Modulation Effect of Hardness on the Friction Coefficient and Its Mechanism Analysis of ZrB2/Mo Multilayers Synthesized by Magnetron Sputtering
Abstract
1. Introduction
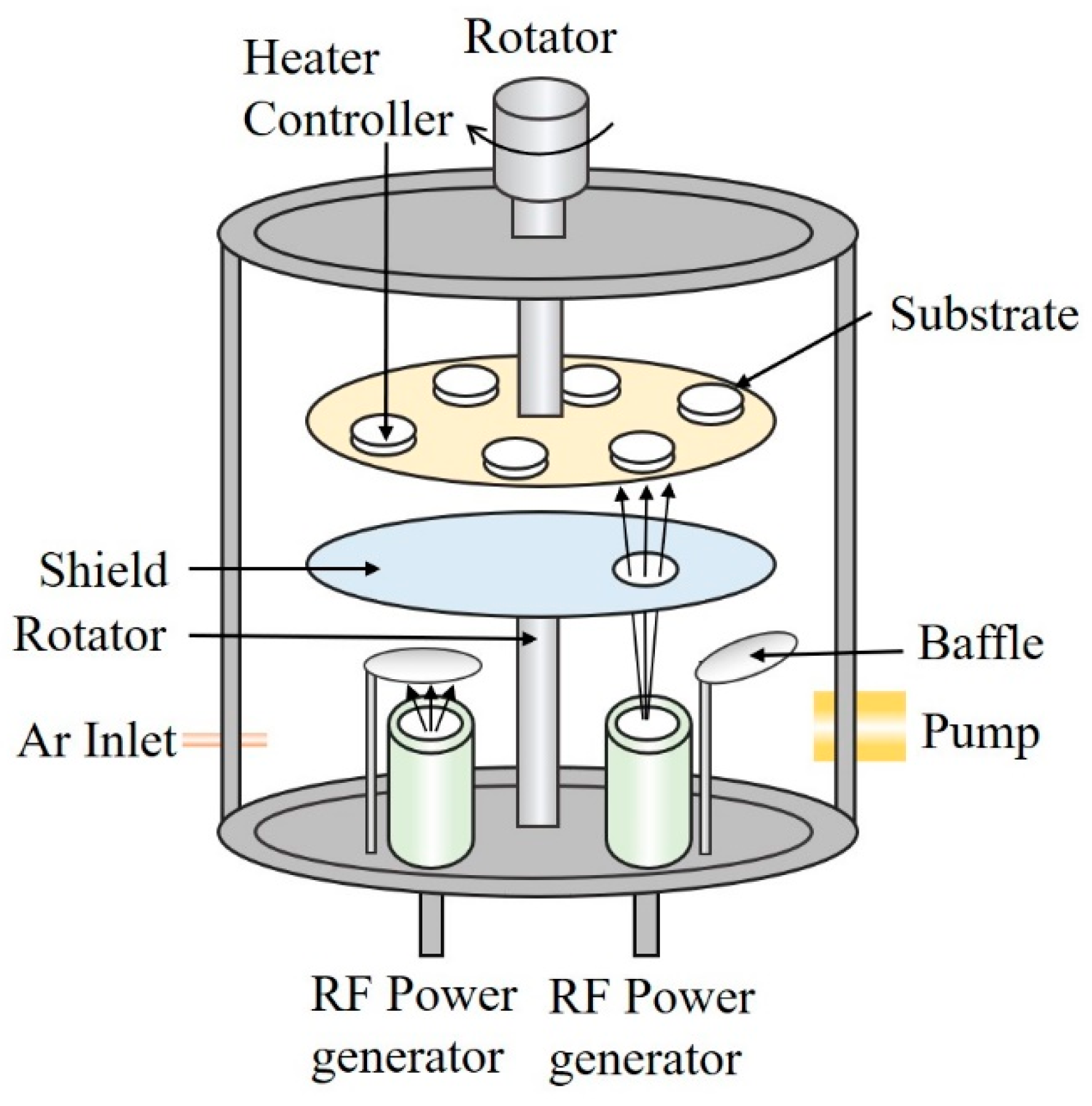
2. Experimental Details
3. Results and Discussion
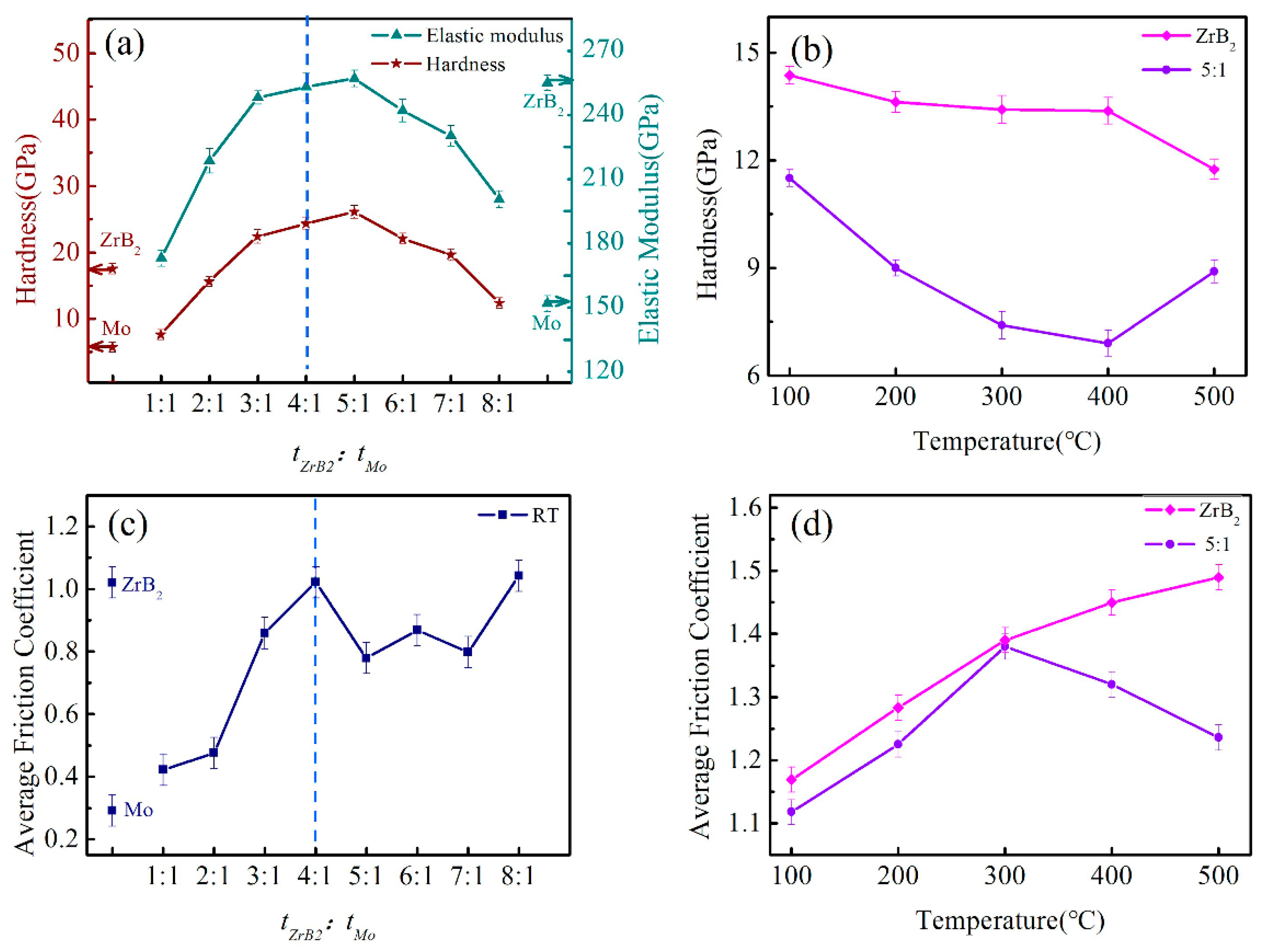
3.1. Friction Properties
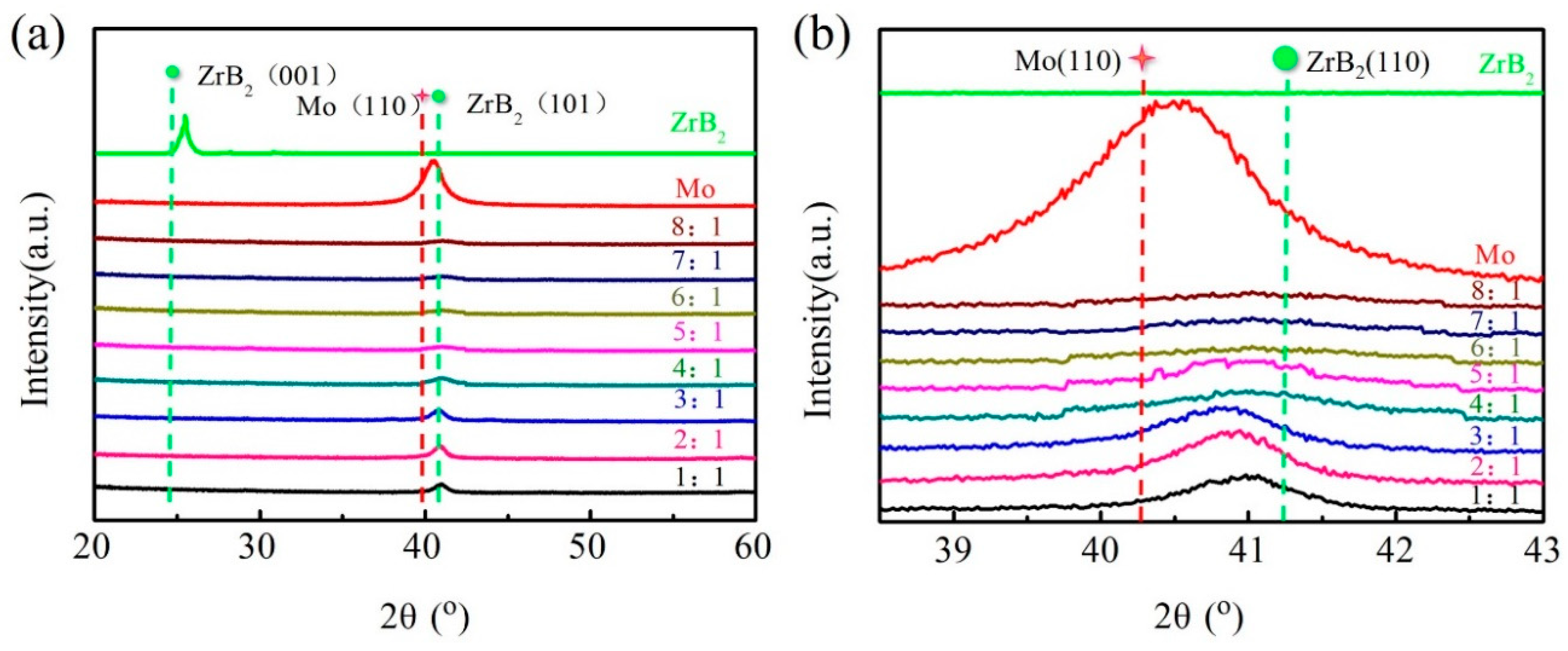
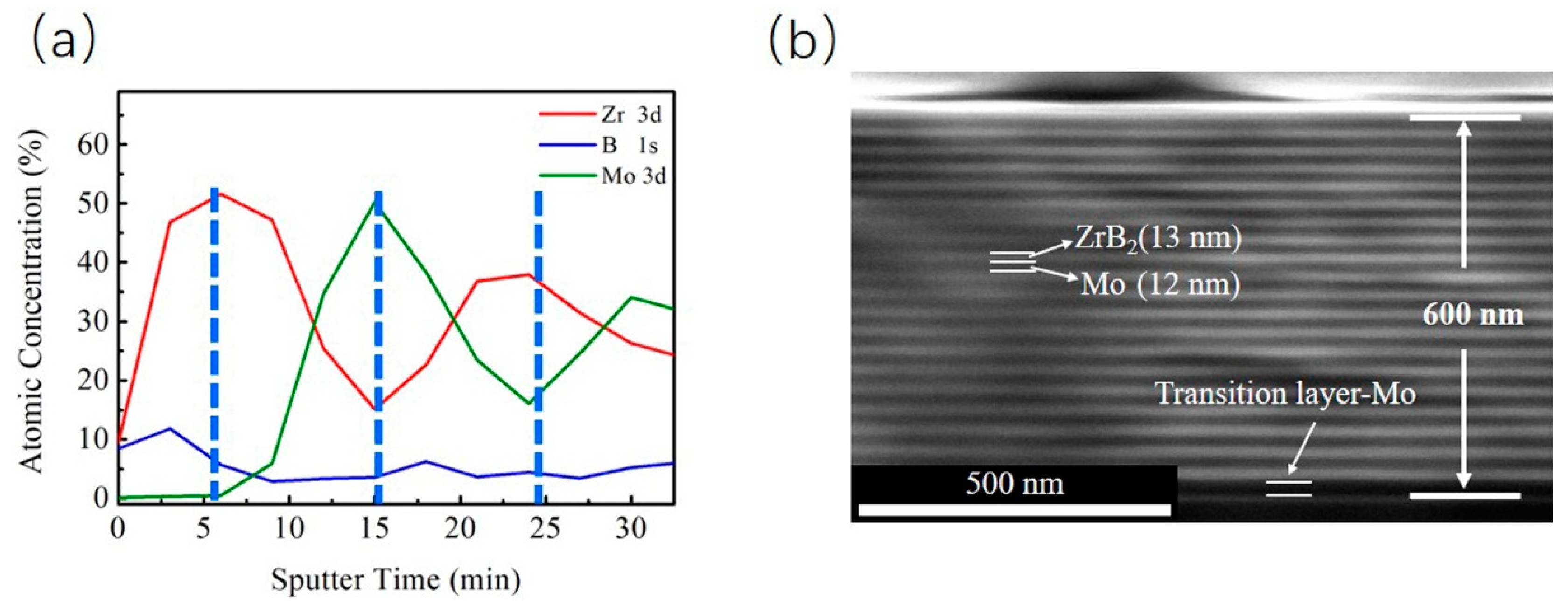
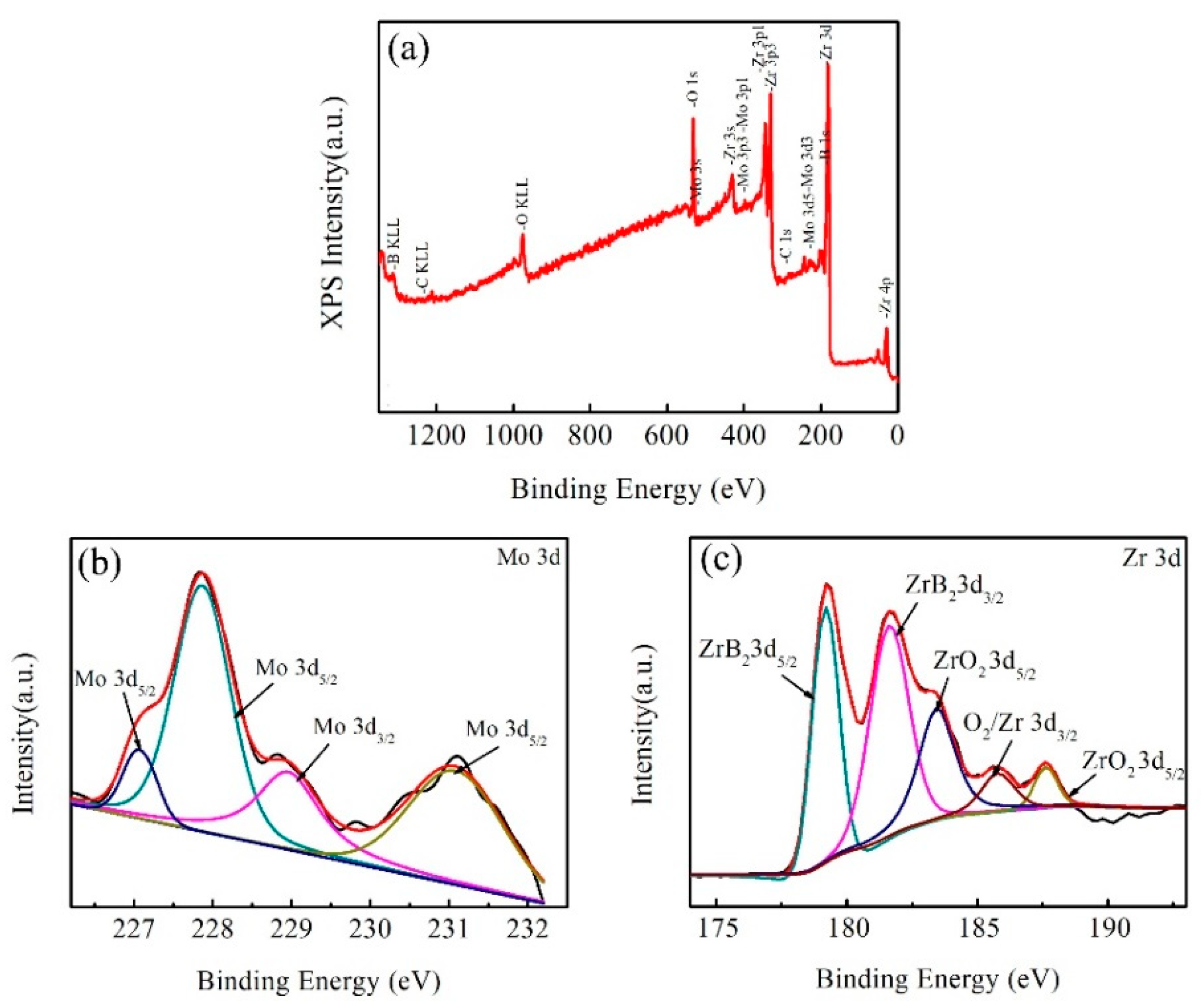
3.2. Mechanical Properties and Microstructures
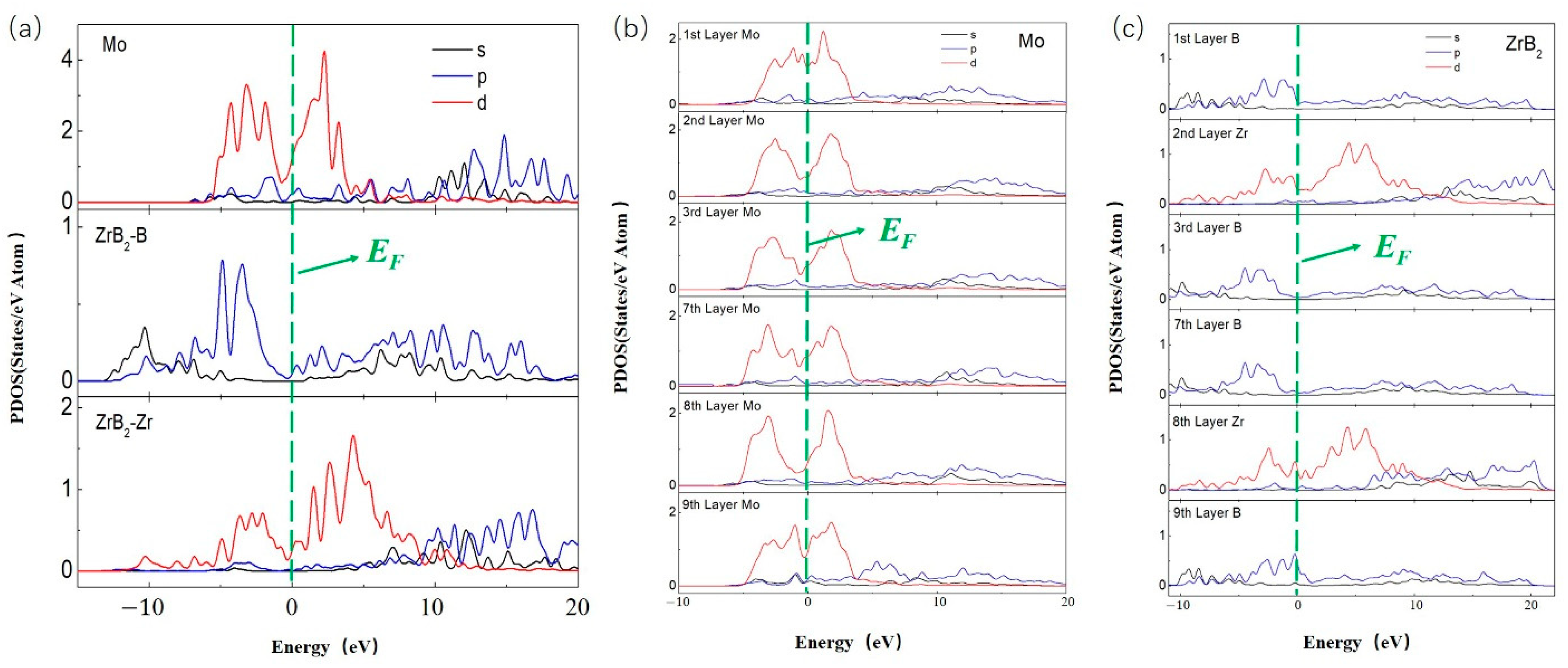
3.3. Mechanism of Friction Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, N.; Gautam, G.; Gautam, R.K.; Mohan, A.; Mohan, S. Wear, friction and profilometer studies of insitu AA5052/ZrB2 composites. Tribol. Int. 2016, 97, 313–326. [Google Scholar] [CrossRef]
- Madhavan, R.; Bellon, P.; Averback, R.S. Wear Resistance of Cu/Ag Multilayers: A Microscopic Study. ACS Appl. Mater. Interfaces 2018, 10, 15288–15297. [Google Scholar]
- Gérard, B. Application of thermal spraying in the automobile industry. Surf. Coat. Technol. 2006, 201, 2028–2031. [Google Scholar]
- Guo, C.; Chen, J.M.; Yao, R.G.; Zhou, J.S. Microstructure and High Temperature Wear Resistance of Laser Cladding NiCoCrAlY/ZrB2 Coating. Rare Metal Mater. Eng. 2013, 42, 1547–1551. [Google Scholar]
- Yu, L.D.; Shuy, G.W.; Vilaithong, T. Friction modification of WC-Co by ion implantation. Surf. Coat. Technol. 2000, 128, 404–409. [Google Scholar] [CrossRef]
- Walck, S.D.; Donley, M.S.; Zabinski, J.S.; Dyhouse, V.J. Characterization of pulsed laser deposited PbO/MoS2 by transmission electron microscopy. J. Mater. Res. 1994, 9, 236–245. [Google Scholar] [CrossRef]
- Walck, S.D.; Zabinski, J.S.; McDevitt, N.T.; Bultman, J.E. Characterization of air-annealed, pulsed laser deposited ZnO-WS2 solid film lubricants by transmission electron microscopy. Thin Solid Films 1997, 305, 130–143. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Luster, B.; Stadler, S.; Kohli, P.; Muratore, C.; Hager, C.; Voevodin, A.A. Adaptive Mo2N/MoS2/Ag tribological nanocom-posite coatings for aerospace applications. Tribol. Lett. 2008, 29, 95–103. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Simonson, W.J.; Ge, Q.; Kohli, P.; Muratore, C.; Voevodin, A.A. Tribological investigation of adaptive Mo2N/MoS2/Ag coatings with high sulfur content. Surf. Coat. Technol. 2009, 203, 1304–1309. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Xiang, H.; Zhou, Y. A Theoretical Investigation on the Anisotropic Surface Stability and Oxygen Adsorption Behavior of ZrB2. J. Am. Ceram. Soc. 2016, 99, 4113–4120. [Google Scholar] [CrossRef]
- Wang, P.; Qi, Y.; Zhou, S.; Hu, P.; Chen, G.; Zhang, X.; Han, W. Polycrystalline ZrB2 coating prepared on graphite by chemical vapor deposition. Phys. Status Solidi (b) 2016, 253, 1590–1595. [Google Scholar] [CrossRef]
- Ma, H.-B.; Zhang, G.-J.; Liu, H.-L.; Liu, J.-X.; Lu, Y.; Xu, F.F. Effect of WC or ZrC addition on thermal residual stresses in ZrB2SiC ceramics. Mater. Des. 2016, 110, 340–345. [Google Scholar] [CrossRef]
- Sha, J.; Li, J.; Wang, S.; Wang, Y.; Zhang, Z.; Dai, J. Toughening effect of short carbon fibers in the ZrB2–ZrSi2 ceramic composites. Mater. Des. 2015, 75, 160–165. [Google Scholar] [CrossRef]
- Balak, Z.; Asl, M.S.; Azizieh, M.; Kafashan, H.; Hayati, R. Effect of different additives and open porosity on fracture toughness of ZrB2-SiC-based composites prepared by SPS. Ceram. Int. 2017, 43, 2209–2220. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, P.; Wei, C.; Han, W.; Zhang, X.; Xu, B. ZrB2 grains synthesized on graphite by chemical vapor deposition. J. Alloys Compd. 2017, 698, 27–32. [Google Scholar] [CrossRef]
- Emami, S.M.; Salahi, E.; Zakeri, M.; Tayebifard, S.A. Effect of composition on spark plasma sintering of ZrB2-SiC-ZrC nanocomposite synthesized by MASPSyn. Ceram. Int. 2017, 43, 111–115. [Google Scholar] [CrossRef]
- Zhou, S.B.; Wang, Z.; Zhang, W. Effect of graphite flake orientation on microstructure and mechanical properties of ZrB2-SiC-graphite composite. J. Alloys Compd. 2009, 485, 181–185. [Google Scholar] [CrossRef]
- Silvestroni, L.; Sciti, D.; Hilmas, G.E.; Fahrenholtz, W.G.; Watts, J. Effect of a weak fiber interface coating in ZrB2 reinforced with long SiC fibers. Mater. Des. 2015, 88, 610–618. [Google Scholar] [CrossRef]
- Sciti, D.; Pienti, L.; Murri, A.N.; Landi, E.; Medri, V.; Zoli, L. From random chopped to oriented continuous SiC fibers-ZrB2 composites. Mater. Des. 2014, 63, 464–470. [Google Scholar] [CrossRef]
- Monteverde, F.; Guicciardi, S.; Bellosi, A. Advances in microstructure and mechanical properties of zirconium diboride based ceramics. Mater. Sci. Eng. A 2003, 346, 310–319. [Google Scholar] [CrossRef]
- Xu, J.; Ju, H.; Yu, L. Effects of Mo Content on the Microstructure and Friction and Wear Properties of Timon Films. Acta Met. Sin. 2012, 48, 1132–1138. [Google Scholar] [CrossRef]
- Hazar, H. Characterization of MoN coatings for pistons in a diesel engine. Mater. Des. 2010, 31, 624–627. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, L.; Patnaik, P.; Zeng, X. Wear resistant TiMoN coatings deposited by magnetron sputtering. Wear 2006, 261, 119–125. [Google Scholar] [CrossRef]
- Mei, F.; Shao, N.; Wei, L.; Dong, Y.; Li, G. Coherent epitaxial growth and superhardness effects of fcc-TiN/hcp-TiB2 nanomultilayers. Appl. Phys. Lett. 2005, 87, 011906. [Google Scholar] [CrossRef]
- Kong, M.; Shao, N.; Dong, Y.; Yue, J.; Li, G. Growth, microstructure and mechanical properties of (Ti, Al)N/VN nanomultilayers. Mater. Lett. 2006, 60, 874–877. [Google Scholar] [CrossRef]
- Liu, C.H.; Du, X.S.; Wang, D.Z.; Huang, N.K.; Yang, B. XPS characterization of the molybdenum before and after H+ ion irradiation. J. Funct. Mater. 2007, 2, 176–178. [Google Scholar]
- Wang, T.-G.; Liu, Y.; Zhang, T.; Kim, D.-I.; Kim, K.H. Influence of Nitrogen Flow Ratio on the Microstructure, Composition, and Mechanical Properties of DC Magnetron Sputtered Zr–B–O–N Films. J. Mater. Sci. Technol. 2012, 28, 981–991. [Google Scholar] [CrossRef]
- Pierson, J.; Billard, A.; Belmonte, T.; Michel, H.; Frantz, C. Influence of oxygen flow rate on the structural and mechanical properties of reactively magnetron sputter-deposited Zr–B–O coatings. Thin Solid Films 1999, 347, 78–84. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, Q.; Li, S.-X.; Qin, Z. Microstructure, corrosion and tribological properties of Ti(CN) multilayer coatings on 35CrMo steel by CVD. Rare Met. 2014, 39, 1314–1320. [Google Scholar] [CrossRef]
- Li, Y.; Zou, W.J.; Li, B.X.; Dong, Q.M. The Frictional Properties and Mechanism of Nano-Diamond/Ni Composite Coatings by Brush-Plating. Adv. Mater. Res. 2011, 291–294, 197–200. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Ma, S.; Xu, K. Effects of annealing temperature on the microstructure and hardness of TiAlSiN hard coatings. Chin. Sci. Bull. 2011, 56, 1727–1731. [Google Scholar] [CrossRef][Green Version]
- Anderson, P.; Li, C. Hall-Petch relations for multilayered materials. Nanostruct. Mater. 1995, 5, 349–362. [Google Scholar] [CrossRef]
- Du, H.J.; Tian, Y.J. Hardening mechanism for Nano-multilayer films. Acta Inorg. Mater. 2006, 4, 769–775. [Google Scholar]
- Kato, M.; Mori, T.; Schwartz, L. Hardening by spinodal modulated structure. Acta Met. 1980, 28, 285–289. [Google Scholar] [CrossRef]
- Shinn, M.; Barnett, S.A. Effect of superlattice layer elastic moduli on hardness. Appl. Phys. Lett. 1994, 64, 61–63. [Google Scholar] [CrossRef]
- Bouaouina, B.; Besnard, A.; Abaidia, S.; Haid, F. Residual stress, mechanical and microstructure properties of multilayer Mo2 N/CrN coating produced by R.F Magnetron discharge. Appl. Surf. Sci. 2017, 395, 117–121. [Google Scholar] [CrossRef]
- Drnovsek, A.; de Figueiredo, M.R.; Vo, H.; Xia, A.; Vachhani, S.J.; Kolozsvari, S.; Hosemann, P.; Franz, R. Correlating high temperature mechanical and tribological properties of CrAlN and CrAlSiN hard coatings. Surf. Coat. Technol. 2019, 372, 361–368. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V.; Lemesheva, M.V.; Shvyndina, N.V.; Levashov, E.A.; Potanin, A.Y. Structure, Mechanical Properties, and Oxidation Resistance of ZrB2, ZrSiB, and ZrSiB/SiBC Coatings. Prot. Met. Phys. Chem. Surf. 2018, 54, 1147–1156. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Zhou, Y.-X.; Rao, Q.-C.; Jin, Z.-H. An investigation of the abrasive wear behavior of ductile cast iron. J. Mater. Process. Technol. 2001, 116, 176–181. [Google Scholar] [CrossRef]
- Tarasov, S.; Rubtsov, V.; Kolubaev, E.; Gnyusov, S.F.; Kudinov, Y.A. Radioscopy of remnant joint line in a friction stir welded seam. Russ. J. Nondestruct. Test. 2015, 51, 573–579. [Google Scholar] [CrossRef]
- Jin, S.X.; Liu, N.; Zhang, S.; Li, D.J. The simulation of interface structure, energy and electronic properties of TaN/ReB2 multilayers using first-principles. Surf. Coat. Technol. 2017, 326, 417–423. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, Z.; Zhang, H.; Du, Z.; Chen, C. First principles calculation of interfacial stability, energy and electronic properties of SiC/ZrB2 interface. J. Phys. Chem. Solids 2017, 107, 162–169. [Google Scholar] [CrossRef]
- Fan, X.; Chen, B.; Zhang, M.; Li, D.; Liu, Z.; Xiao, C. First-principles calculations on bonding characteristic and electronic property of TiC (111)/TiN (111) interface. Mater. Des. 2016, 112, 282–289. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, D.; Liu, S. The simulation of adhesion, stability, electronic structure of W/ZrB2 interface using first-principles. Surf. Coat. Technol. 2013, 228, S583–S587. [Google Scholar] [CrossRef]



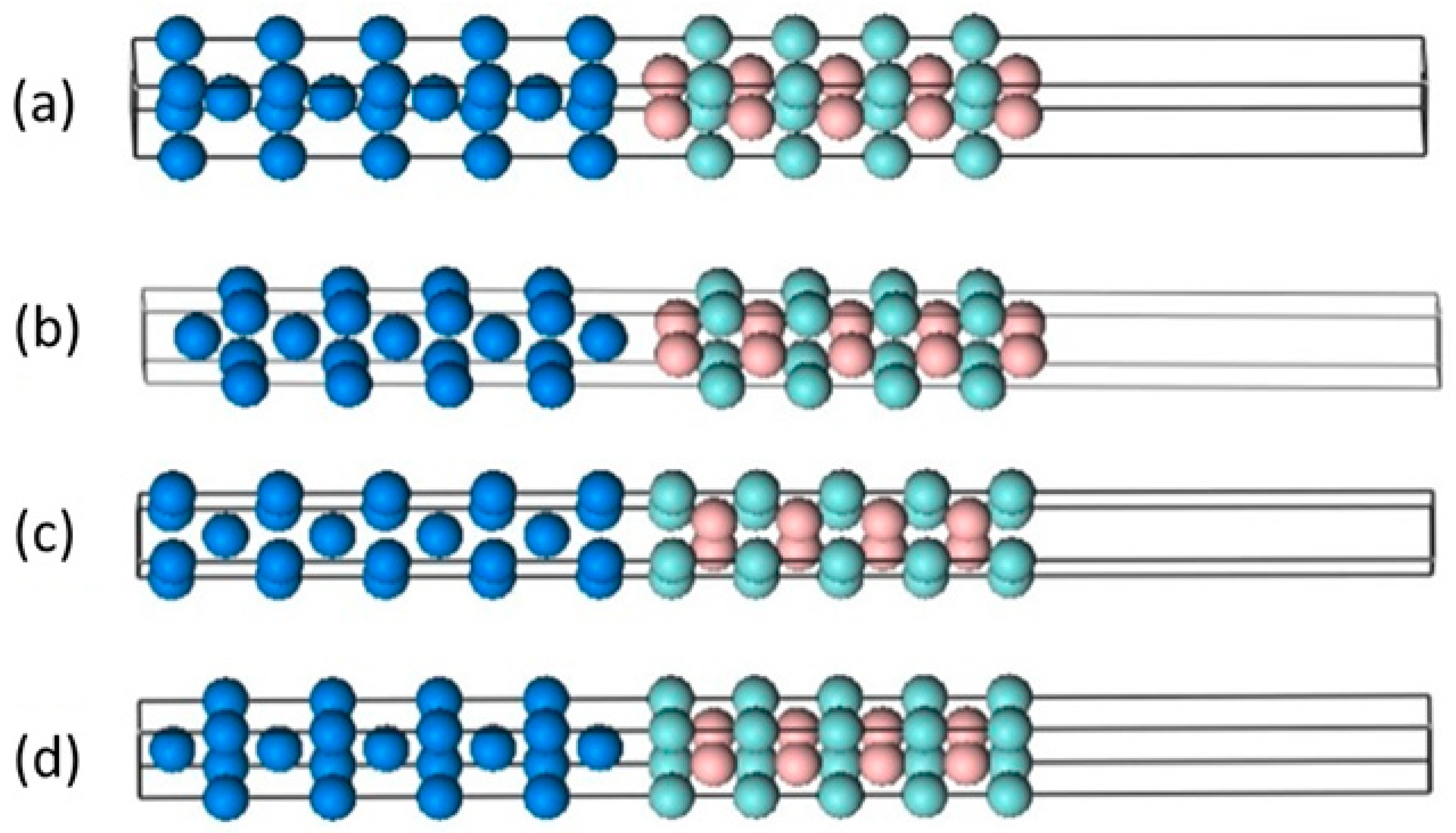

| Stacking | d (Å) | Wad (J/m2) | γ (eV/Å2) | |
|---|---|---|---|---|
| B-terminated | Model 1 | 2.958 | 6.227 | −0.1690 |
| Model 2 | 1.070 | 6.233 | −0.1693 | |
| Zr-terminated | Model 3 | 3.000 | 2.778 | 0.0463 |
| Model 4 | 3.000 | 4.755 | −1.2351 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Dong, L.; Wu, J.; Li, D. Modulation Effect of Hardness on the Friction Coefficient and Its Mechanism Analysis of ZrB2/Mo Multilayers Synthesized by Magnetron Sputtering. Crystals 2021, 11, 69. https://doi.org/10.3390/cryst11010069
Zhang T, Dong L, Wu J, Li D. Modulation Effect of Hardness on the Friction Coefficient and Its Mechanism Analysis of ZrB2/Mo Multilayers Synthesized by Magnetron Sputtering. Crystals. 2021; 11(1):69. https://doi.org/10.3390/cryst11010069
Chicago/Turabian StyleZhang, Tingjia, Lei Dong, Jie Wu, and Dejun Li. 2021. "Modulation Effect of Hardness on the Friction Coefficient and Its Mechanism Analysis of ZrB2/Mo Multilayers Synthesized by Magnetron Sputtering" Crystals 11, no. 1: 69. https://doi.org/10.3390/cryst11010069
APA StyleZhang, T., Dong, L., Wu, J., & Li, D. (2021). Modulation Effect of Hardness on the Friction Coefficient and Its Mechanism Analysis of ZrB2/Mo Multilayers Synthesized by Magnetron Sputtering. Crystals, 11(1), 69. https://doi.org/10.3390/cryst11010069




