Performance Comparison of Eichhornia crassipes and Salvinia natans on Azo-Dye (Eriochrome Black T) Phytoremediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
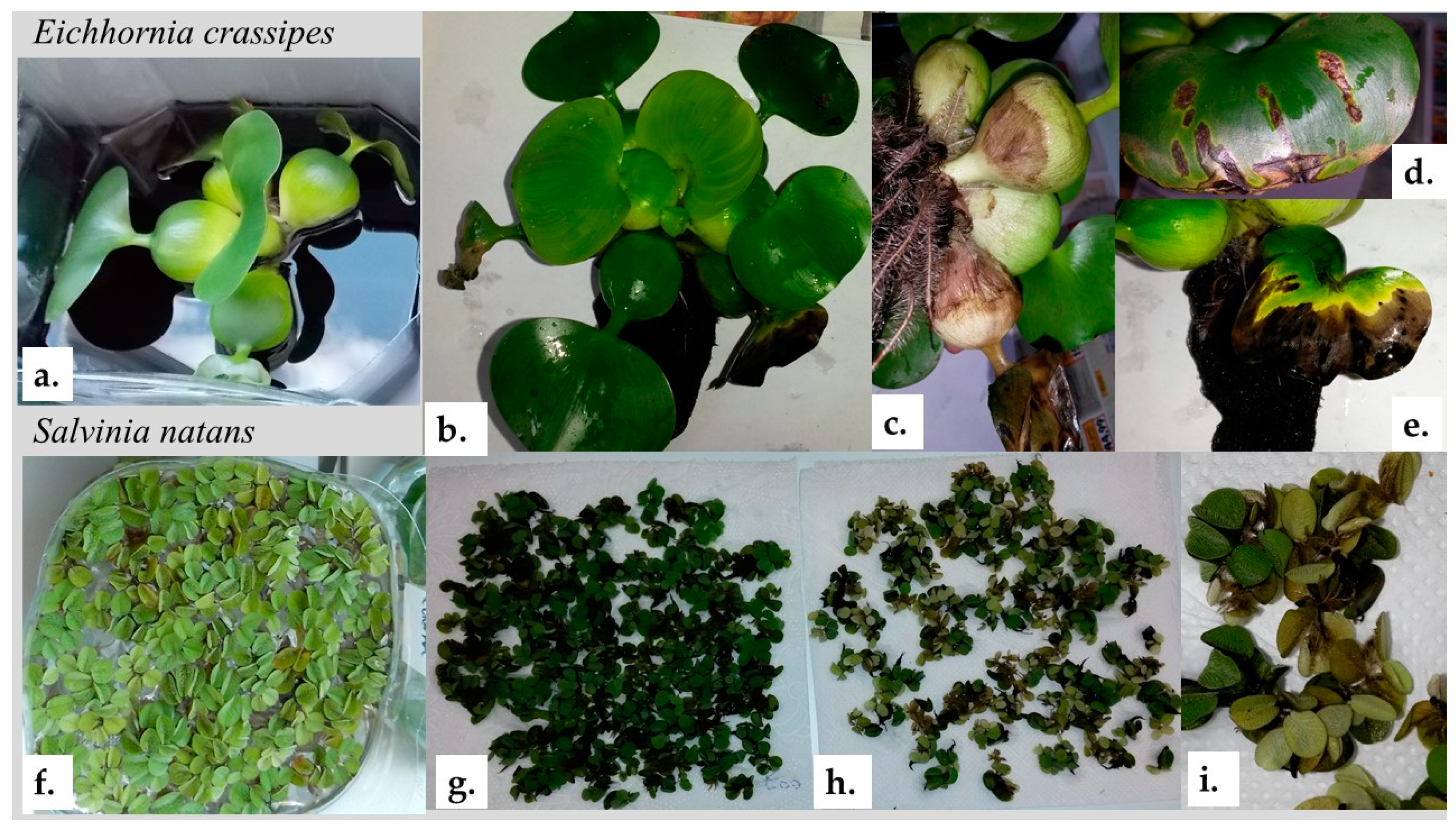
2.2. Phytoremediation Studies
2.3. Photocatalytic Investigations
2.4. Scanning Electron Microscopy and Elemental Analysis
2.5. Photosynthetic Pigment Determination
2.6. Enzyme Activity Assay of Polyphenol-Oxidase (PPO)
2.7. Dye Toxicity
3. Results
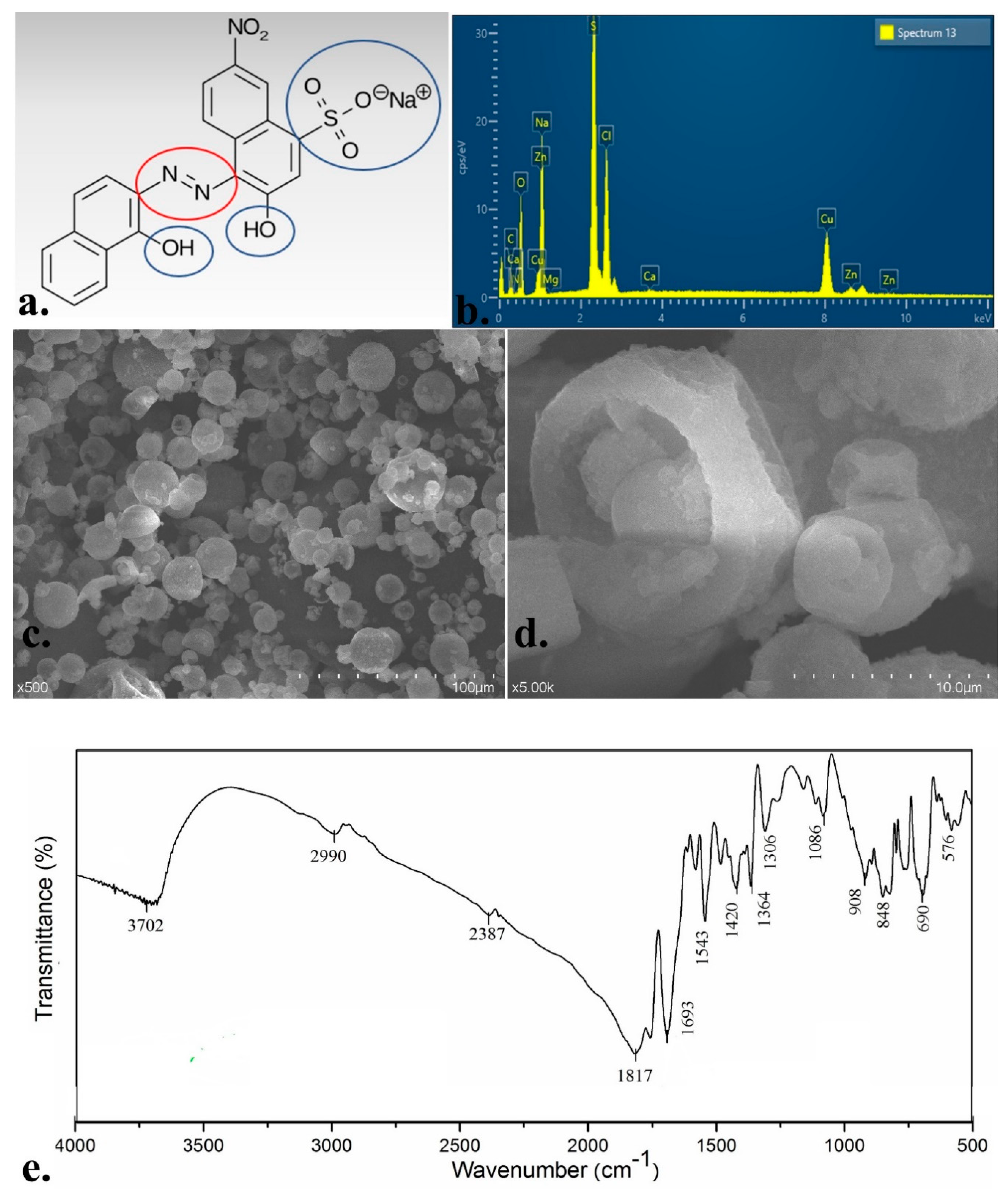
3.1. EBT Dye Solid-State Characteristics
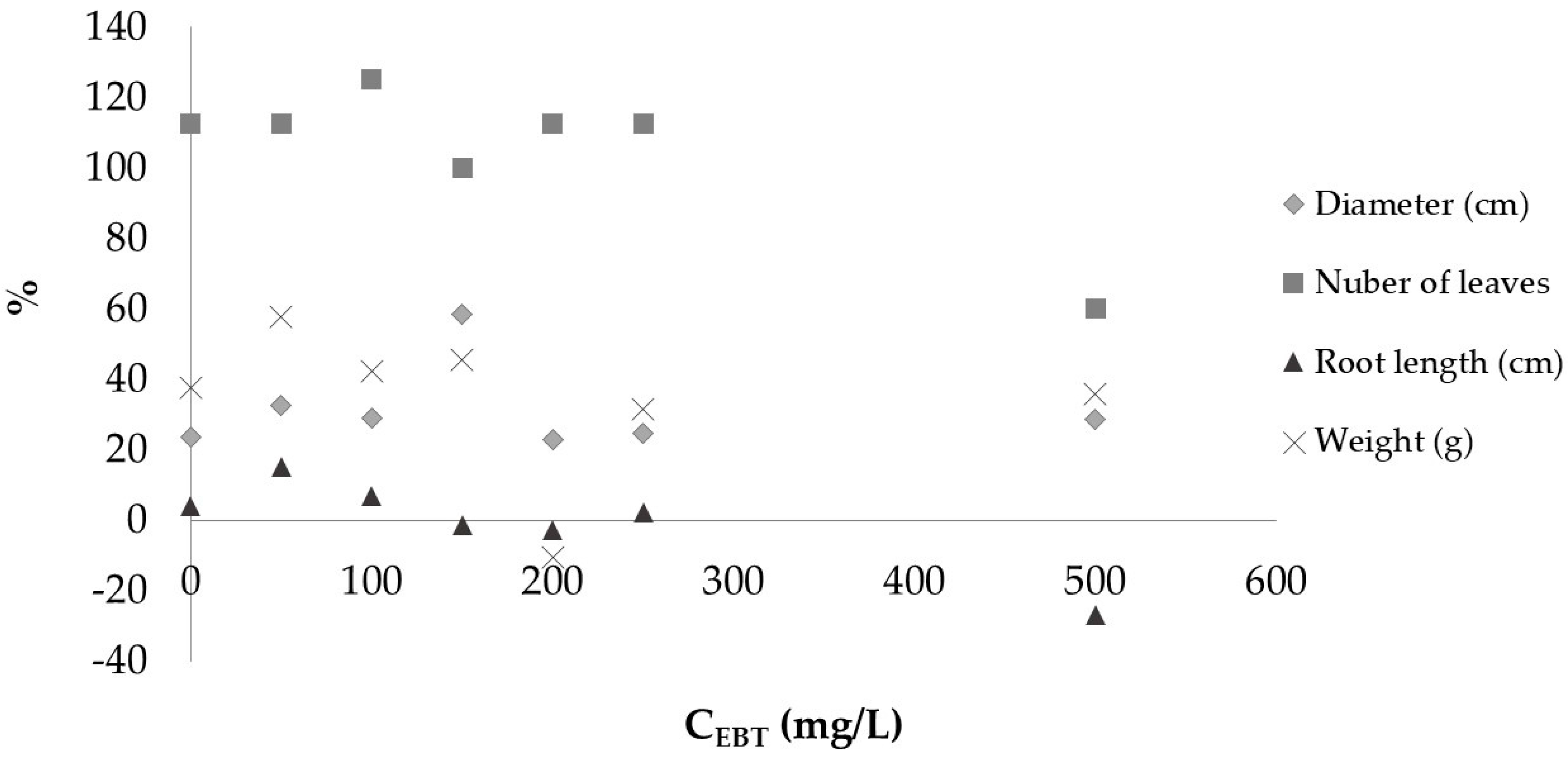
3.2. Seedling Growth Test
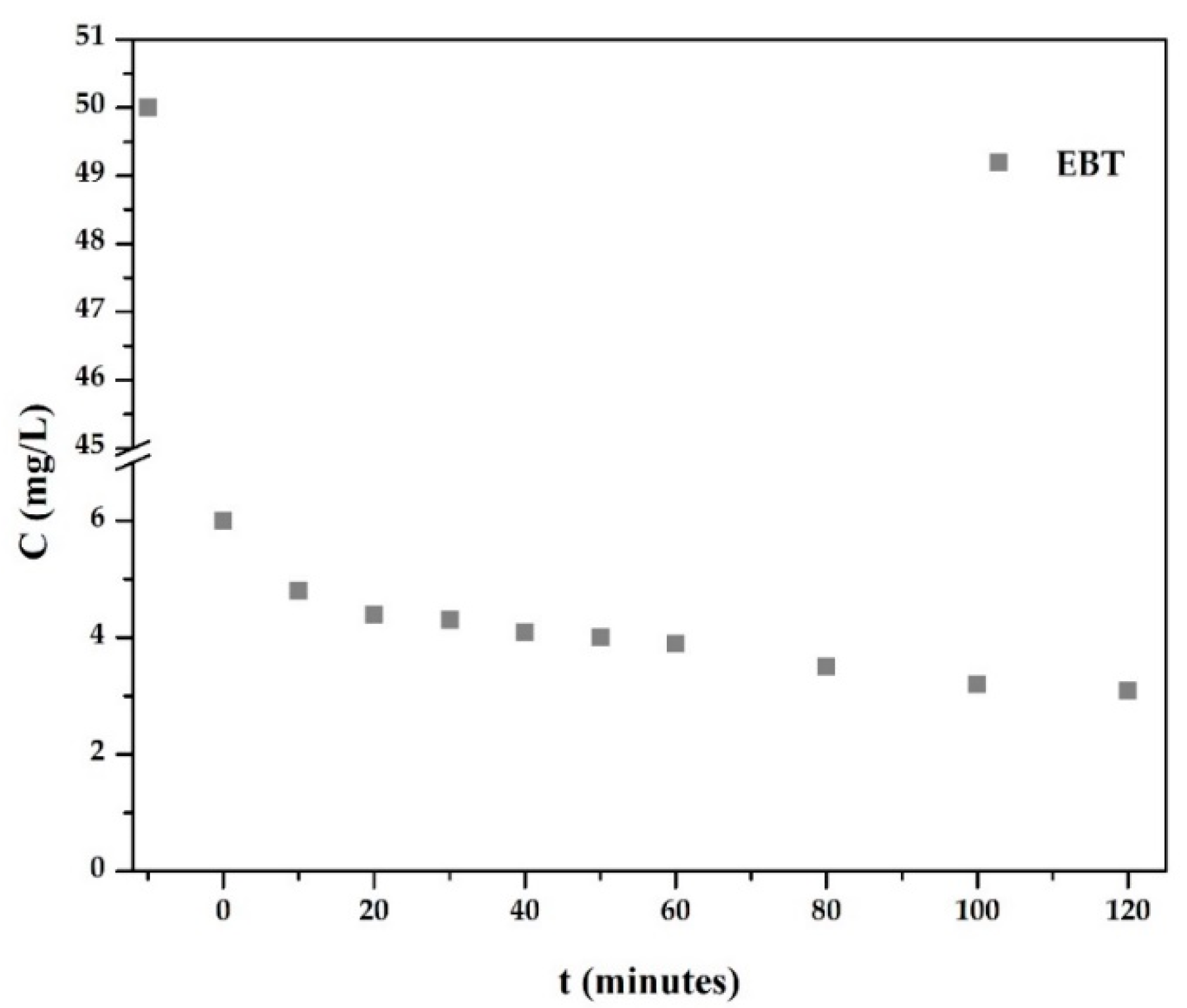
3.3. Photocatalytic Decomposition and Photolysis
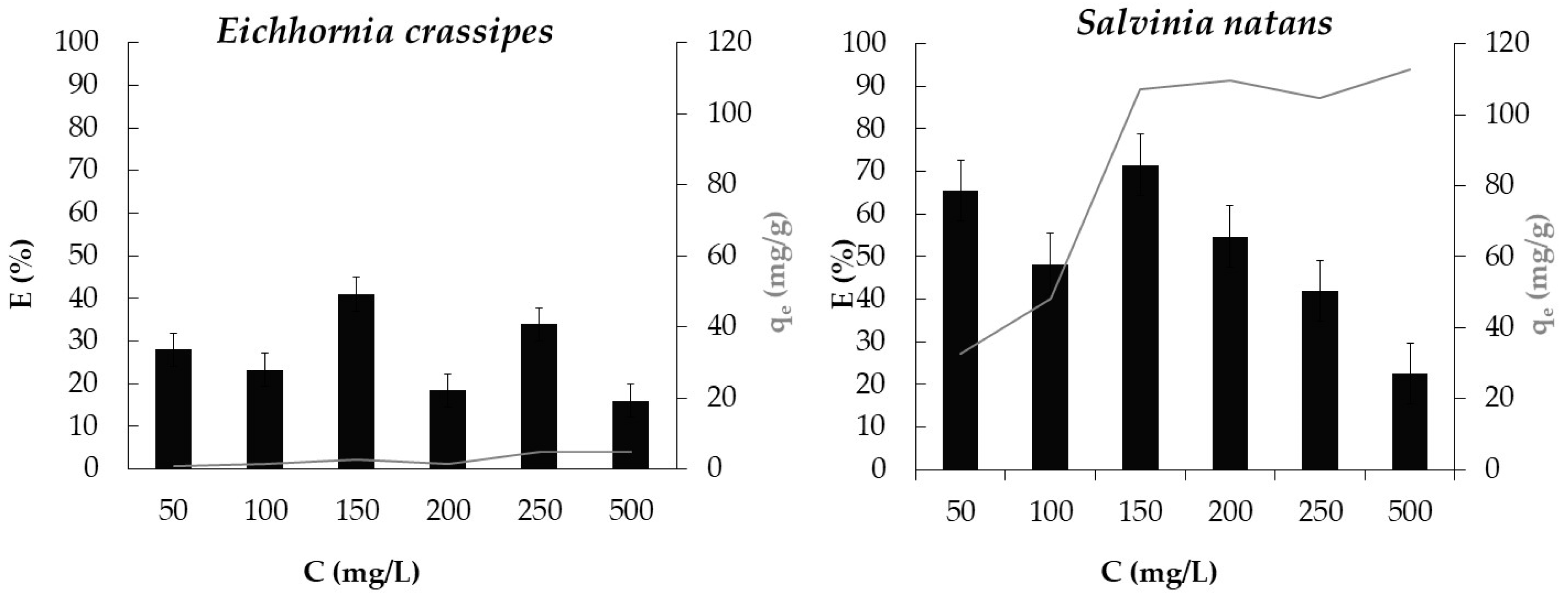
3.4. Effect of Dye Concentration and of Plant Species
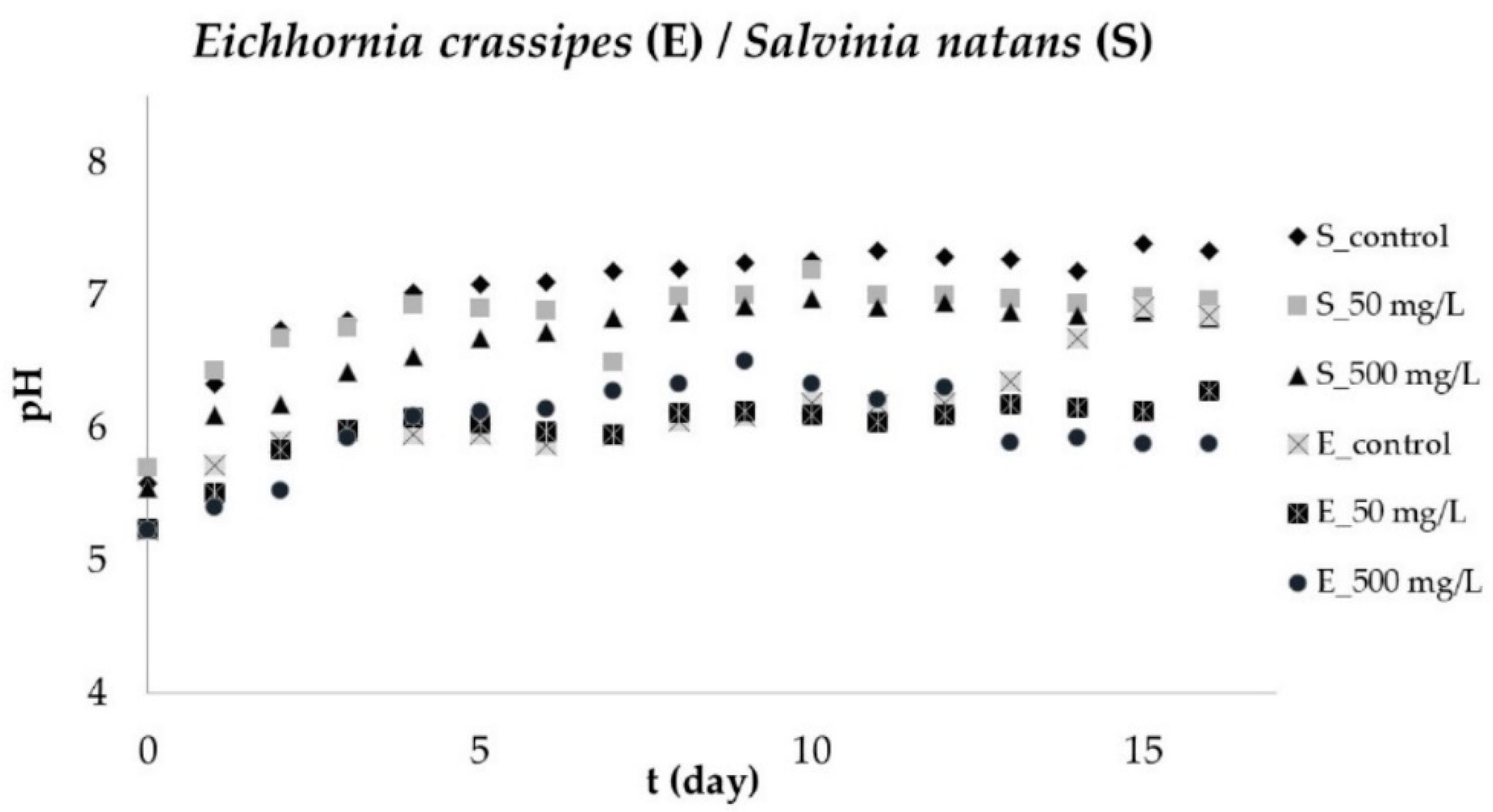
3.5. pH Changes of Aqueous Solution
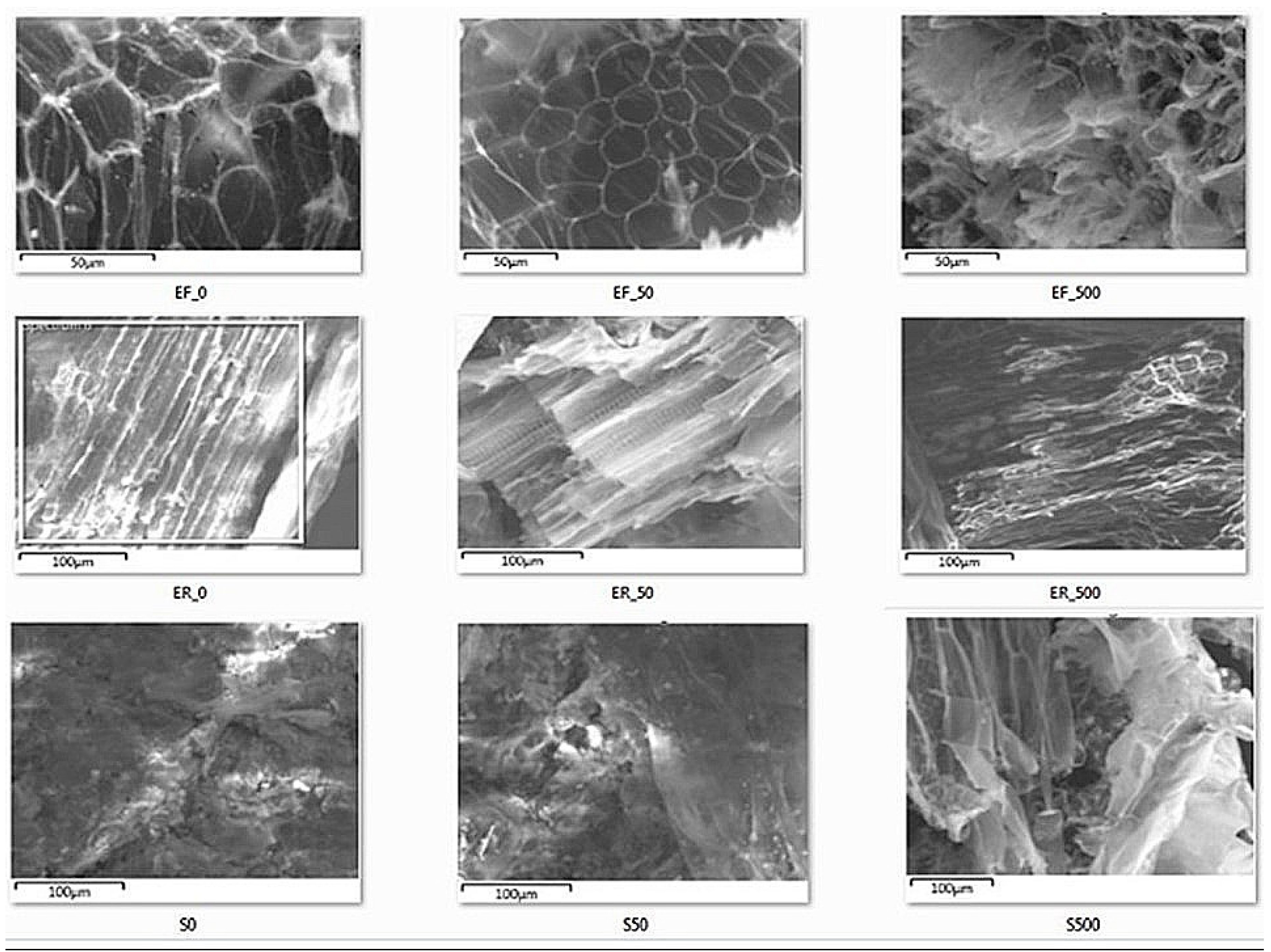
3.6. Morphology Studies
3.7. Elemental Analyses
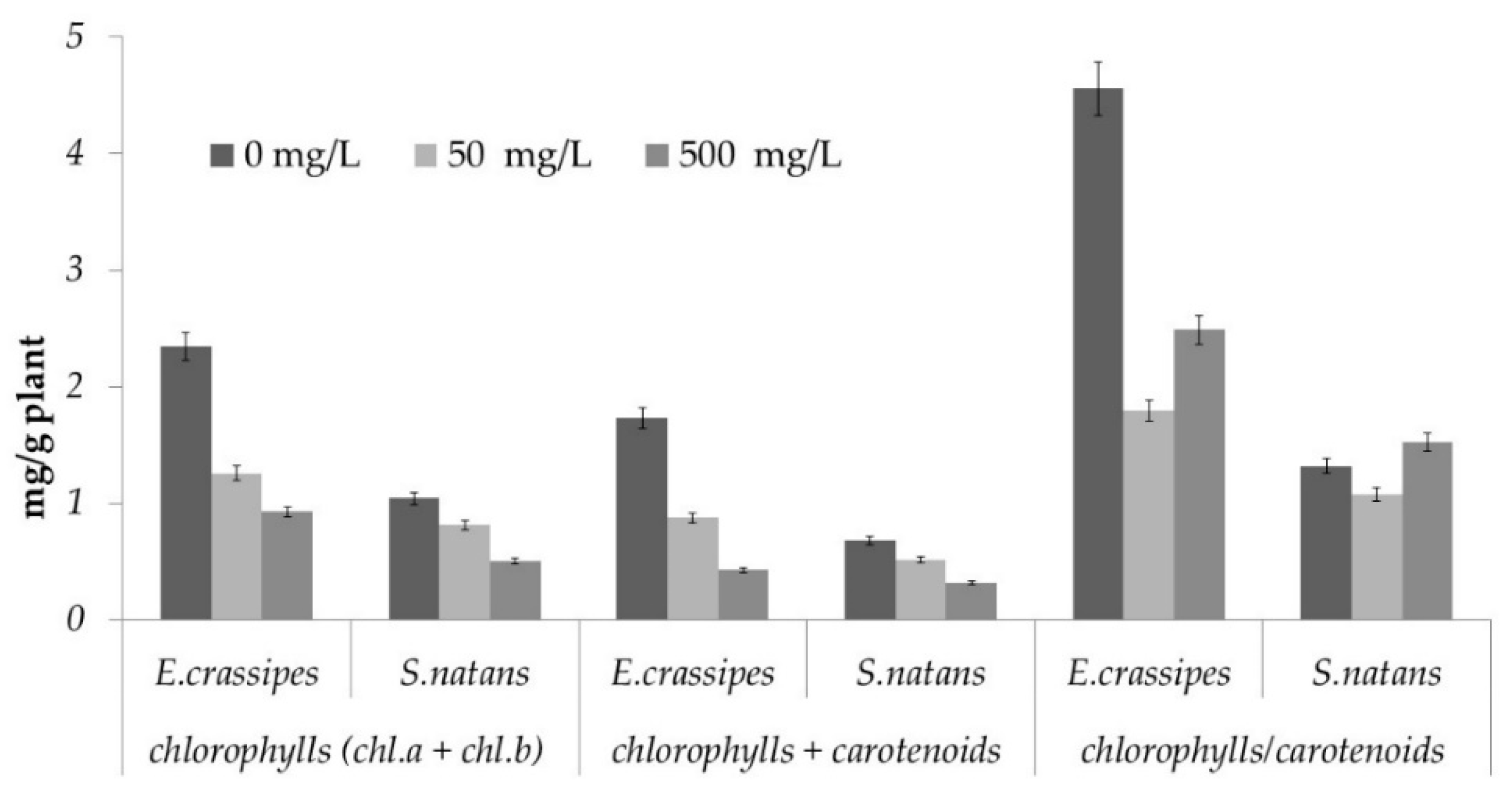
3.8. Photosynthetic Pigment Determination
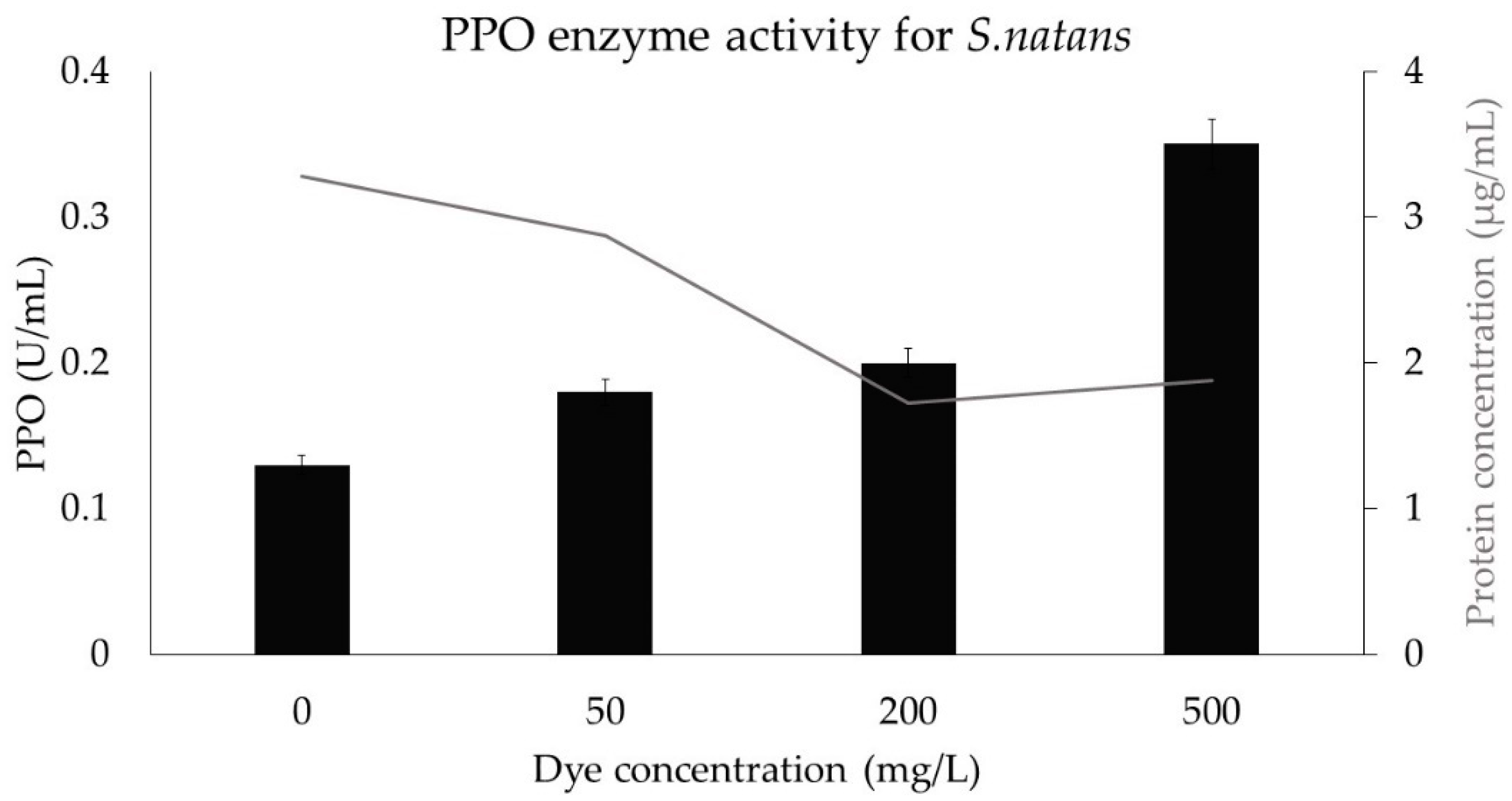
3.9. Stress Protein Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adki, V.S.; Jadhav, J.P.; Bapat, V.A. Exploring the Phytoremediation Potential of Cactus (nopalea Cochenillifera Salm. Dyck.) Cell Cultures for Textile Dye Degradation. Int. J. Phytoremediat. 2012, 14, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Chou, C.; Lin, Y.-C.; Lin, P.-J.; Ho, J.-Y.; Lee Hu, T. Kinetic characteristics of bacterial azo-dye decolorization by Pseudomonas luteola. Water Res. 2001, 35, 2841–2850. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Dave, P.; Kaur, S.; Khosla, E. Removal of Eriochrome black-T by adsorption on to eucalyptus bark using green technology. Indian J. Chem. Technol. 2011, 18, 53–60. [Google Scholar]
- Moeinpour, F.; Alimoradi, A.; Kazemi, M. Efficient removal of Eriochrome black-T from aqueous solution using NiFe2O4 magnetic nanoparticles. J. Environ. Health Sci. Eng. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Zubair, M. A Comparative Study on the Adsorption of Eriochrome Black T Dye from Aqueous Solution on Graphene and Acid-Modified Graphene. Arab. J. Sci. Eng. 2018, 43, 2167–2179. [Google Scholar] [CrossRef]
- El-Dars, F.; Ibrahim, H.M.; Farag, H.A.; Zakaria Abdelwahhab, M.; Shalabi, M. Adsorption Kinetics of Bromophenol Blue and Eriochrome Black T using Bentonite Carbon Composite Material. Int. J. Sci. Eng. Res. 2015, 6, 679–688. [Google Scholar]
- Ghosh, A.; Dastidar, M.G.; Sreekrishnan, T.R. Response surface modeling of bioremediation of acid black 52 dye using Aspergillus flavus. Water Sci. Technol. 2017, 75, 2864–2874. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; Makhfouk, M.E. Removal of Methylene Blue and Eriochrome Black T from aqueous solutions by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320–326. [Google Scholar] [CrossRef]
- Simon, L.; Vincze, G.; Varga, C.; Szabó, B.; Koncz, J. Passive phytoextraction of toxic elements from sewage sludge compost by Salix viminalis energy plants. Acta Phytopathol. Entomol. Hung. 2012, 47, 285–291. [Google Scholar] [CrossRef]
- Simon, L.; Széles, É.; Kovács, B.; Prokisch, J.; Győri, Z. Phytoextraction of selenium from contaminated soils with Indian mustard, fodder radish and alfalfa. Trace elements in the food chain. In Proceedings of the International Symposium on Trace Elements in the Food Chain, Budapest, Hungary, 25–27 May 2006; pp. 40–44. [Google Scholar]
- Kumari, M.; Tripathi, B.D. Effect of aeration and mixed culture of Eichhornia crassipes and Salvinia natans on removal of wastewater pollutants. Ecol. Eng. 2014, 62, 48–53. [Google Scholar] [CrossRef]
- Saha, P.; Mondal, A.; Sarkar, S. Phytoremediation of cyanide containing steel industrial wastewater by Eichhornia crassipes. Int. J. Phytoremediat. 2018, 20, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Tabinda, A.B.; Arif, R.A.; Yasar, A.; Baqir, M.; Rasheed, R.; Mahmood, A.; Iqbal, A. Treatment of textile effluents with Pistia stratiotes, Eichhornia crassipes and Oedogonium sp. Int. J. Phytoremediat. 2019, 21, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Photosynthetic performance of Salvinia natans exposed to chromium and zinc rich wastewater. Braz. J. Plant Physiol. 2008, 20, 61–70. [Google Scholar] [CrossRef]
- Schneller, J.J. Salviniaceae. In Pteridophytes and Gymnosperms; Kramer, K.U., Green, P.S., Eds.; Springer: Berlin, Heidelberg, 1990; pp. 256–258. [Google Scholar]
- Sculthorpe, C.D. The Biology of Aquatic Vascular Plants; Edward Arnold: London, UK, 1967. [Google Scholar]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Tan, K.A.; Morad, N.; Ooi, J.Q. Phytoremediation of Methylene Blue and Methyl Orange Using Eichhornia crassipes. IJESD 2016, 7, 724–728. [Google Scholar] [CrossRef]
- Ng, Y.S.; Chan, D.J.C. Wastewater phytoremediation by Salvinia molesta. J. Water Process Eng. 2017, 15, 107–115. [Google Scholar] [CrossRef]
- Török, A.; Buta, E.; Indolean, C.; Tonk, S.; Silaghi-Dumitrescu, L.; Majdik, C. Biological removal of triphenylmethane dyes from aqueous solution by Lemna minor. Acta Chim. Slov. 2015, 62, 452–461. [Google Scholar] [CrossRef][Green Version]
- Baia, L.; Orbán, E.; Fodor, S.; Hampel, B.; Kedves, E.Z.; Saszet, K.; Székely, I.; Karácsonyi, É.; Réti, B.; Berki, P.; et al. Preparation of TiO2/WO3 composite photocatalysts by the adjustment of the semiconductors’ surface charge. Mater. Sci. Semicond. Process. 2016, 42, 66–71. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Cheema, S.; Sommerhalter, M. Characterization of polyphenol oxidase activity in Ataulfo mango. Food Chem. 2015, 171, 382–387. [Google Scholar] [CrossRef]
- Vincze, E.B.; Salamon, R.V.; Kovacs, E.; Mara, G. Effect of metal tolerant plant growth promoting rhizobacteria on bean growth, cadmium and zinc uptake and stress responses. Environ. Eng. Manag. J. 2018, 17, 803–811. [Google Scholar]
- Veszélyes Hulladékok Vizsgálata | KÖRnyezetvédelmi INFOrmáció. Available online: https://enfo.hu/etanfolyam/5491 (accessed on 6 October 2019).
- Rápó, E.; Szép, R.; Keresztesi, Á.; Suciu, M.; Tonk, S. Adsorptive Removal of Cationic and Anionic Dyes from Aqueous Solutions by Using Eggshell Household Waste as Biosorbent. Acta Chim. Slov. 2018, 65, 709–717. [Google Scholar] [CrossRef]
- Rápó, E.; Aradi, L.E.; Szabó, Á.; Posta, K.; Robert, S.; Szende, T. Adsorption of Remazol Brilliant Violet-5R Textile Dye from Aqueous Solutions by Using Eggshell Waste Biosorbent. Sci. Rep. 2020, 10, 8385. [Google Scholar]
- Sahoo, J.K.; Konar, M.; Rath, J.; Kumar, D.; Sahoo, H. Magnetic hydroxyapatite nanocomposite: Impact on eriochrome black-T removal and antibacterial activity. J. Mol. Liq. 2019, 294, 111596. [Google Scholar] [CrossRef]
- Hunger, K.; Gregory, P.; Miederer, P.; Berneth, H.; Heid, C.; Mennicke, W. Important Chemical Chromophores of Dye Classes. In Industrial Dyes; John Wiley & Sons, Ltd.: Weinheim, Germany, 2004; pp. 13–112. ISBN 978-3-527-60201-8. [Google Scholar]
- da Silva, Á.R.L.; Jhones dos Santos, A.; dos Santos, E.V.; da Silva, D.R.; Martínez-Huitle, C.A. Theoretical and experimental study of the influence of cation–Eriochrome complexes on the BDD anodic oxidation of Eriochrome Black T solutions. Electrochem. Commun. 2020, 112, 106668. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: Zürich, Switzerland, 2003; ISBN 978-3-906390-23-9. [Google Scholar]
- Rápó, E.; Posta, K.; Suciu, M.; Szép, R.; Tonk, S. Adsorptive Removal of Remazol Brilliant Violet-5R Dye from Aqueous Solutions using Calcined Eggshell as Biosorbent. Acta Chim. Slov. 2019, 66, 648–658. [Google Scholar] [CrossRef]
- Naphthalene. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C91203&Type=IR-SPEC&Index=1 (accessed on 5 May 2020).
- 1,5-Dihydroxy Naphthalene(83-56-7) IR2. Available online: https://www.chemicalbook.com/SpectrumEN_83-56-7_ir2.htm (accessed on 15 May 2020).
- Ahmed, F.; Dewani, R.; Pervez, M.K.; Mahboob, S.J.; Soomro, S.A. Non-destructive FT-IR analysis of mono azo dyes. Bulg. Chem. Commun. 2016, 48, 71–77. [Google Scholar]
- Veerakumar, P.; Jeyapragasam, T.; Surabhi; Salamalai, K.; Maiyalagan, T.; Lin, K.-C. Functionalized Mesoporous Carbon Nanostructures for Efficient Removal of Eriochrome Black-T from Aqueous Solution. J. Chem. Eng. Data 2019, 64, 1305–1321. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Yavari, S.; Modirshahla, N. Investigation on adsorption capacity of TiO2-P25 nanoparticles in the removal of a mono-azo dye from aqueous solution: A comprehensive isotherm analysis. Chem. Ind. Chem. Eng. Q. 2014, 20, 97–107. [Google Scholar] [CrossRef]
- Tahir, U.; Yasmin, A.; Khan, U.H. Phytoremediation: Potential flora for synthetic dyestuff metabolism. J. King Saud Univ. Sci. 2016, 28, 119–130. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, X. Effect of different plants on azo-dye wastewater bio-decolorization. Procedia Environ. Sci. 2013, 18, 540–546. [Google Scholar] [CrossRef]
- Muthunarayanan, V.; Santhiya, M.; Swabna, V.; Geetha, A. Phytodegradation of textile dyes by Water Hyacinth (Eichhornia crassipes) from aqueous dye solutions. Int. J. Environ. Sci. 2011, 1, 1702–1717.41. [Google Scholar]
- Torbati, S.; Khataee, A.R.; Movafeghi, A. Application of watercress (Nasturtium officinale R. Br.) for biotreatment of a textile dye: Investigation of some physiological responses and effects of operational parameters. Chem. Eng. Res. Des. 2014, 92, 1934–1941. [Google Scholar] [CrossRef]
- Valipour, A.; Raman, V.K.; Ahn, Y.-H. Effectiveness of Domestic Wastewater Treatment Using a Bio-Hedge Water Hyacinth Wetland System. Water 2015, 7, 329–347. [Google Scholar] [CrossRef]
- Téllez, T.R.; López, E.; Granado, G.; Pérez, E.; López, R.; Guzmán, J. The Water Hyacinth, Eichhornia crassipes: An invasive plant in the Guadiana River Basin (Spain). Aquat. Invasions 2008, 3, 42–53. [Google Scholar] [CrossRef]
- Netten, J.J.C.; Arts, G.H.P.; Gylstra, R.; van Nes, E.H.; Scheffer, M.; Roijackers, R.M.M. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttallii mesocosms. Fundam. App. Lim. 2010, 177, 125–132. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Q.; Xu, G.; Guo, S. New insight into the strategy for nitrogen metabolism in plant cells. Int. Rev. Cell Mol. Biol. 2014, 310, 1–37. [Google Scholar]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant 2008, 133, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 2010, 348, 7. [Google Scholar] [CrossRef]
- Allen, B.; David, P. Handbook of Plant Nutrition; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2015; ISBN 978-1-4398-8197-2. [Google Scholar]
- Fageria, N.K. Nutrient Uptake in Crop Plants; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-367-38688-7. [Google Scholar]
- Mills, H.A.; Jones, J.B. Plant Analysis Handbook II: A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Pub.: Athens, GA, USA, 1996; ISBN 978-1-878148-05-6. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Oki, Y.; Ito, M.; Ueki, K. Studies on the Growth and Reproduction of Water hyacinth, Eichhornia crassipes (Mart.) Solms. J. Weed Sci. Technol. 1978, 23, 120–125. [Google Scholar] [CrossRef]
| Properties of the Dye | |
|---|---|
| Dye names | Eriochrome Black T, Mordant Black 11 |
| Molecular formula | C20H12N3O7SNa |
| M. weight | 461.381 g/mol |
| Max wavelength | 530 nm |
| Colour index | 14645 |
| Colour | Powder: black, deep purple |
| Aqueous solution after protonated form: blue | |
| Aqueous solution after making complex with C2+ and Mg 2+: red | |
| Characteristics | Mono-azo |
| Sample (mg/L EBT) | Lettuce Seed | ||
|---|---|---|---|
| Root Length (cm) | Number of Germinated Seeds | Root Growth Inhibition (%) | |
| 0 | 1.2 | 19.5 | |
| 50 | 1.5 | 19.5 | −23.7 |
| 100 | 1.2 | 15.5 | 4.4 |
| 150 | 1.0 | 17.0 | 21.7 |
| 200 | 0.9 | 16.0 | 30.2 |
| 250 | 0.9 | 18.0 | 30.4 |
| 500 | 0.8 | 18.0 | 32.2 |
| Plant | Dye Name (Colour Index Number) | Initial Concentration (mg/L) | Efficiency (%) | Reference |
|---|---|---|---|---|
| Lemna minor | Malachite Green (C.I. 11294) | 40 | 98.0 | [23] |
| Lemna minor | Cristal Violet (C.I. 42555) | 40 | 96.0 | [23] |
| Sesbania cannabina | Acid red B (C.I. 14680) | 100 | 44.23 | [41] |
| Sesbania cannabina | Acid red B (C.I. 14680) | 500 | 22.58 | [41] |
| Sesbania cannabina | Acid red B (C.I. 14680) | 1000 | 12.23 | [41] |
| Sesbania cannabina | Acid scarlet GR (C.I. 27290) | 1000 | 8.66 | [41] |
| Eichhornia crassipes | Reactive Black 5 (C.I. 20505) | 10 | 99.5 | [42] |
| Eichhornia crassipes | Reactive Red 198 (C.I. 18221) | 10 | 95.0 | [42] |
| Nasturtium officinale | Acid Blue 92 (C.I. 13390) | 5 | 79.1 | [43] |
| Eichhornia crassipes | Eriochrome Black T (C.I. 14645) | 150 | 33.0 | Present study |
| Salvinia natans | Eriochrome Black T (C.I. 14645) | 150 | 77.5 | Present study |
| Bioconcentration Factors (%) | ||||
|---|---|---|---|---|
| Eichhornia crassipes | Salvinia natans | |||
| Plant in CEBT = 50 mg/L | Plant in CEBT = 500 mg/L | Plant in CEBT = 50 mg/L | Plant in CEBT = 500 mg/L | |
| Mg | −40.5 | −81.2 | −55.6 | −75.2 |
| P | −65 | −78.6 | −39.5 | −69 |
| S | −38.1 | −66.3 | −63 | −68.9 |
| K | 7 | −75 | −48.4 | −66.5 |
| Ca | 17.5 | −68.4 | −64.2 | −88.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rápó, E.; Posta, K.; Csavdári, A.; Vincze, B.É.; Mara, G.; Kovács, G.; Haddidi, I.; Tonk, S. Performance Comparison of Eichhornia crassipes and Salvinia natans on Azo-Dye (Eriochrome Black T) Phytoremediation. Crystals 2020, 10, 565. https://doi.org/10.3390/cryst10070565
Rápó E, Posta K, Csavdári A, Vincze BÉ, Mara G, Kovács G, Haddidi I, Tonk S. Performance Comparison of Eichhornia crassipes and Salvinia natans on Azo-Dye (Eriochrome Black T) Phytoremediation. Crystals. 2020; 10(7):565. https://doi.org/10.3390/cryst10070565
Chicago/Turabian StyleRápó, Eszter, Katalin Posta, Alexandra Csavdári, Boglárka Éva Vincze, Gyöngyvér Mara, Gábor Kovács, Imane Haddidi, and Szende Tonk. 2020. "Performance Comparison of Eichhornia crassipes and Salvinia natans on Azo-Dye (Eriochrome Black T) Phytoremediation" Crystals 10, no. 7: 565. https://doi.org/10.3390/cryst10070565
APA StyleRápó, E., Posta, K., Csavdári, A., Vincze, B. É., Mara, G., Kovács, G., Haddidi, I., & Tonk, S. (2020). Performance Comparison of Eichhornia crassipes and Salvinia natans on Azo-Dye (Eriochrome Black T) Phytoremediation. Crystals, 10(7), 565. https://doi.org/10.3390/cryst10070565