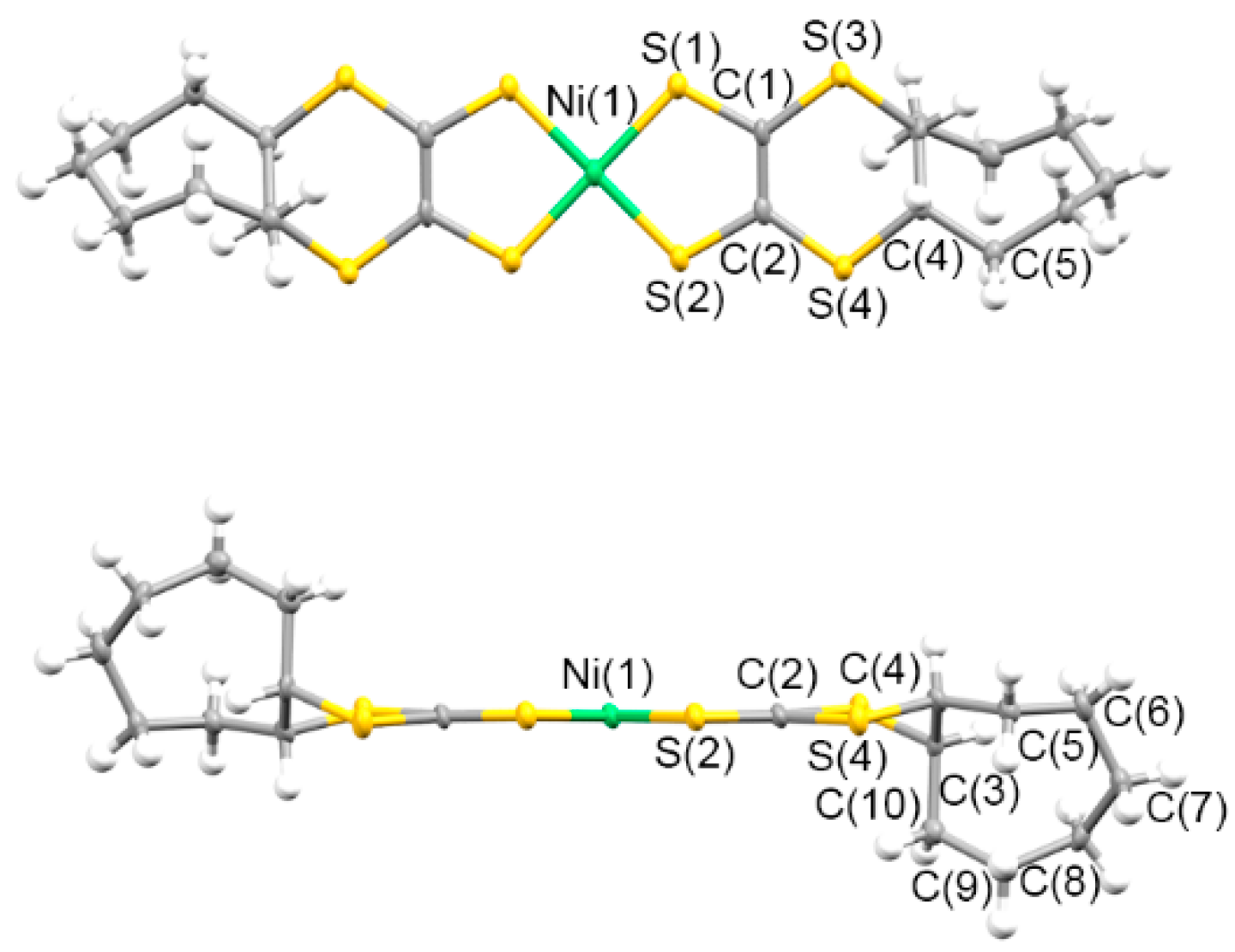
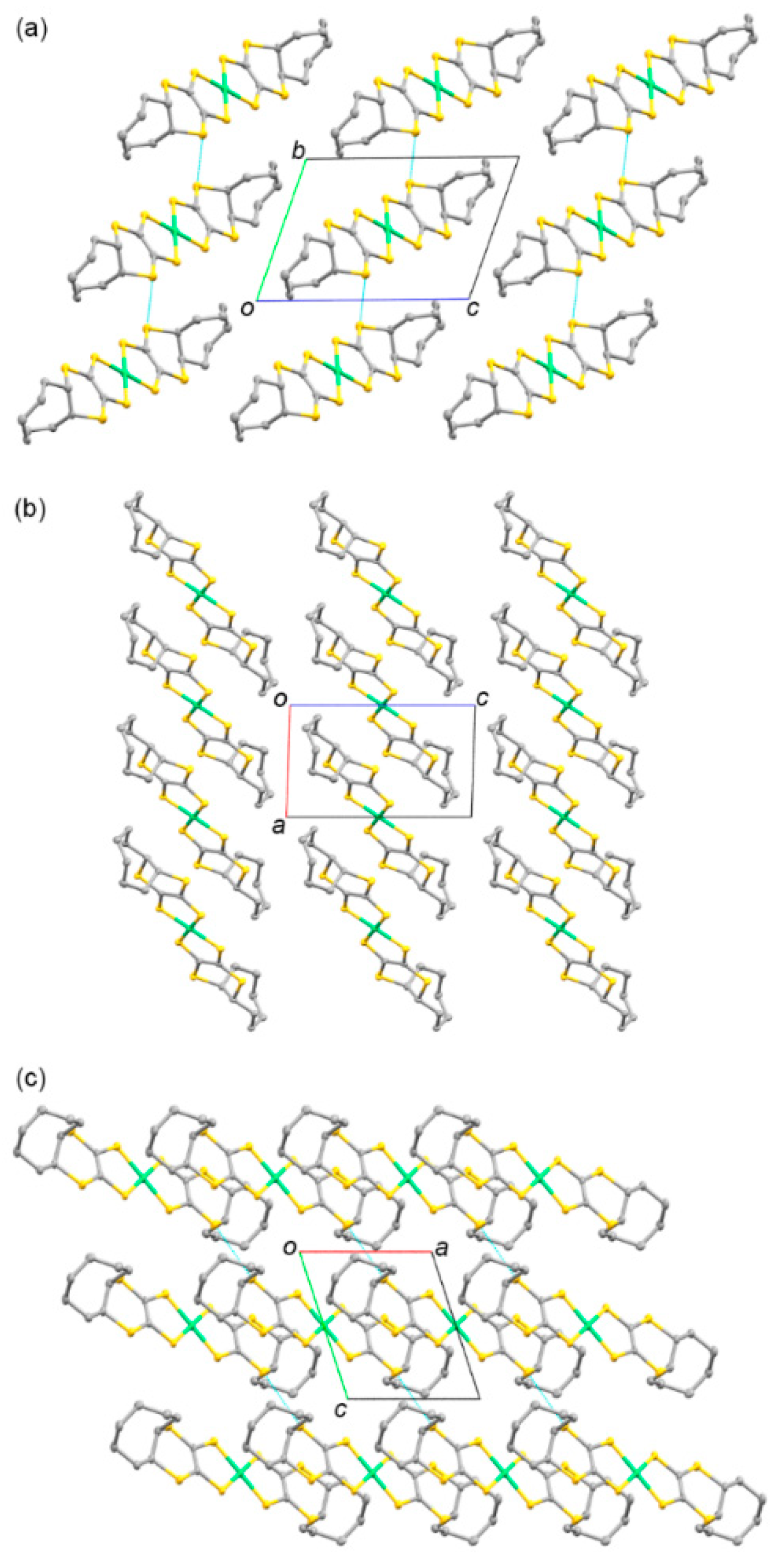
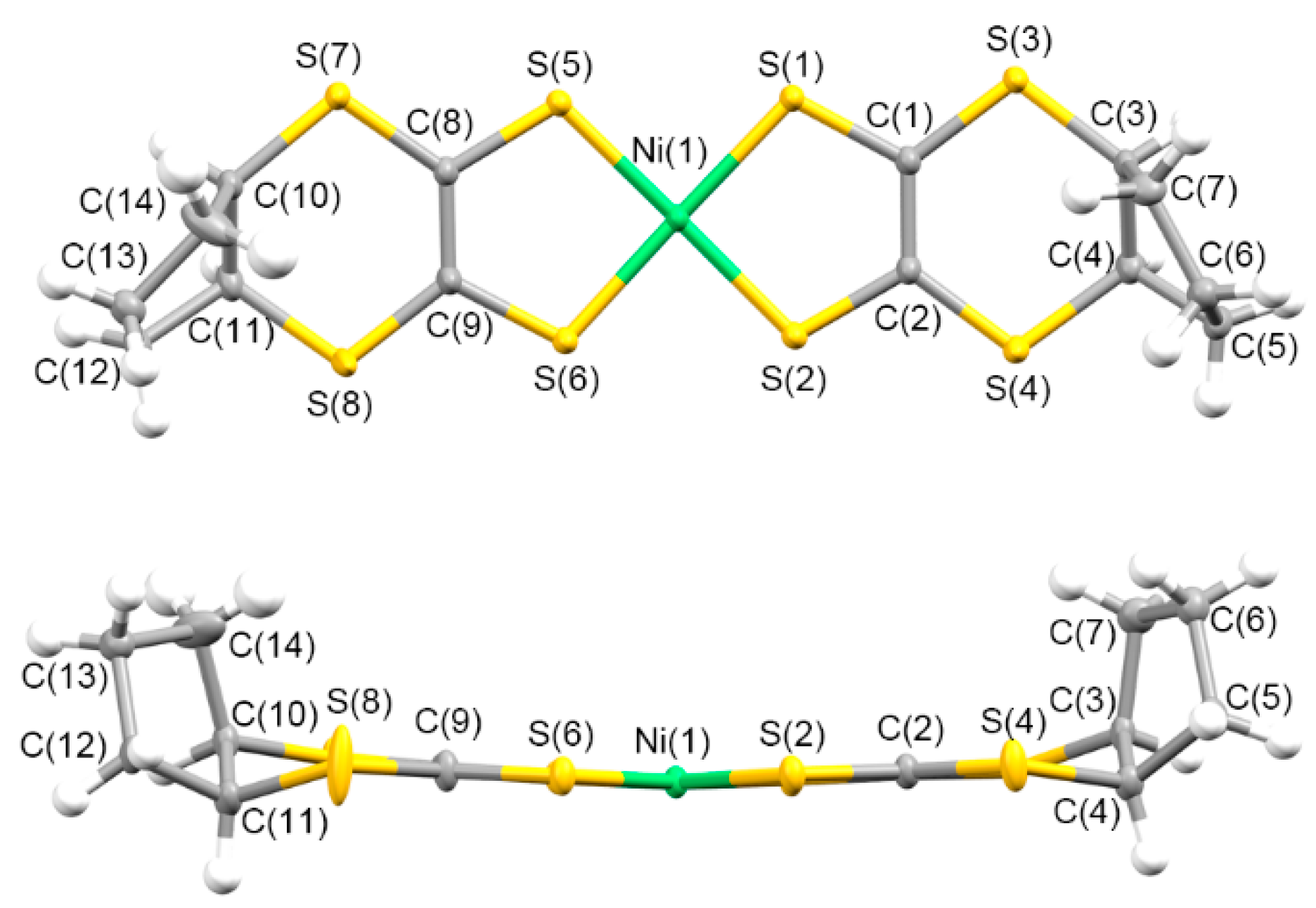
3.1.2. Molecular and Crystal Structures of Neutral Complexes 5–8
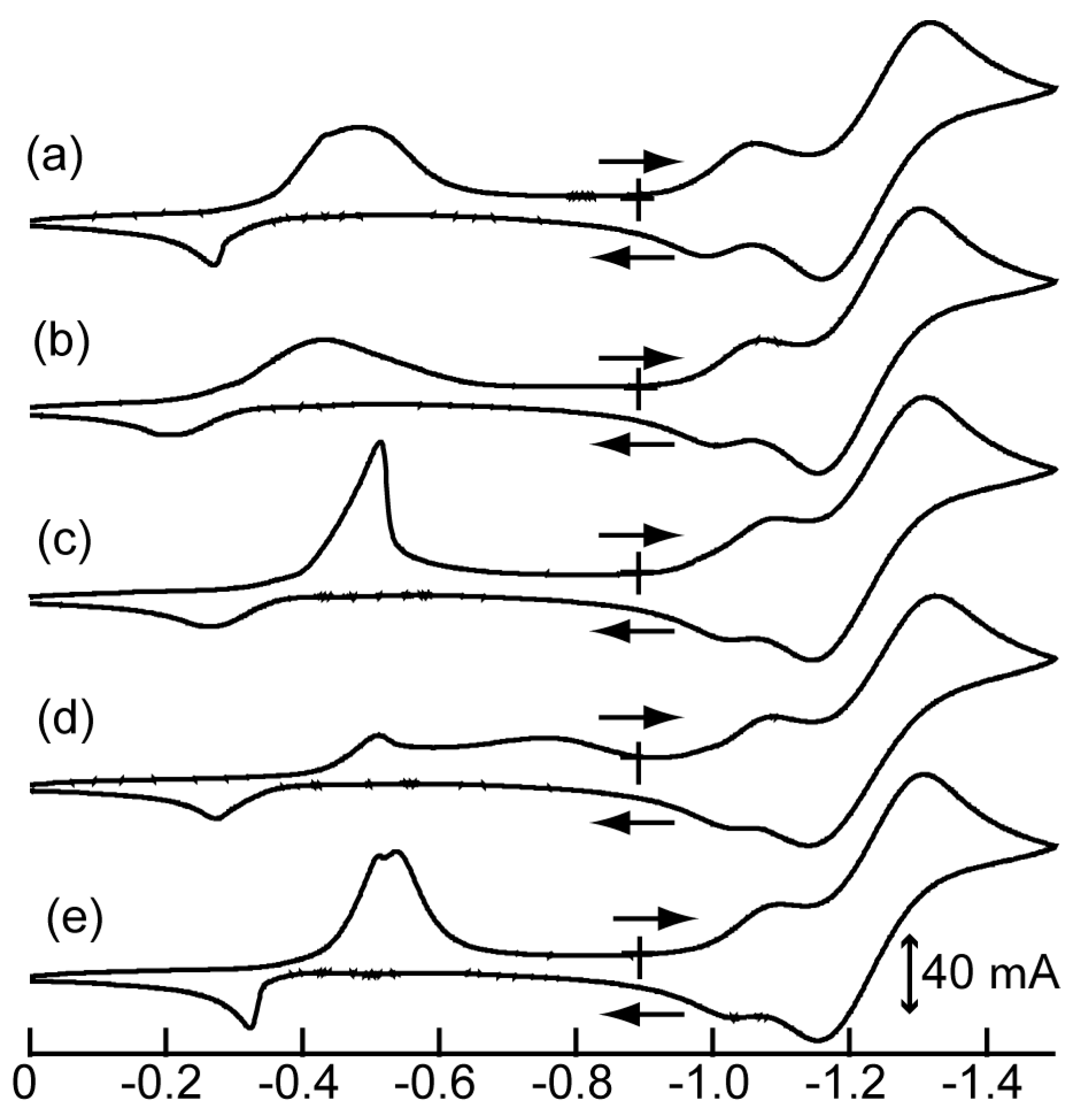
3.2. Electrochemical Properties of 1–4
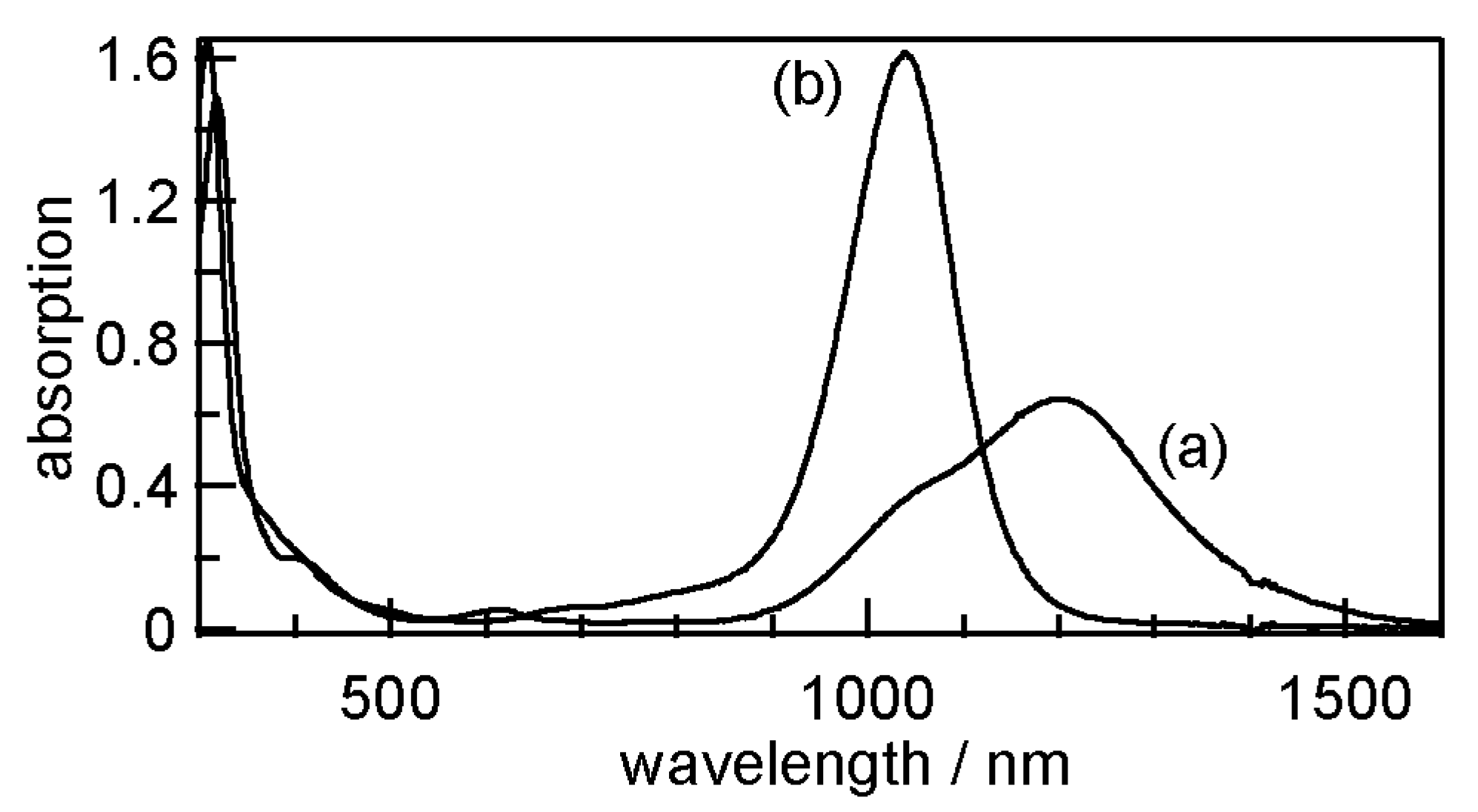
3.3. Electronic Absorption Spectra of 1–8
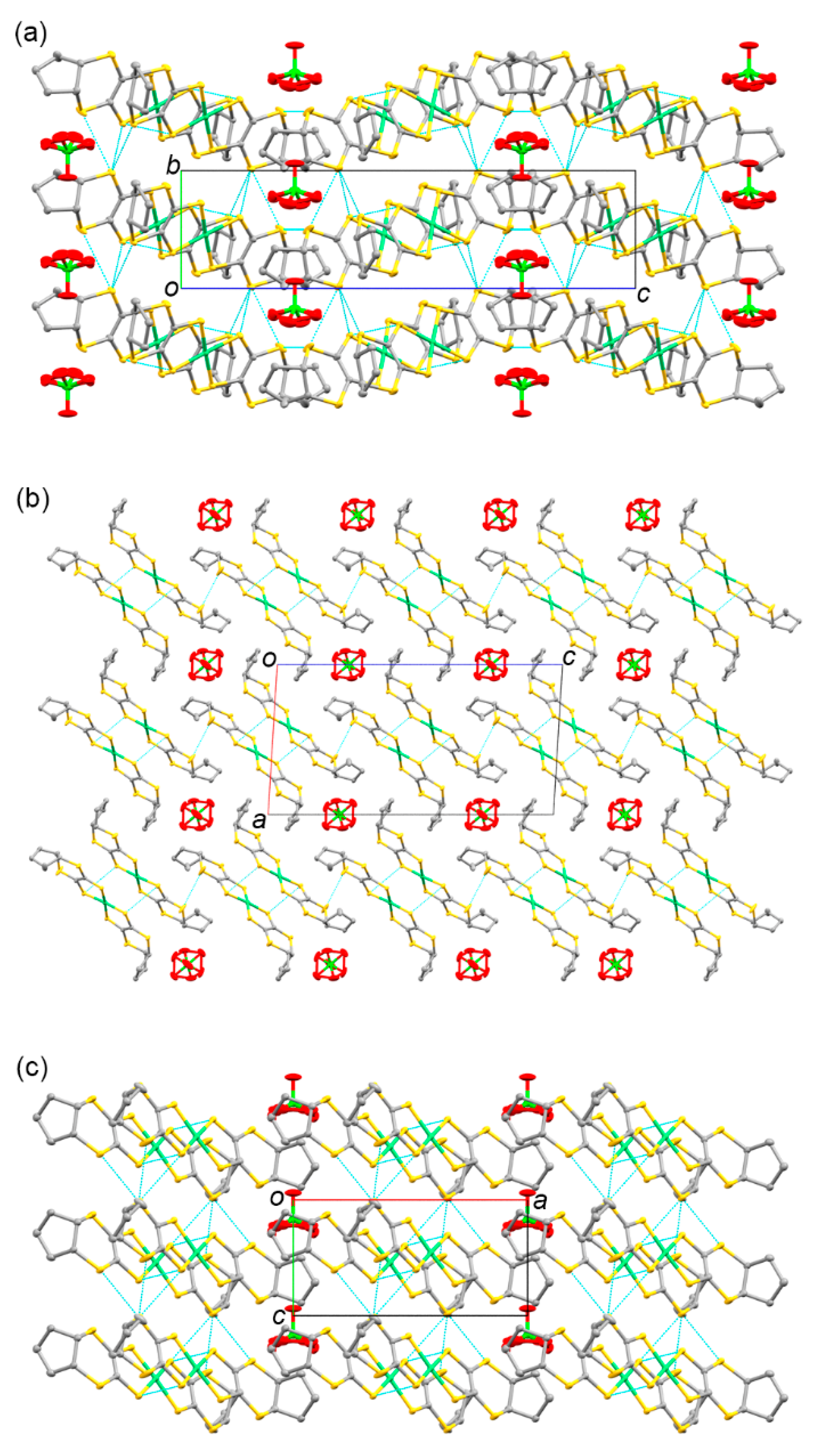
3.4. Structures and Physical Properties of Radical Cation Crystals 9 and 10
3.4.1. Molecular and Crystal Structures of 9 and 10
3.4.2. Molecular Orbitals and Energy Band Calculations of 9 and 10
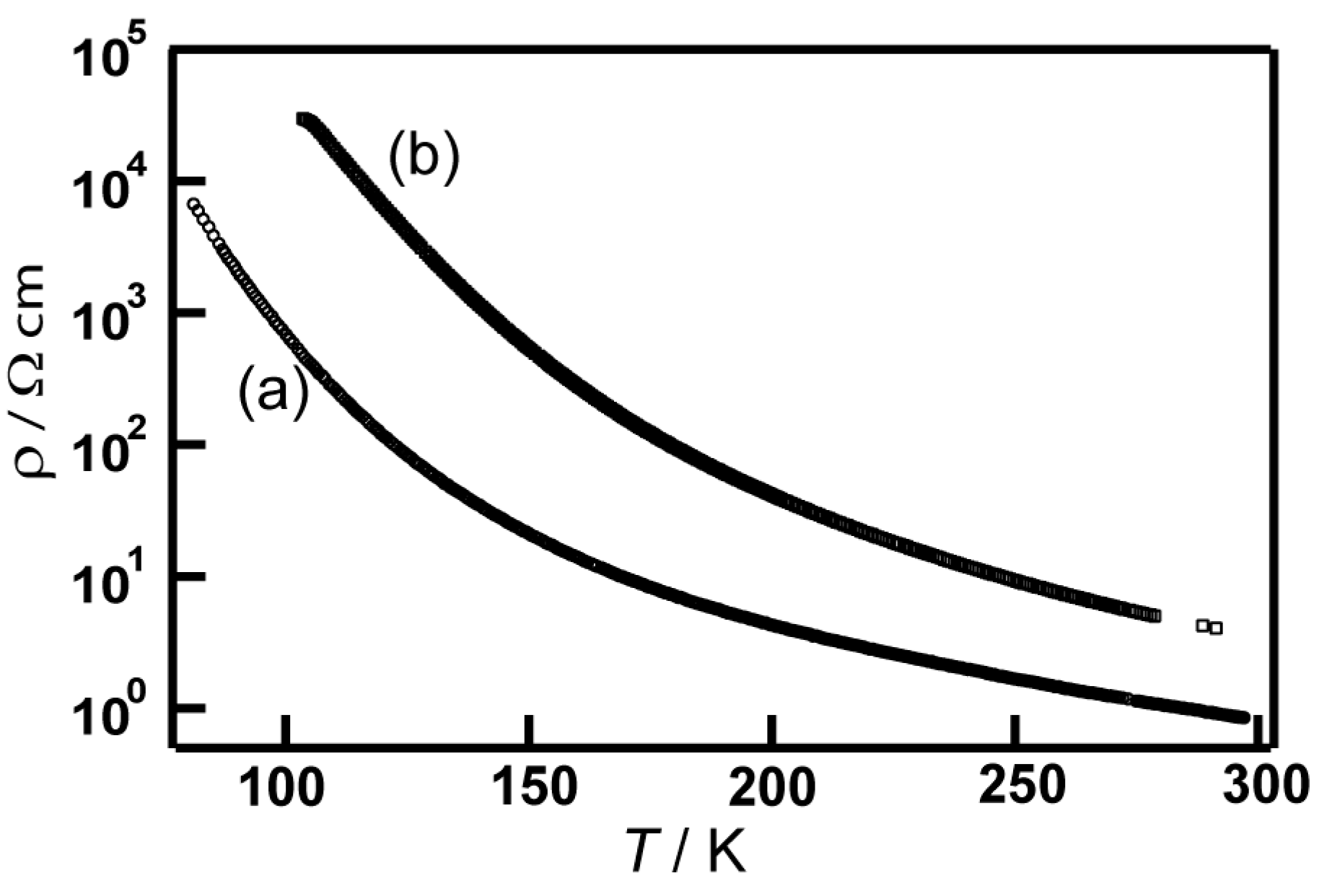
3.4.3. Electrical Conductivities and Magnetic Properties of 9 and 10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Narita, M.; Pittman, C.U., Jr. Preparation of tetrathiafulvalenes (TTF) and their selenium analogs -Tetraselenafulvalenes (TSeF). Synthesis 1976, 489–514. [Google Scholar] [CrossRef]
- Kistenmacher, T.J.; Phillips, T.E.; Cowan, D.O. The crystal structure of the 1:1 radical cation-radical anion salt of 2,2′-bis-l,3-dithiole (TTF) and 7,7,8,8-tetracyanoquinodimethane (TCNQ). Acta Cryst. 1974, B30, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Coleman, L.B.; Cohen, M.J.; Sandman, D.J.; Yamagishi, F.G.; Garito, A.F.; Heeger, A.J. Superconducting fluctuations and the peierls instability in an organic solid. Solid State Commun. 1973, 12, 1125–1132. [Google Scholar] [CrossRef]
- Ferraris, J.; Cowan, D.O.; Walatka, V.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948–949. [Google Scholar] [CrossRef]
- Jerome, D.; Mazaud, A.; Ribault, M.; Bechgaard, K. Superconductivity in a synthetic organic conductor (TMTSF)2PF6. J. Phys. Lett. 1980, 41, L95. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic Superconductors (Including Fullerenes); Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar] [CrossRef]
- Ishiguro, T.; Yamaji, K.; Saito, G. Organic Superconductors, 2nd ed.; Springer Ser. Solid-State Sci.; Springer: Berlin, Germany, 1998; Volume 18. [Google Scholar]
- Fabre, J.M. From the organic molecule to superconductors. J. Phys. IV 2000, 10, Pr3-19–Pr3-33. [Google Scholar] [CrossRef]
- Bechgaard, K.; Jacobsen, C.S.; Mortensen, V.; Thorup, N. The properties of five highly conducting salts: (TMTSF)2X, X = PF6−, AsF6−, SbF6−, BF4− and NO3−, derived from tetramethyltetraselenafulvalene (TMTSF). J. Solid State Commun. 1980, 33, 1119–1125. [Google Scholar] [CrossRef]
- Urayama, H.; Yamochi, H.; Saito, G.; Nozawa, K.; Sugano, T.; Kinoshita, M.; Sato, S.; Oshima, K.; Kawamoto, A.; Tanaka, J. A New ambient pressure organic superconductor based on BEDT-TTF with Tc higher than 10K (Tc = 10.4 K). Chem. Lett. 1988, 55–58. [Google Scholar] [CrossRef]
- Williams, J.M.; Kini, A.M.; Wang, H.H.; Carlson, K.D.; Geiser, U.; Montgomery, L.K.; Pyrka, G.J.; Watkins, D.M.; Kommers, J.K.; Boryschuk, S.J.; et al. Semiconductor-semiconductor transition (42 K) to the highest-Tc organic superconductor, x-(ET)2Cu[N(CN)2]Cl (Tc = 12.5 K). Inorg. Chem. 1990, 29, 3272–3274. [Google Scholar] [CrossRef]
- Papavassiliou, G.C.; Mousdis, G.A.; Zambounis, J.S.; Terzis, A.; Hountas, A.; Hilti, B.; Mayer, C.W.; Pfeiffer, J. Low temperature measurements of the electrical conductivities of some charge transfer salts with the asymmetric donors MDT-TTF, EDT-TTF and EDT-DSDTF. (MDT-TTF)2AuI2, a new superconductor (Tc = 3.5 K at ambient pressure). Synth. Met. 1988, 27, B379–B383. [Google Scholar] [CrossRef]
- Kikuchi, K.; Murata, K.; Honda, Y.; Namiki, T.; Saito, K.; Ishiguro, T.; Kobayashi, K.; Ikemoto, I. On ambient-pressure superconductivity in organic conductors: Electrical properties of (DMET)2I3, (DMET)2I2Br and (DMET)2IBr2. J. Phys. Soc. Jpn. 1987, 56, 3436–3439. [Google Scholar] [CrossRef]
- Shibaeva, R.P.; Yagubskii, E.B. Molecular conductors and superconductors based on trihalides of BEDT-TTF and some of Its analogues. Chem. Rev. 2004, 104, 5347–5378. [Google Scholar] [CrossRef]
- Geiser, U.; Schlueter, J.A. Conducting organic radical cation salts with organic and organometallic anions. Chem. Rev. 2004, 104, 5203–5242. [Google Scholar] [CrossRef]
- Kobayashi, H.; Cui, H.-B.; Kobayashi, A. Organic metals and superconductors based on BETS (BETS = Bis(ethylenedithio)tetraselenafulvalene). Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-Field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tomita, H.; Naito, T.; Kobayashi, A.; Sakai, F.; Watanabe, T.; Cassoux, P. New BETS Conductors with Magnetic Anions (BETS) bis(ethylenedithio)tetraselenafulvalene). J. Am. Chem. Soc. 1996, 118, 368–377. [Google Scholar] [CrossRef]
- Aoyagi, I.; Katsuhara, M.; Mori, T. Syntheses and structures of highly soluble bis(ethylenedithio)tetrathiafulvalene molecules with alkyl chains. Sci. Tech. Adv. Mater. 2004, 5, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y. Effect of methyl substitution on conformation and molecular arrangement of BEDT-TTF derivatives in the crystalline environment. Bull. Chem. Soc. Jpn. 1993, 66, 513–522. [Google Scholar] [CrossRef]
- Riobé, F.; Avarvari, N. Electroactive oxazoline ligands. Coord. Chem. Rev. 2010, 254, 1523–1533. [Google Scholar] [CrossRef]
- Awheda, I.; Krivickas, J.; Yang, S.; Martin, L.; Guziak, M.A.; Brooks, A.C.; Pelletier, F.; Le Kerneau, M.; Day, P.; Horton, P.N.; et al. Synthesis of new chiral organosulfur donors with hydrogen bonding functionality and their first charge transfer salts. Tetrahedron 2013, 69, 8738–8750. [Google Scholar] [CrossRef] [Green Version]
- Biet, T.; Fihey, A.; Cauchy, T.; Vanthuyne, N.; Roussel, C.; Crassous, J.; Avarvar, N. Ethylenedithio-tetrathiafulvalene-helicenes: Electroactive helical precursors with switchable chiroptical properties. Chem. A Eur. J. 2013, 19, 13160–13167. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Pop, F.; Melan, C.; Brooks, A.C.; Martin, L.; Horton, P.; Auban-Senzier, P.; Rikken, G.L.J.A.; Avarvari, N.; Wallis, J.D. Charge transfer complexes and radical cation salts of chiral methylated organosulfur donors. CrystEngComm 2014, 16, 3906–3916. [Google Scholar] [CrossRef] [Green Version]
- Chas, M.; Lemarié, M.; Guleab, M.; Avarvari, N. Chemo- and enantioselective sulfoxidation of bis(ethylenedithio)-tetrathiafulvalene (BEDT-TTF) into chiral BEDT-TTF-sulfoxide. Chem. Commun. 2008, 220–222. [Google Scholar] [CrossRef]
- Chas, M.; Riobé, F.; Sancho, R.; Minguíllon, C.; Avarvari, N. Selective monosulfoxidation of tetrathiafulvalenes into chiral TTF-sulfoxides. Chirality 2009, 21, 818–825. [Google Scholar] [CrossRef]
- Karrer, A.; Wallis, J.D.; Dunitz, J.D.; Hilti, B.; Mayer, C.W.; Biirkle, M.; Pfeiffer, J. Structures and electrical properties of some new organic conductors derived from the donor molecule TMET (S,S,S,S- Bis(dimethylethy1enedithio)tetrathiafulvalene). Helv. Chim. Acta 1987, 70, 942–953. [Google Scholar] [CrossRef]
- Krivickas, S.J.; Ichikawa, A.; Takahashi, K.; Tajima, H.; Wallis, J.D.; Mori, H. One-dimensional antiferromagnetic behavior of a chiral molecular crystal, α’-(S,S-DMBEDT-TTF)2PF6. Synth. Met. 2011, 161, 1563–1565. [Google Scholar] [CrossRef]
- Krivickas, S.J.; Hashimoto, C.; Yoshida, J.; Ueda, A.; Takahashi, K.; Wallis, J.D.; Mori, H. Synthesis of racemic and chiral BEDT-TTF derivatives possessing hydroxy groups and their achiral and chiral charge transfer complexes. Beilstein J. Org. Chem. 2015, 11, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Madalan, A.M.; Réthoré, C.; Fourmigué, M.; Canadell, E.; Lopes, E.B.; Almeida, M.; Auban-Senzier, P.; Avarvari, N. Order versus disorder in chiral tetrathiafulvalene-oxazoline radical-cation salts: Structural and theoretical investigations and physical properties. Chem. Eur. J. 2010, 16, 528–537. [Google Scholar] [CrossRef]
- Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y.; Tokumoto, M. Crystal ctructure and conductivity of chiral radical ion salts (Me2ET)2X. Bull. Chem. Soc. Jpn. 1993, 66, 1949–1954. [Google Scholar] [CrossRef]
- Réthoré, C.; Avarvari, N.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M. Chiral molecular metals: Syntheses, structures, and properties of the AsF6-salts of racemic (±)-, (R)-, and (S)-tetrathiafulvalene-oxazoline derivatives. J. Am. Chem. Soc. 2005, 127, 5748–5749. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Allain, M.; Auban-Senzier, P.; Martínez-Lillo, J.; Lloret, F.; Julve, M.; Canadell, E.; Avarvari, N. Enantiopure conducting salts of dimethylbis(ethylenedithio)tetrathiafulvalene (DM-BEDT-TTF) with the hexachlororhenate(IV) anion. Eur. J. Inorg. Chem. 2014, 24, 3855–3862. [Google Scholar] [CrossRef]
- Avarvari, N.; Wallis, J.D. Strategies towards chiral molecular conductors. J. Mater. Chem. 2009, 19, 4061–4076. [Google Scholar] [CrossRef] [Green Version]
- Zambounis, J.S.; Muyer, C.W.; Huuenstein, K.; Hilti, B.; HoJherr, W.; Pfegfer, J.; Biirkle, M.; Rihs, G. Crystal structure and electrical properties of κ-((S,S)-DMSEDT-TTF)2ClO4. Adv. Mater. 1992, 4, 33–35. [Google Scholar] [CrossRef]
- Yang, S.; Brooks, A.C.; Martin, L.; Day, P.; Li, H.; Horton, P.; Malec, L.; Wallis, J.D. Novel enantiopure bis(pyrrolo)tetrathiafulvalene donors exhibiting chiral crystal packing arrangements. CrystEngComm 2009, 11, 993–996. [Google Scholar] [CrossRef]
- Wallis, J.D.; Karrer, A.; Dunitz, J.D. Chiral metals? A chiral substrate for organic conductors and superconductors. Helv. Chim. Acta 1986, 69, 69–70. [Google Scholar] [CrossRef]
- Short, J.I.; Blundell, T.J.; Krivickas, S.J.; Yang, S.; Wallis, J.D.; Akutsu, H.; Nakazawa, Y.; Martin, L. Chiral molecular conductor with an insulator–metal transition close to room temperature. Chem. Commun. 2020, 56, 9497–9500. [Google Scholar] [CrossRef]
- Saad, A.; Jeannin, O.; Fourmigué, M. Chiral, flexible binaphthol-substituted tetrathiafulvalenes. Tetrahedron 2011, 67, 3820–3829. [Google Scholar] [CrossRef]
- Pop, F.; Auban-Senzier, P.; Canadell, E.; Avarvari, N. Anion size control of the packing in the metallic versus semiconducting chiral radical cation salts (DM-EDT-TTF)2XF6 (X = P, As, Sb). Chem. Commun. 2016, 52, 12438–12441. [Google Scholar] [CrossRef] [Green Version]
- Pop, F.; Auban-Senzier, P.; Frąckowiak, A.; Ptaszyński, K.; Olejniczak, I.; Wallis, J.D.; Canadell, E.; Avarvari, N. Chirality driven metallic versus semiconducting behavior in a complete series of radical cation salts based on dimethyl-ethylenedithio-tetrathiafulvalene (DM-EDT-TTF). J. Am. Chem. Soc. 2013, 135, 17176–17186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán-Mascarós, J.R.; Coronado, E.; Goddard, P.A.; Singleton, J.; Coldea, A.I.; Wallis, J.D.; Coles, S.J.; Alberola, A. A chiral ferromagnetic molecular metal. J. Am. Chem. Soc. 2010, 132, 9271–9273. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Maejima, T.; Suzuki, H.; Chiba, R.; Mori, H.; Kawamoto, T.; Mori, T.; Moriyama, H.; Nishio, Y.; Kajita, K. A new organic superconductor β-(meso-DMBEDT-TTF)2PF6. Chem. Commun. 2004, 2454–2455. [Google Scholar] [CrossRef]
- Kimura, S.; Suzuki, H.; Maejima, T.; Mori, H.; Yamaura, J.; Kakiuchi, T.; Sawa, H.; Moriyama, H. Checkerboard-type charge-ordered state of a pressure-induced superconductor, β-(meso-DMBEDT-TTF)2PF6. J. Am. Chem. Soc. 2006, 128, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamashita, K.; Suto, M.; Maejima, T.; Kimura, S.; Mori, H.; Nishio, Y.; Kajita, K.; Moriyama, H. Control of electronic state in the 2D hydrogen-bonded system: β″-(CnDT-EDO-TTF)2(PF6)x (n = 5, 6, 7, 8). Synth. Met. 2004, 144, 89–95. [Google Scholar] [CrossRef]
- Kimura, S.; Suzuki, H.; Maejima, T.; Suto, M.; Yamashita, K.; Ichikawa, S.; Mori, H.; Moriyama, H.; Mochida, T.; Nishio, Y.; et al. Crystal structures and electrical conductivities controlled by CH/n interactions. J. Phys. IV 2004, 114, 521–522. [Google Scholar] [CrossRef]
- Kato, R. Conducting metal dithiolene complexes: Structural and electronic properties. Chem. Rev. 2004, 104, 5319–5346. [Google Scholar] [CrossRef]
- Kobayashi, A.; Fujiwara, E.; Kobayashi, H. Single-component molecular metals with extended-TTF Dithiolate ligands. Chem. Rev. 2004, 104, 5243–5264. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Okano, Y.; Kobayashi, H.; Suzuki, W.; Kobayashi, A. A three-dimensional synthetic metallic crystal composed of single-component molecules. Science 2001, 291, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.M.; Pan, B.L.; Song, B.Q.; Huang, Y.Y.; Zeng, J.Y.; Yu, Y.J.; Cheng, E.J.; Wang, L.S.; Dai, D.Z.; Kato, R.; et al. Absence of magnetic thermal conductivity in the quantum spin liquid candidate EtMe3Sb[Pd(dmit)2]2. Phys. Rev. Lett. 2019, 123, 247204. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois-Hope, P.; Laliberté, F.; Lefrançois, E.; Grissonnanche, G.; de Cotret, S.R.; Gordon, R.; Kitou, S.; Sawa, H.; Cui, H.; Kato, R.; et al. Thermal conductivity of the quantum spin liquid candidate EtMe3Sb[Pd(dmit)2]2: No evidence of mobile gapless excitations. Phys. Rev. X 2019, 9, 041051. [Google Scholar] [CrossRef] [Green Version]
- Itou, T.; Watanabe, E.; Maegawa, S.; Tajima, A.; Tajima, N.; Kubo, K.; Kato, R.; Kanoda, K. Slow dynamics of electrons at a metal–Mott insulator boundary in an organic system with disorder. Sci. Adv. 2017, 3, e1601594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyagawa, K.; Kanoda, K. NMR studies on two-dimensional molecular conductors and superconductors: Mott transition in κ-(BEDT-TTF)2X. Chem. Rev. 2004, 104, 5635–5653. [Google Scholar] [CrossRef]
- Kato, R.; Cui, H.B.; Tsumuraya, T.; Miyazaki, T.; Suzumura, Y. Emergence of the Dirac electron system in a single-component molecular conductor under high pressure. J. Am. Chem. Soc. 2017, 139, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Fox, S.B.; Yagubskii, E.B.; Kushch, L.A.; Kotov, A.I.; Whangbo, M.-H. Direct observation of the electron-donating property of the 5,6-dihydro-1,4-dithiin-2,3-dithiolate (dddt) ligands in square planar M(dddt)2 complexes (M = Ni, Pd, Pt, and Au). J. Am. Chem. Soc. 1997, 119, 7601–7602. [Google Scholar] [CrossRef]
- Cui, H.; Tsumuraya, T.; Yeung, H.H.-M.; Coates, C.S.; Warren, M.R.; Kato, R. High Pressure Crystal Structure and Electrical Properties of a Single Component Molecular Crystal [Ni(dddt)2] (dddt = 5,6-dihydro-1,4-dithiin-2,3-dithiolate). Molecules 2019, 24, 1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushch, L.A.; Gritsenko, V.V.; Buravov, L.I.; Khomenko, A.G.; Shilov, G.V.; Dyachenko, O.A.; Merzhanov, V.A.; Yagubskii, E.B.; Rousseau, R.; Canadell, E. The M(dddt)2 family of conducting complexes: [Ni(dddt)2]3(AuBr2)2, the first quasi-two-dimensional metal stable down to at least 1.3 K. J. Mater. Chem. 1995, 5, 1633–1638. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Kato, R.; Suzumura, Y. Effective Hamiltonian of topological nodal line semimetal in single-component molecular conductor [Pd(dddt)2] from first-principles. J. Phys. Soc. Jpn. 2018, 87, 113701-1–113701-5. [Google Scholar] [CrossRef]
- Kato, R.; Suzumura, Y. Novel Dirac electron in single-component molecular conductor [Pd(dddt)2] (dddt = 5,6-dihydro-1,4-dithiin-2,3-dithiolate). J. Phys. Soc. Jpn. 2017, 86, 064705. [Google Scholar] [CrossRef]
- Suzumura, Y.; Cui, H.B.; Kato, R. Conductivity and resistivity of Dirac electrons in single-component molecular conductor [Pd(dddt)2]. J. Phys. Soc. Jpn. 2018, 87, 084702. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, H.; Wang, Z.F.; Yang, J.; Liu, F. Pressure-induced organic topological nodal-line semimetal in the three-dimensional molecular crystal Pd(dddt)2. Phys. Rev. B 2018, 97, 155138. [Google Scholar] [CrossRef] [Green Version]
- Suzumura, Y.; Tsumuraya, T.; Kato, R.; Matsuura, H.; Ogata, M. Role of Velocity Field and Principal Axis of Tilted Dirac Cones in Effective Hamiltonian of Non-Coplanar Nodal Loop. J. Phys. Soc. Jpn. 2019, 88, 124704. [Google Scholar] [CrossRef]
- Suzumura, Y.; Kato, R.; Ogata, M. Electric transport of nodal line semimetals in single-component molecular conductors. Crystals 2020, 10, 862. [Google Scholar] [CrossRef]
- Kato, R.; Kashimura, Y.; Sawa, H.; Okano, Y. Synthesis, structure, and electrochemical properties of new “unsymmetrical” metal dithiolate complexes. Chem. Lett. 1997, 26, 921–922. [Google Scholar] [CrossRef]
- Kashimura, Y.; Okano, Y.; Yamaura, J.; Kato, R. Synthesis, structures, and physical properties of molecular conductors based on unsymmetrical metal-dithiolene complexes. Synth. Met. 1999, 103, 2123–2124. [Google Scholar] [CrossRef]
- Kini, A.M.; Geiser, U.; Wang, H.-H.; Lykke, K.R.; Williams, J.M.; Campana, C.F. Alternative synthesis of (z),(z)-4,5;4’,5’-bis(1,4-dioxanediyl-2,3-dithio)tetrathiafulvalene (cis,cis-BDDT-TTF), and preparation, epr properties and structure of the radical cation salt (cis,cis-BDDT-TTF)2I3. J. Mater. Chem. 1995, 5, 1647–1652. [Google Scholar] [CrossRef]
- Kotov, A.I.; Buravov, L.I.; Konovalikhin, S.V.; Dyachenko, O.A.; Yagubskii, E.B.; Malfant, I.; Courcet, T.; Cassoux, P.; Akimoto, J.; Honda, K.; et al. BEDT-TTF Derivatives with one and two dioxane rings and their conductive salts. Synth. Met. 1999, 102, 1630–1631. [Google Scholar] [CrossRef]
- Nishikawa, H.; Morimoto, T.; Kodama, T.; Ikemoto, I.; Kikuchi, K.; Yamada, J.; Yoshino, H.; Murata, K. New organic superconductors consisting of an unprecedented π-electron donor. J. Am. Chem. Soc. 2002, 124, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sato, Y.; Kikuchi, K.; Kodama, T.; Ikemoto, I.; Yamada, J.; Oshio, H.; Kondo, R.; Kagoshima, S. Charge ordering and pressure-induced superconductivity in β’’-(DODHT)2PF6. Phys. Rev. B 2005, 72, 052510-1–052510-4. [Google Scholar] [CrossRef]
- Svenstrup, N.; Becher, J. The organic chemistry of 1,3-Dithiole-2-thione-4,5-dithiolate (DMIT). Synthesis 1995, 215–235. [Google Scholar] [CrossRef]
- Walker, I.R. Nonmagnetic piston–cylinder pressure cell for use at 35 kbar and above. Rev. Sci. Instrum. 1999, 70, 3402–3412. [Google Scholar] [CrossRef]
- Ishii, Y.; Tamura, M.; Kato, R.; Hedo, M.; Uwatoko, Y.; Môri, N. Effect of hydrostatic pressure on molecular conductors, Pd(dmit)2 salts. Synth. Met. 2005, 152, 389–392. [Google Scholar] [CrossRef]
- Albright, T.A.; Hoffmann, P.; Hoffmann, R. Conformational preferences and rotational barriers in polyene-ML3 transition metal complexes. J. Am. Chem. Soc. 1977, 99, 7546–7557. [Google Scholar] [CrossRef]
- Chen, M.M.L.; Hoffmann, R. Sulfuranes. Theoretical aspects of bonding, substituent site preferences, and geometrical distortions. J. Am. Chem. Soc. 1976, 98, 1647–1653. [Google Scholar] [CrossRef]
- Komiya, S.; Albright, T.A.; Hoffmann, R. Reductive elimination and isomerization of organogold complexes. Theoretical studies of trialkylgold species as reactive intermediates. J. Am. Chem. Soc. 1976, 98, 7255–7265. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software system, version 1.171.40.67a; Rigaku Corporation: Wroclaw, Poland, 2012. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Khodorkovsky, V.Y.; Becker, J.Y.; Bernstein, J. [2 + 4] Cycloaddition to tetrathiafulvalene (TTF): A new route to multisulfur TTF derivatives. Synthesis 1992, 1071–1072. [Google Scholar] [CrossRef]
- Gillman, J.; Hartke, K. Thion- und Dithioester, 40. [2 + 4]-Cycloadditionen von Tetrathiooxalsäuredimethylester an cyclisch konjugierte, mehrfach ungesättigte Verbindungen. Chem. Ber. 1986, 119, 2859–2867. [Google Scholar] [CrossRef]
- Medne, R.S.; Katsens, Y.Y.; Kraupsha, I.L.; Neiland, O.Y. Bis(1,2-cyclopentylenedithio)- and bis(1,2-cyclohexylenedithio)tetrathiafulvalenes. Chem. Heterocycl. Compd. 1991, 27, 1053–1055. [Google Scholar] [CrossRef]
- Kotov, A.I.; Faulmann, C.; Cassoux, P.; Yagubskii, E.B. New π-donor precursor molecule for the preparation of organic metals, 4,5;4’,5’-bis(l,4-dioxanediyl-2,3-dithio)-tetrathiafulvalene: Synthesis and properties. J. Org. Chem. 1994, 59, 2626–2629. [Google Scholar] [CrossRef]
- Bai, J.-F.; Zuo, J.-L.; Shi, F.-N.; Jin, R.; Shen, Z.; You, X.-Z. Synthesis and crystal structure of 4,5-(cis-cyclohexylenedithio)-1,3-dithiole-2-one. J. Chem. Crystallogr. 1999, 29, 719–723. [Google Scholar] [CrossRef]
- Kim, H.; Kobayashi, A.; Sasaki, Y.; Kato, R.; Kobayashi, H.; Nakamura, T.; Nogami, T.; Shirota, Y. Crystal and molecular structure of tetrabutylammonium bis(1,4-dithiin-2,3-dithiolato)nickelate(III), (Bun4N)[Ni(ddt)2]. Bull. Chem. Soc. Jpn. 1988, 61, 2559–2562. [Google Scholar] [CrossRef] [Green Version]
- Bigoli, F.; Deplano, P.; Devillanova, F.A.; Lippolis, V.; Lukes, P.J.; Mercuri, M.L.; Pellinghelli, M.A.; Trogu, E.F. New neutral nickel dithiolene complexes derived from 1,3-dialkylimidazolidine-2,4,5-trithione, showing remarkable near-IR absorption. J. Chem. Soc. Chem. Commun. 1995, 371–372. [Google Scholar] [CrossRef]
- Bigoli, F.; Deplano, P.; Mercuri, M.L.; Pellinghelli, M.A.; Pintus, G.; Trogu, E.F.; Zonnedda, G.; Wang, H.H.; Williams, J.M. Novel oxidation and reduction products of the neutral nickel-dithiolene Ni(Pr2itimdt)2 (Pr2itimdt is the monoanion of 1,3-diisopropylimidazolidine-2,4,5-trithione). Inorg. Chim. Acta 1998, 273, 175–183. [Google Scholar] [CrossRef]
- Bigoli, F.; Deplano, P.; Devillanova, F.A.; Ferraro, J.R.; Lippolis, V.; Lukes, P.J.; Mercuri, M.L.; Pellinghelli, M.A.; Trogu, E.F.; Williams, J.M. Syntheses, X-ray crystal structures, and spectroscopic properties of new nickel-dithiolenes and related compounds. Inorg. Chem. 1997, 36, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Hill, C.A.S.; Underhill, A.E.; Malik, K.M.A.; Hursthouse, M.B.; Karaulov, A.I.; Møller, J. Synthesis, physical properties and x-ray crystal structures of a series of nickel complexes based on n-alkylthio-substituted ethylene-1,2-dithiolene ligands. J. Mater. Chem. 1994, 4, 1861–1866. [Google Scholar] [CrossRef]
- Zuo, J.-L.; Yao, T.-M.; You, F.; You, X.-Z.; Fun, H.-K.; Yip, B.-C. Syntheses, characterization and non-linear optical properties of nickel complexes of multi-sulfur 1,2-dithioiene with strong near-IR absorption. J. Mater. Chem. 1996, 6, 1633–1637. [Google Scholar] [CrossRef]
- Zuo, J.-L.; You, F.; You, X.-Z.; Fun, H.-K. Syntheses and properties of the neutral nickel complexes of 1,2-dithiolates MEDT and PHDT. The crystal structure of [Ni(MEDT)2]. Polyhedron 1997, 16, 1465–1469. [Google Scholar] [CrossRef]
- Proctor, K.A.; Boyle, P.D.; Bereman, R.D. The synthesis and characterization of new neutral metal complexes containing the tetrathioethylene unit. J. Coord. Chem. 1996, 39, 43–58. [Google Scholar] [CrossRef]
- Schultz, A.J.; Wang, H.H.; Soderholm, L.C.; Sifter, T.L.; Williams, J.M.; Bechgaard, K.; Whangbo, M.H. Crystal structures of bis(5,6-dihydro-1,4-dithiin-2,3-dithiolato)gold and tetrabutylammonium bis(5,6-dihydro-1,4-dithiin-2,3-dithiolato)nickelate(1-) and the ligandlike character of the isoelectronic radicals [Au(DDDT)2]0 and [Ni(DDDT)2]-. Inorg. Chem. 1987, 26, 3757–3761. [Google Scholar] [CrossRef]
- Ji, Y.; Zuo, J.-L.; Chen, L.; Tian, Y.-Q.; Song, Y.; Li, Y.-Z.; You, X.-Z. Synthesis, characterization and optical limiting effect of nickel complexes of multi-sulfur 1,2-dithiolene. J. Phys. Chem. Solids 2005, 66, 207–212. [Google Scholar] [CrossRef]
- Bai, J.-F.; Zuo, J.-L.; Tan, W.-L.; Ji, W.; Shen, Z.; Fun, H.-K.; Chinnakali, K.; Razak, I.A.; You, X.-Z.; Che, C.-M. Synthesis, structure and optical limiting effect of two new nickel complexes containing strongly bound geometrically fixed multi-sulfur 1,2-dithiolene ligands showing remarkable near-IR absorption. J. Mater. Chem. 1999, 9, 2419–2423. [Google Scholar] [CrossRef]
- Mueller-Westerhoff, U.T.; Vance, B.; Yoon, D.I. The synthesis of dithiolene dyes with strong near-IR absorption. Tetrahedron 1991, 47, 909–932. [Google Scholar] [CrossRef]
- Herman, Z.S.; Kirchner, R.F.; Loew, G.H.; Mueller-Westerhoff, U.T.; Nazzal, A.; Zerner, M.C. Electronic spectra and structure of bis(ethylene-1,2-dithiolato)nickel and bis-(propene-3-thione-1-thiolato)nickel. Inorg. Chem. 1982, 21, 46–56. [Google Scholar] [CrossRef]
- Tan, W.L.; Ji, W.; Zuo, J.L.; Bai, J.F.; You, X.Z.; Lim, J.H.; Yang, S.; Hagan, D.J.; Van Stryland, E.W. Optical-limiting properties of neutral nickel-dithiolenes. Appl. Phys. B 2000, 70, 809–812. [Google Scholar] [CrossRef]
- Lee, H.-J.; Noh, D.-Y. Electrochemistry and strong near-IR absorption of the [Ni(dphdt)2]n complexes (n = −1, 0; dphdt = 5,6-diphenyl-1,4-dithiin-2,3-dithiolate); X-ray crystal structure of (Ph4P)[Ni(dphdt)2](CH2Cl2). Polyhedron 2000, 19, 425–429. [Google Scholar] [CrossRef]
- Nagapetyan, S.S.; Shklover, V.E.; Struchkov, Y.T.; Kotov, A.I.; Yagubskii, E.B.; Ukhin, L.Y. Synthesis andthe X-ray study of [Ni(DDDT)2]3(ClO4)2. Trimeric cationic dithiolate complex—a precursor of low-dimensional metals of a new type. Dokl. Akad. Nauk SSSR 1990, 310, 94–98. [Google Scholar]
- Yagubskii, E.B. Effect of metal (M) and counter ion nature on crystal structure and conducting properties of M(dddt)2 cation complexes. Synth. Met. 2003, 133–134, 385–387. [Google Scholar] [CrossRef]
- Kotov, A.I.; Buravov, L.I.; Yagubskii, E.B.; Khasanov, S.S.; Zorina, L.V.; Shibaeva, R.P.; Canadell, E. Comparative study of BEDT-TTF and Ni(dddt)2 electroconducting salts with the HXO4 (X = Se, S) anions. Synth. Met. 2001, 124, 357–362. [Google Scholar] [CrossRef]
- Yagubskii, E.B.; Kotov, A.I.; Khomenko, A.G.; Buravov, L.I.; Schegolev, A.I.; Shibaeva, R.P. [Ni(dddt)2]3(HSO4)2, the first metal among the M(dddt)2 salts. Synth. Met. 1992, 46, 255–259. [Google Scholar] [CrossRef]
- Yagubskii, E.B.; Kotov, A.I.; Laukhina, E.E.; Ignatiev, A.A.; Buravov, L.I.; Khomenko, A.G.; Shklover, V.E.; Nagapetyan, S.S.; Struchkov, Y.T. New class of metal BIS-dithiolene electroconducting solids: Cation complexes of metals with dddt. Synth. Met. 1991, 42, 2515–2522. [Google Scholar] [CrossRef]
- Kubo, K.; Nakao, A.; Ishii, Y.; Yamamoto, T.; Tamura, M.; Kato, R.; Yakushi, K.; Matsubayashi, G. Electrical Properties and Electronic States of Molecular Conductors Based on Unsymmetrical Organometallic-Dithiolene Gold(III) Complexes. Inorg. Chem. 2008, 47, 5495–5502. [Google Scholar] [CrossRef] [PubMed]







| Complex | Observed (Calculation Result) (%) | Complex | Observed (Calculation Result) (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Formula | C | H | N | Formula | C | H | Ni | S |
| 1 | 48.32 | 6.91 | 1.89 | 5 | 33.59 | 3.14 | 11.58 | 51.33 |
| C30H52NNiS8 | (48.56) | (7.06) | (1.89) | C14H16NiS8 | (33.66) | (3.23) | (11.75) | (51.36) |
| 2 | 49.65 | 7.26 | 1.85 | 6 | 36.21 | 3.70 | 11.08 | 48.60 |
| C32H56NNiS8 | (49.91) | (7.33) | (1.82) | C16H20NiS8 | (36.43) | (3.82) | (11.13) | (48.63) |
| 3 | 50.88 | 7.50 | 1.67 | 7 | 38.87 | 4.26 | 10.44 | 46.40 |
| C34H60NNiS8 | (51.17) | (7.58) | (1.76) | C18H24NiS8 | (38.91) | (4.35) | (10.56) | (46.17) |
| 4 | 51.90 | 7.71 | 1.70 | 8 | 41.00 | 4.81 | 9.81 | 43.86 |
| C36H64NNiS8 | (52.34) | (7.81) | (1.70) | C20H28NiS8 | (41.16) | (4.84) | (10.06) | (43.95) |
| L2 | L3 | L4 | |
|---|---|---|---|
| Chemical formula | C7.2H8S4 | C8H9.60S4 | C7.34H9.34S3.34 |
| Crystal size/mm3 | 0.40 × 0.10 × 0.04 | 0.30 × 0.06 × 0.03 | 0.40 × 0.03 × 0.02 |
| Formula weight | 222.78 | 234.00 | 204.45 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c | P2/c |
| a/Å | 12.7079(3) | 5.3224(3) | 11.2835(7) |
| b/Å | 9.8356(2) | 21.7855(10) | 6.8878(3) |
| c/Å | 18.7846(4) | 10.5687(5) | 17.1727(13) |
| α/° | 90 | 90 | 90 |
| β/° | 105.640(2) | 95.793(5) | 106.533(7) |
| γ/° | 90 | 90 | 90 |
| V/Å3 | 2260.95(9) | 1219.19(11) | 1279.46(14) |
| Z | 10 | 5 | 6 |
| T/K | 93 | 93 | 93 |
| μ(Mo Kα)/mm−1 | 0.980 | 0.913 | 0.874 |
| Dcalc/g cm−3 | 1.636 | 1.594 | 1.592 |
| F(000) | 1152 | 608 | 640 |
| λ (Mo-Kα)/Å | 0.71073 | 0.71073 | 0.71073 |
| Measured 2θ range/° | 4.75–60.98 | 3.938–60.722 | 3.766–60.41 |
| No. of reflections collected | 22,206 | 10,849 | 11,351 |
| Independent reflections | 16,862 | 7823 | 6705 |
| Observed reflections with I > 2.00 σ(I) | 5456 | 2882 | 2973 |
| Rint | 0.0191 | 0.0291 | 0.0255 |
| R(F2) (I > 2.00σ(I)) a | 0.0211 | 0.0293 | 0.0267 |
| wR(F2) (all data) b | 0.0537 | 0.0740 | 0.0654 |
| Goodness of fit (GOF) | 1.064 | 1.056 | 1.041 |
| CCDC number | 2,103,657 | 2,103,658 | 2,103,659 |
| 5 | 6 | 7 | 8 | |
|---|---|---|---|---|
| Chemical formula | C14H16NiS8 | C16H20NiS8 | C7.2H9.6Ni0.4S3.2 | C6.67H9.32Ni0.33S2.66 |
| Crystal size/mm3 | 0.14 × 0.11 × 0.06 | 0.16 × 0.09 × 0.06 | 0.07 × 0.04 × 0.01 | 0.18 × 0.07 × 0.07 |
| Formula weight | 499.46 | 527.51 | 222.22 | 194.41 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/n | P21 | P21/n | P-1 |
| a/Å | 6.2830(2) | 8.0303(3) | 12.5305(6) | 6.8973(10) |
| b/Å | 9.8825(3) | 8.8084(4) | 6.7500(3) | 8.6190(13) |
| c/Å | 15.2978(4) | 14.8128(5) | 13.6312(6) | 11.5730(15) |
| α/° | 90 | 90 | 90 | 69.507(13) |
| β/° | 100.685(3) | 105.033(4) | 108.057(5) | 85.237(11) |
| γ/° | 90 | 90 | 90 | 71.298(13) |
| V/Å3 | 933.40(5) | 1011.91(7) | 1096.15(9) | 610.09(16) |
| Z | 2 | 2 | 5 | 3 |
| T/K | 93 | 93 | 93 | 93 |
| μ(Mo Kα)/mm−1 | 1.928 | 1.783 | 1.651 | 1.486 |
| Dcalc/g cm−3 | 1.777 | 1.731 | 1.683 | 1.587 |
| F(000) | 512 | 544 | 576 | 304 |
| λ (Mo-Kα)/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Measured 2θ range/° | 4.938–60.77 | 5.224–60.742 | 5.376–61.08 | 3.75–60.606 |
| No. of reflections collected | 10,661 | 9330 | 9850 | 9364 |
| Independent reflections | 7606 | 6979 | 6434 | 3699 |
| Observed reflections with I > 2.00σ(I) | 2215 | 4857 | 2436 | 2568 |
| Rint | 0.0182 | 0.0187 | 0.0226 | 0.0267 |
| R(F2) (I > 2.00σ(I)) a | 0.0237 | 0.0499 | 0.0252 | 0.0827 |
| wR(F2) (all data) b | 0.0595 | 0.1305 | 0.0544 | 0.1977 |
| GOF | 1.056 | 1.215 | 1.051 | 1.185 |
| Flack parameter | 0.328(7) | |||
| CCDC number | 2,103,660 | 2,103,661 | 2,103,662 | 2,103,663 |
| 9 | 10 | |
|---|---|---|
| Chemical formula | C7H8Cl0.25Ni0.50OS4 | C7H8F1.5Ni0.5P0.25S4 |
| Crystal size/mm3 | 0.12 × 0.12 × 0.08 | 0.14 × 0.07 × 0.01 |
| Formula weight | 274.59 | 285.97 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P2/c | P2/c |
| a/Å | 12.6888(3) | 12.6865(4) |
| b/Å | 6.25150(10) | 6.3020(2) |
| c/Å | 24.1229(6) | 24.3145(8) |
| α/° | 90 | 90 |
| β/° | 93.462(2) | 92.900(3) |
| γ/° | 90 | 90 |
| V/Å3 | 1910.03(7) | 1941.46(11) |
| Z | 8 | 8 |
| T/K | 93 | 93 |
| μ(Mo Kα)/mm−1 | 1.969 | 1.928 |
| Dcalc/g cm−3 | 1.910 | 1.957 |
| F(000) | 1122 | 1162 |
| λ (Mo-Kα)/Å | 0.71073 | 0.71073 |
| Measured 2θ range/° | 4.782–60.788 | 4.518–60.73 |
| No. of reflections collected | 16,993 | 17,389 |
| Independent reflections | 12,771 | 9840 |
| Observed reflections with I > 2.00σ(I) | 4756 | 4142 |
| Rint | 0.0135 | 0.0338 |
| R(F2) (I > 2.00σ(I)) a | 0.0302 | 0.0335 |
| wR(F2) (all data) b | 0.0659 | 0.0737 |
| GOF | 1.068 | 1.024 |
| CCDC number | 2,103,664 | 2,103,665 |
| 5 | 6 | 7 | 8 | |
|---|---|---|---|---|
| N(1)-S(1) | 2.1291(4) | 2.134(2) | 2.1350(4) | 2.1341(18) |
| Ni(1)-S(2) | 2.1340(4) | 2.134(2) | 2.1343(4) | 2.1404(17) |
| Ni(1)-S(5) | 2.137(2) | |||
| Ni(1)-S(6) | 2.138(2) | |||
| S(1)-C(1) | 1.7147(15) | 1.705(8) | 1.7143(15) | 1.719(7) |
| S(2)-C(2) | 1.7153(15) | 1.716(9) | 1.7084(16) | 1.708(7) |
| S(3)-C(1) | 1.7406(15) | 1.736(9) | 1.7393(16) | 1.748(7) |
| S(4)-C(2) | 1.7406(15) | 1.733(9) | 1.7418(15) | 1.750(7) |
| S(5)-C(9) | 1.715(9) | |||
| S(6)-C(10) | 1.699(9) | |||
| S(7)-C(9) | 1.739(8) | |||
| S(8)-C(10) | 1.755(9) | |||
| S(3)-C(3) | 1.8239(15) | 1.819(9) | 1.8486(15) | 1.829(7) |
| S(4)-C(4) | 1.8126(15) | 1.823(9) | 1.8291(16) | 1.820(7) |
| C(1)-C(2) | 1.386(2) | 1.413(12) | 1.389(2) | 1.380(10) |
| C(3)-C(4) | 1.524(2) | 1.549(12) | 1.525(2) | 1.508(10) |
| S(7)-C(11) | 1.818(9) | |||
| S(8)-C(12) | 1.832(9) | |||
| C(9)-C(10) | 1.400(12) | |||
| C(11)-C(12) | 1.523(12) | |||
| S(1)-Ni(1)-S(2) | 91.652(14) | 91.79(10) | 91.959(15) | 91.68(7) |
| S(5)-Ni(1)-S(6) | 91.50(9) | |||
| S(1)-Ni(1)-S(2′) a) | 88.347(14) | 88.040(15) | 88.32(7) | |
| S(1)-Ni(1)-S(5) | 88.79(9) | |||
| S(1)-Ni(1)-S(6) | 87.97(9) | |||
| C(1)-C(2)-S(2) | 119.03(11) | 118.3(7) | 119.86(12) | 119.5(5) |
| C(1)-C(2)-S(4) | 127.43(12) | 127.8(7) | 122.23(12) | 126.2(5) |
| C(2)-C(1)-S(1) | 119.29(11) | 119.4(7) | 118.98(12) | 119.5(5) |
| C(2)-C(1)-S(3) | 126.84(12) | 125.6(7) | 126.44(12) | 126.6(5) |
| C(9)-C(10)-S(6) | 120.3(7) | |||
| C(9)-C(10)-S(8) | 125.6(7) | |||
| C(10)-C(9)-S(5) | 117.8(6) | |||
| C(10)-C(9)-S(7) | 128.3(7) |
| E1/21/V | E1/22/V | ΔE/V | |
|---|---|---|---|
| (Bu4N)[Ni(dddt)2] | −1.24 | −1.02 | 0.22 |
| 1 | −1.23 | −1.03 | 0.20 |
| 2 | −1.23 | −1.05 | 0.18 |
| 3 | −1.23 | −1.05 | 0.18 |
| 4 | −1.23 | −1.06 | 0.17 |
| λmax/nm | Energy gap/eV | ε/dm3 mol−1 | |
|---|---|---|---|
| 1 | 1036 | 1.19 | 12,000 |
| 2 | 1198 | 1.03 | 10,000 |
| 3 | 1144 | 1.08 | 13,000 |
| 4 | 1201 | 1.03 | 13,000 |
| 5 | 982 | 1.26 | 32,000 |
| 6 | 1035 | 1.20 | 32,000 |
| 7 | 1031 | 1.20 | 39,000 |
| 8 | 1040 | 1.19 | 49,000 |
| S (× 103) | 9 | 10 |  |
| a1 | −2.7 | −1.9 | |
| a2 | −10 | −10 | |
| b1 | −6.9 | −3.8 | |
| b2 | −0.14 | −0.7 | |
| b3 | −0.14 | −0.7 | |
| b4 | 1.2 | −0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, K.; Sadahiro, M.; Arata, S.; Hoshino, N.; Kadoya, T.; Akutagawa, T.; Kato, R.; Yamada, J.-i. Donor-Type Nickel–Dithiolene Complexes Fused with Bulky Cycloalkane Substituents and Their Application in Molecular Conductors. Crystals 2021, 11, 1154. https://doi.org/10.3390/cryst11101154
Kubo K, Sadahiro M, Arata S, Hoshino N, Kadoya T, Akutagawa T, Kato R, Yamada J-i. Donor-Type Nickel–Dithiolene Complexes Fused with Bulky Cycloalkane Substituents and Their Application in Molecular Conductors. Crystals. 2021; 11(10):1154. https://doi.org/10.3390/cryst11101154
Chicago/Turabian StyleKubo, Kazuya, Mamoru Sadahiro, Sonomi Arata, Norihisa Hoshino, Tomofumi Kadoya, Tomoyuki Akutagawa, Reizo Kato, and Jun-ichi Yamada. 2021. "Donor-Type Nickel–Dithiolene Complexes Fused with Bulky Cycloalkane Substituents and Their Application in Molecular Conductors" Crystals 11, no. 10: 1154. https://doi.org/10.3390/cryst11101154
APA StyleKubo, K., Sadahiro, M., Arata, S., Hoshino, N., Kadoya, T., Akutagawa, T., Kato, R., & Yamada, J.-i. (2021). Donor-Type Nickel–Dithiolene Complexes Fused with Bulky Cycloalkane Substituents and Their Application in Molecular Conductors. Crystals, 11(10), 1154. https://doi.org/10.3390/cryst11101154






