Synthesis, Single Crystal X-Ray Structure, Hirshfeld Surface Analysis, DFT Computations, Docking Studies on Aurora Kinases and an Anticancer Property of 3-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-6-methoxy-4H-chromen-4-one
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Crystal Structure Determination
2.3. In Silico Docking with Aurora Kinases
3. Results and Discussion
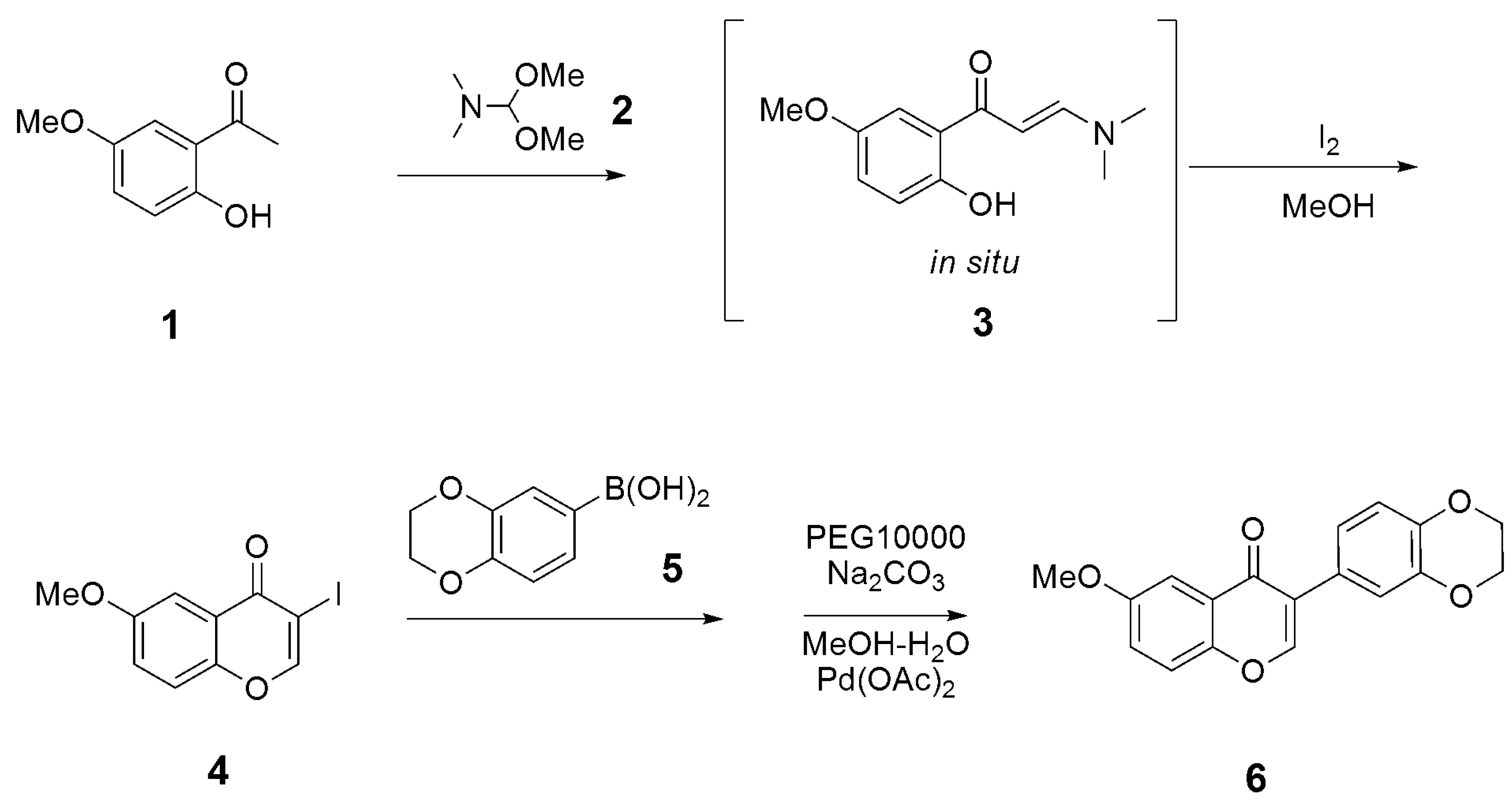
3.1. Synthesis
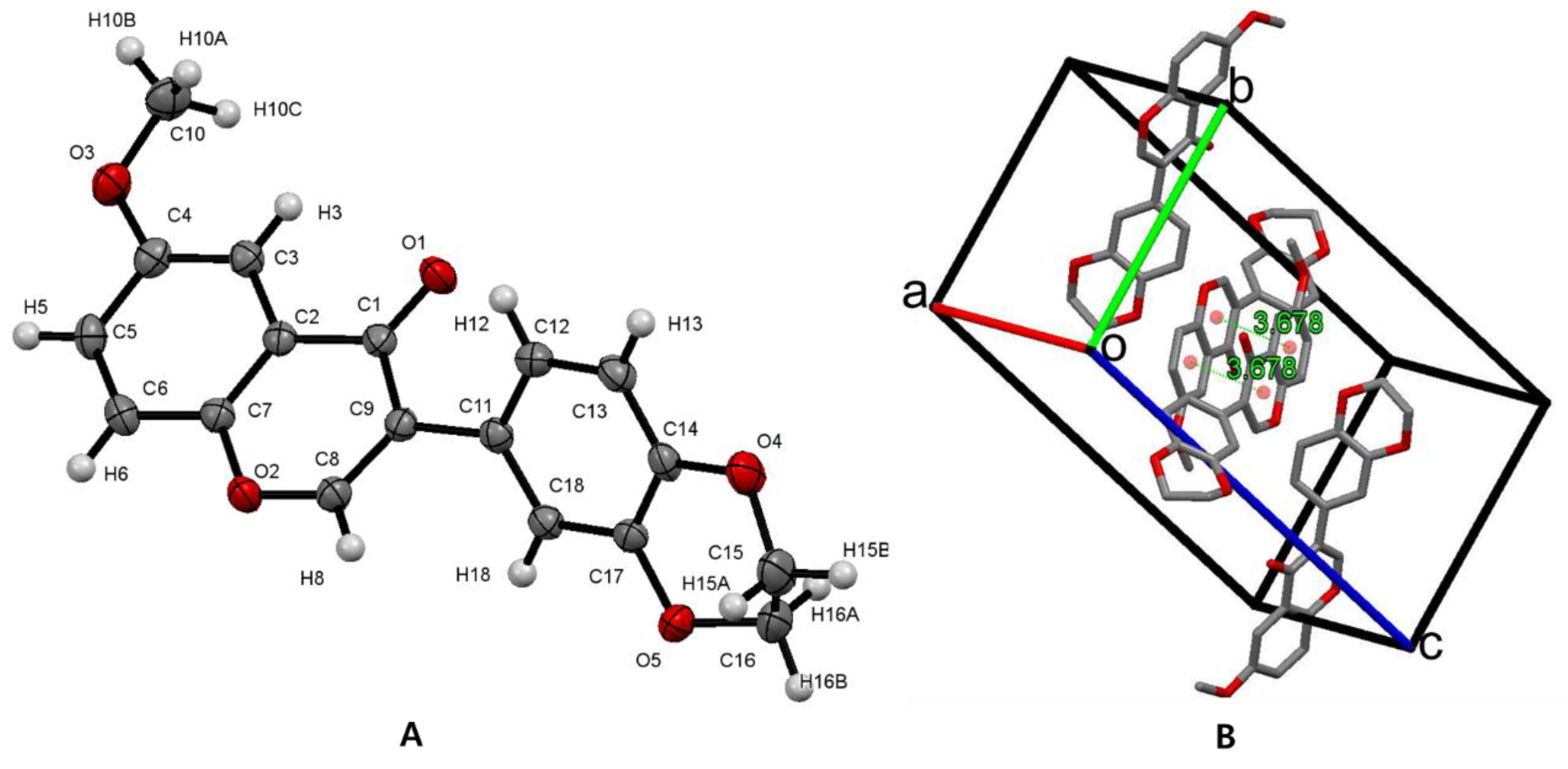
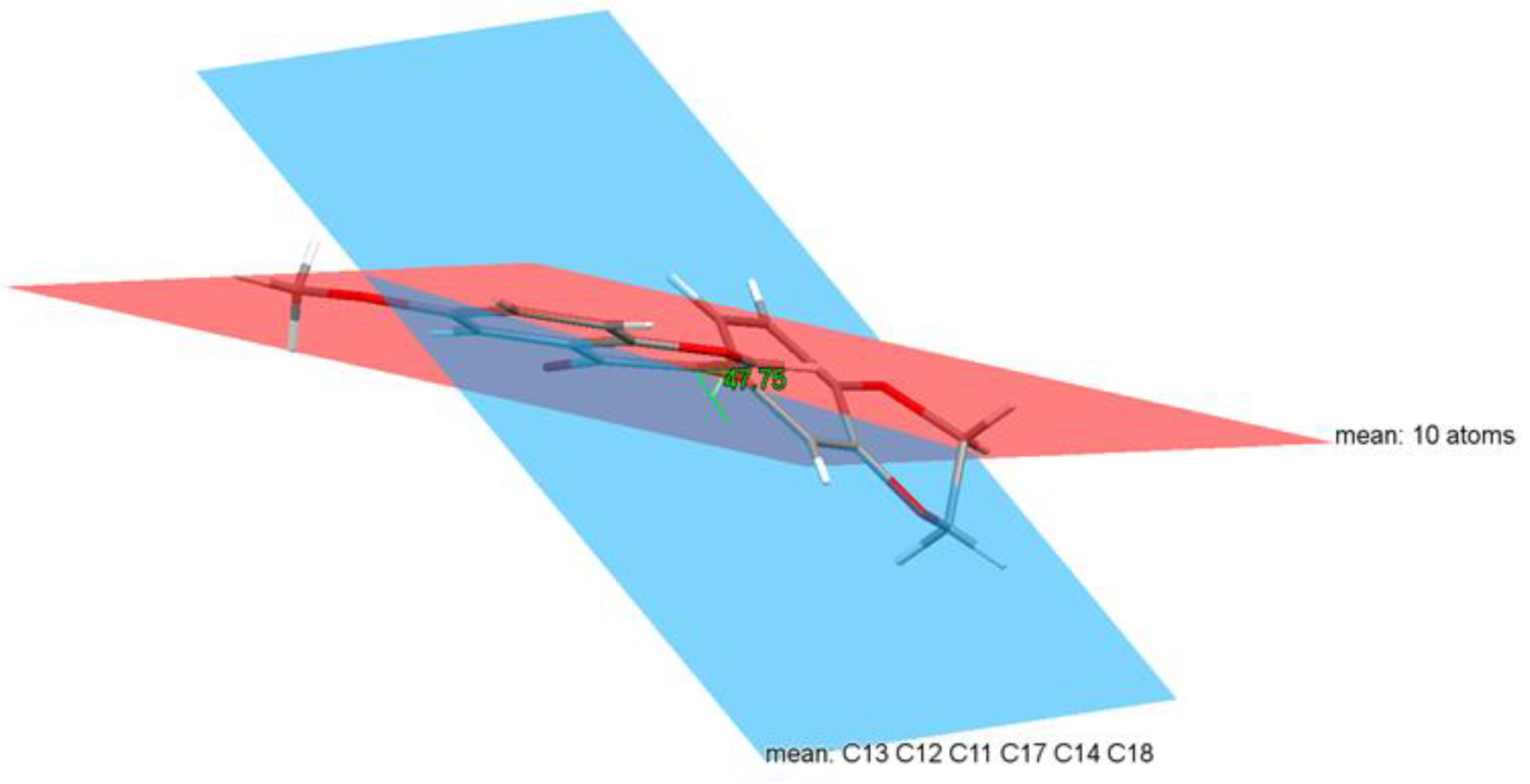
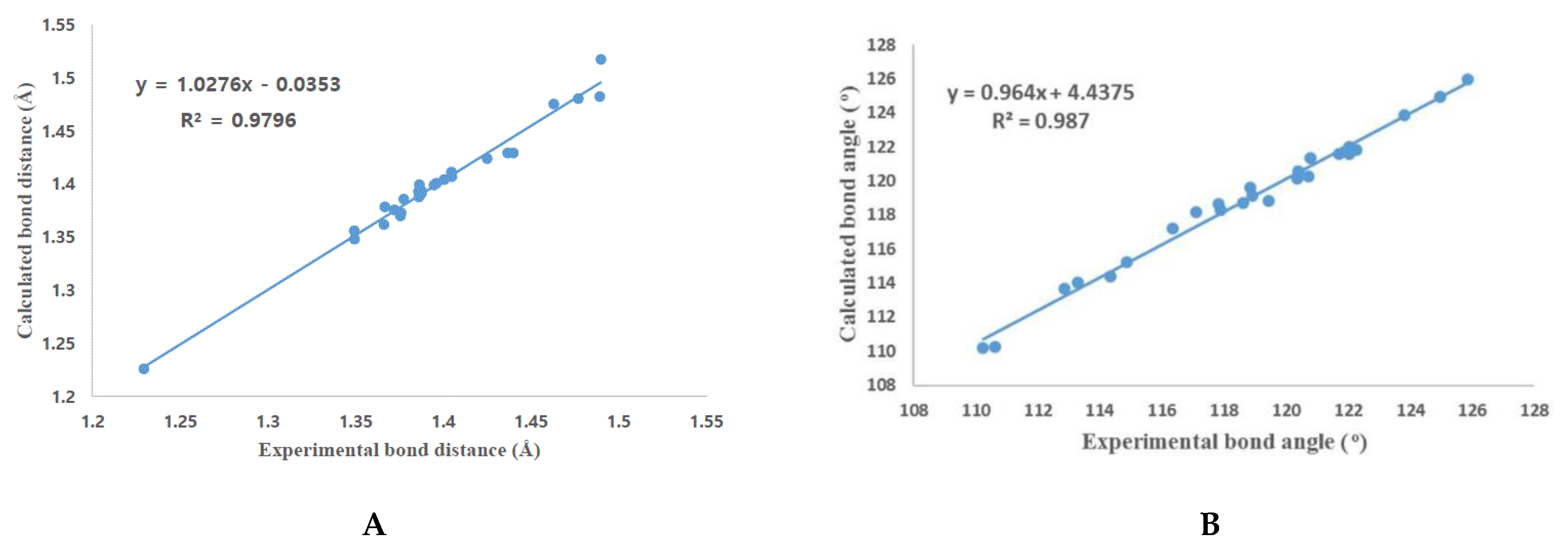
3.2. Crystal Structure of Isoflavone Compound 6
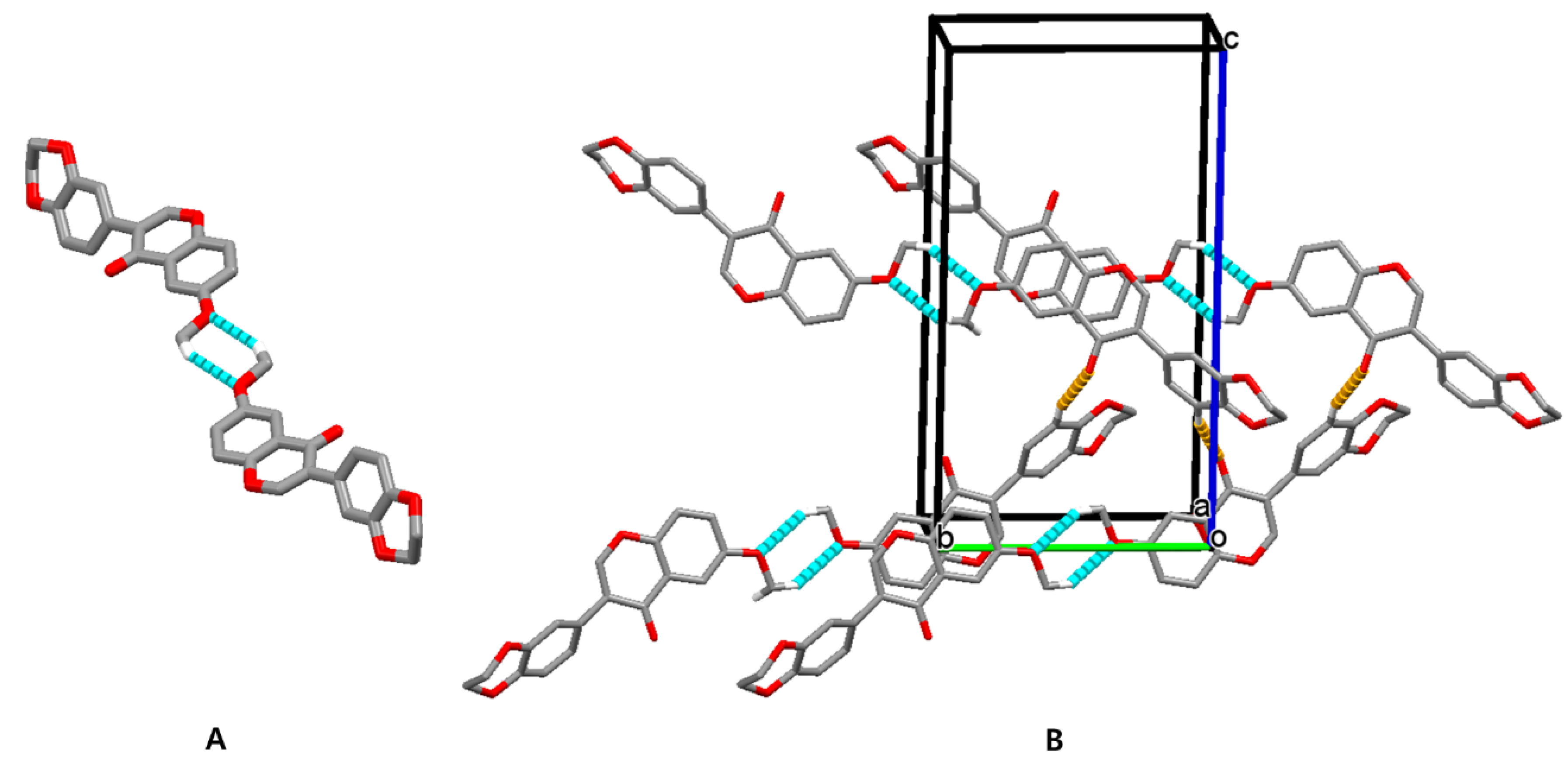
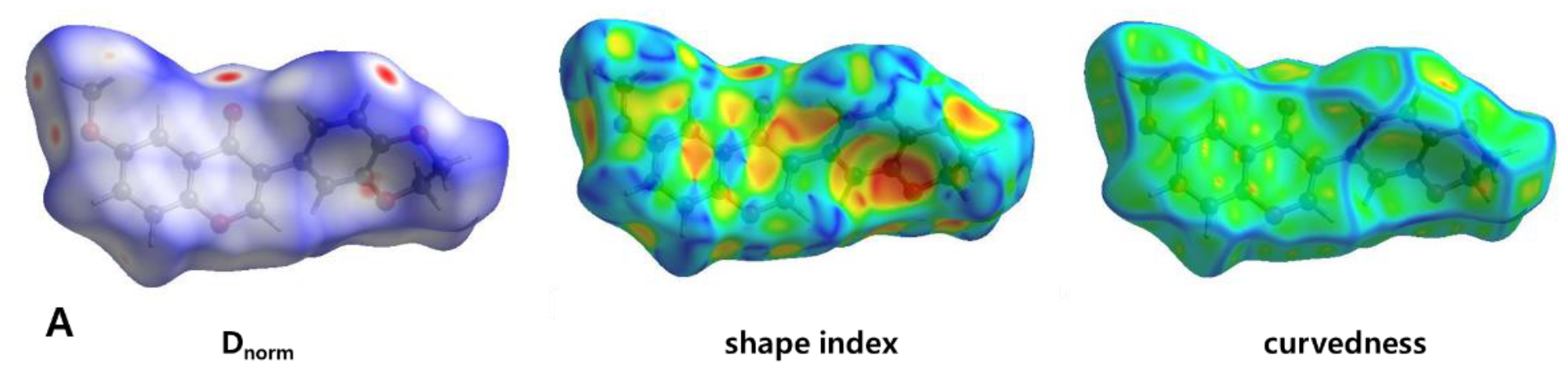
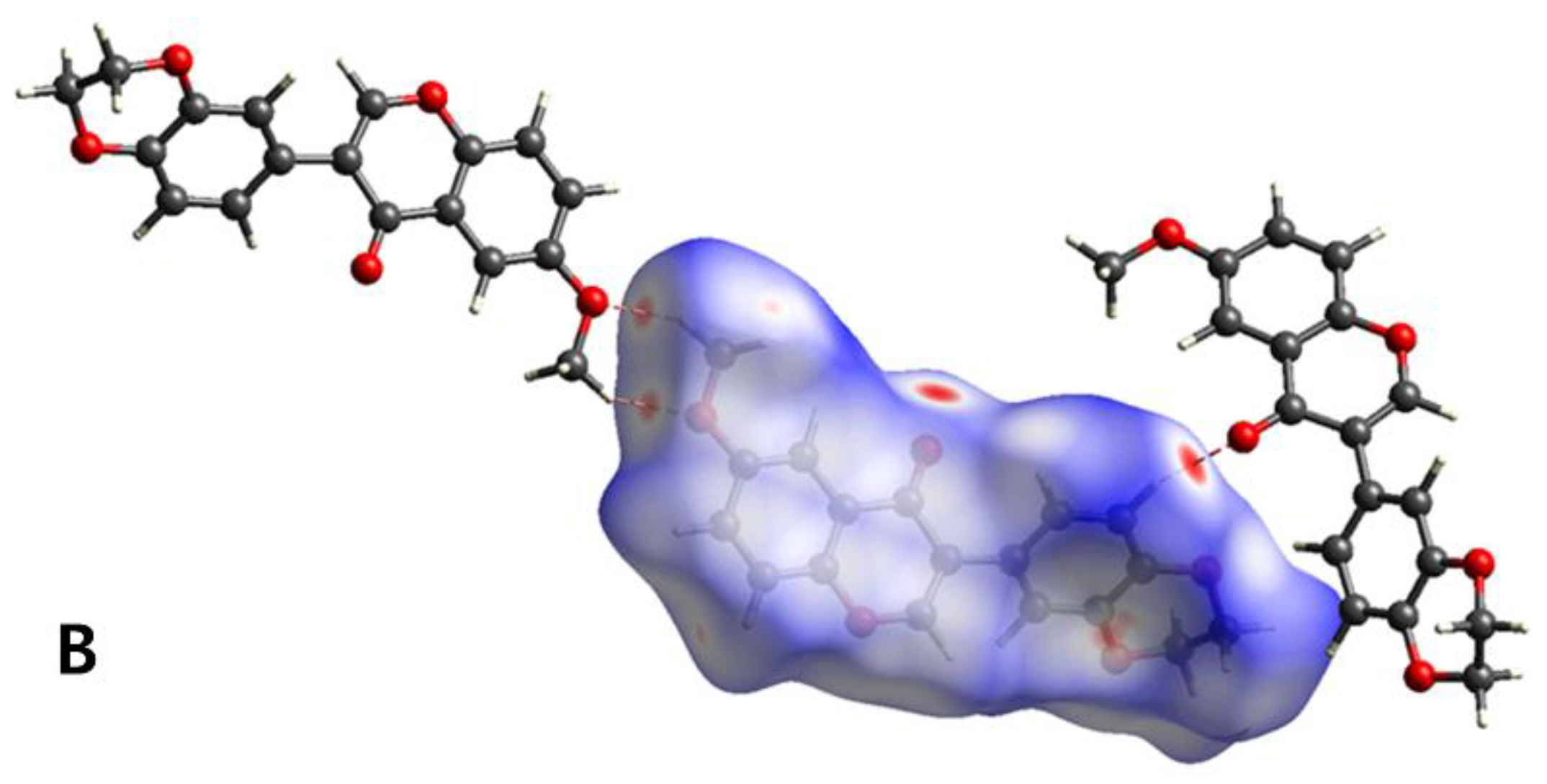
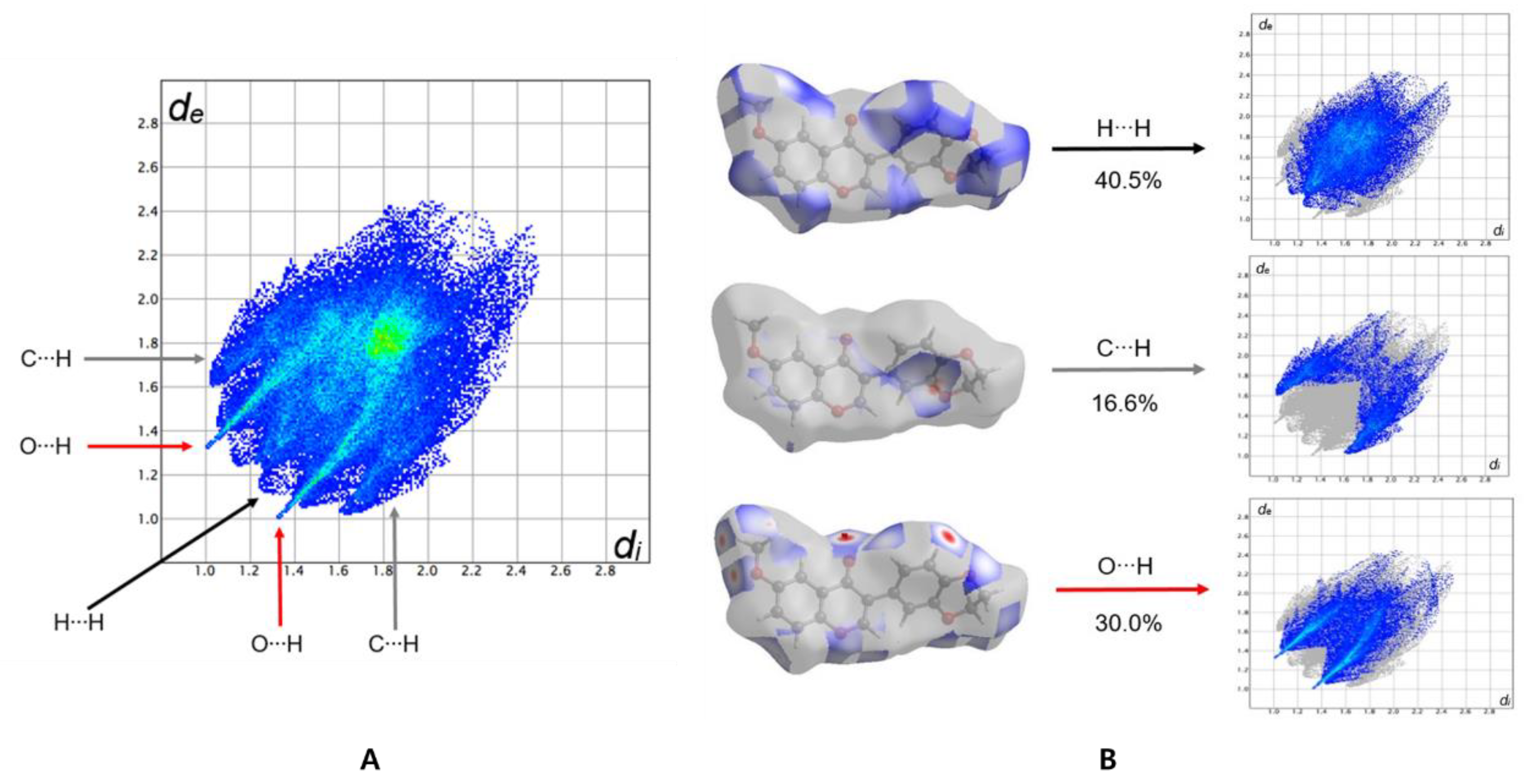
3.3. Hirshfeld Surface Analysis
3.4. DFT Calculation
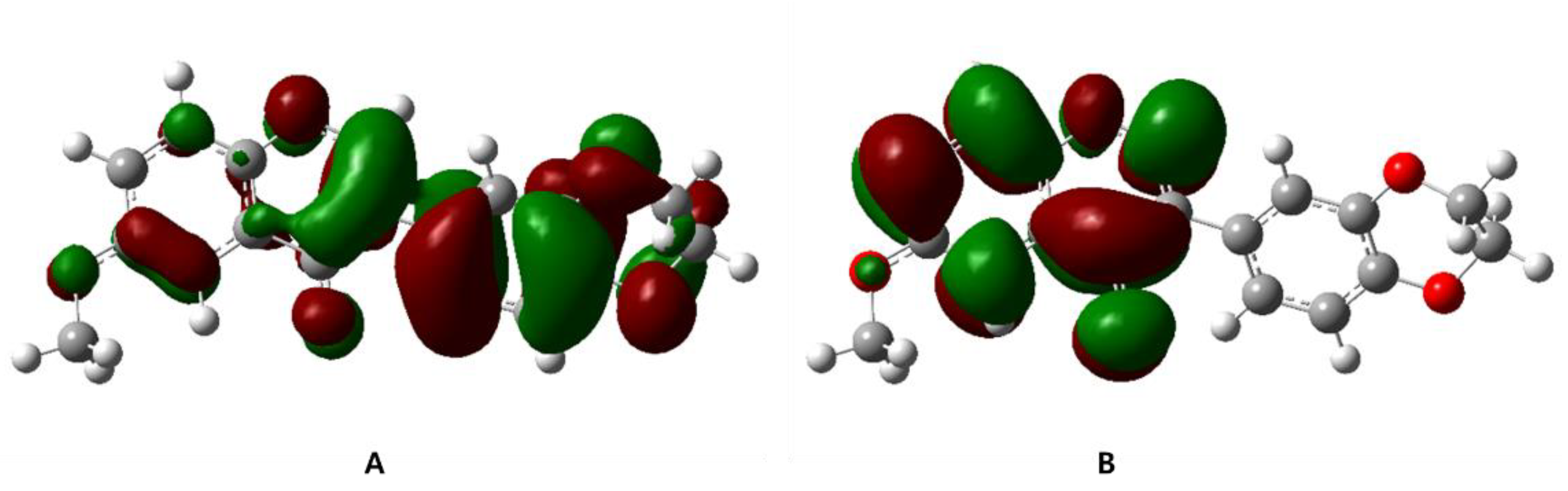
3.5. Frontier Molecular Orbital Calculation
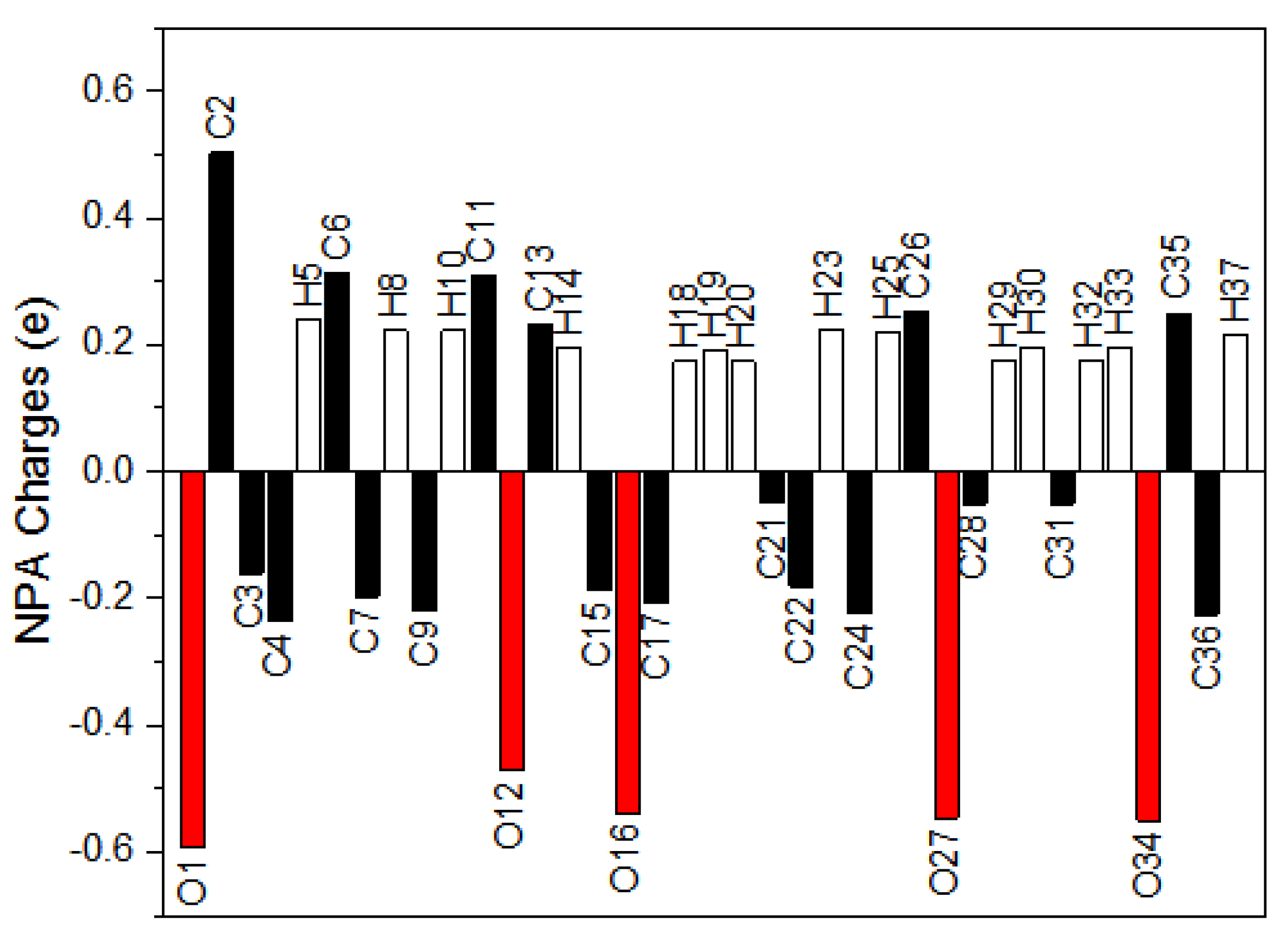
3.6. Natural Population Analysis (NPA)
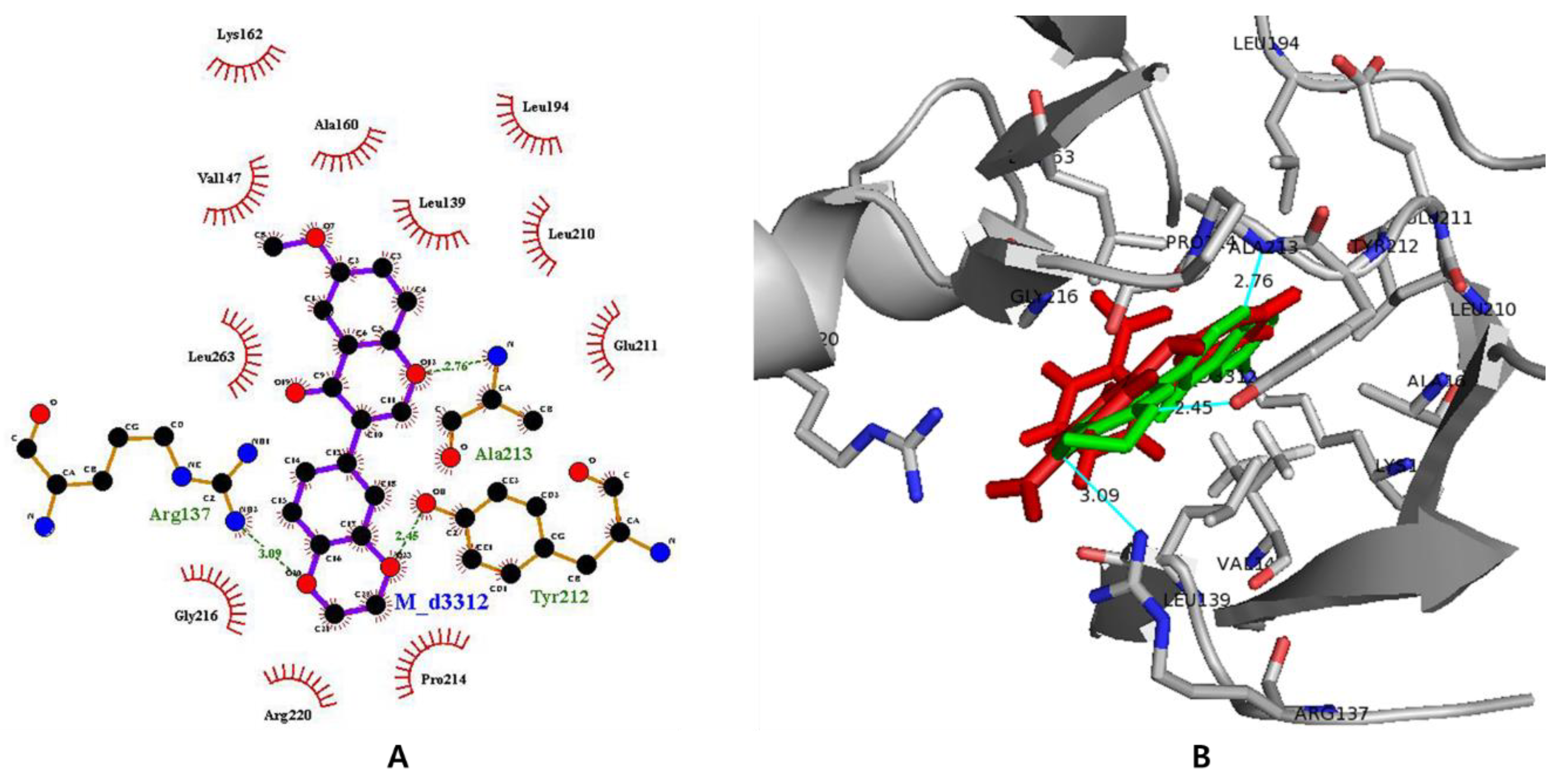
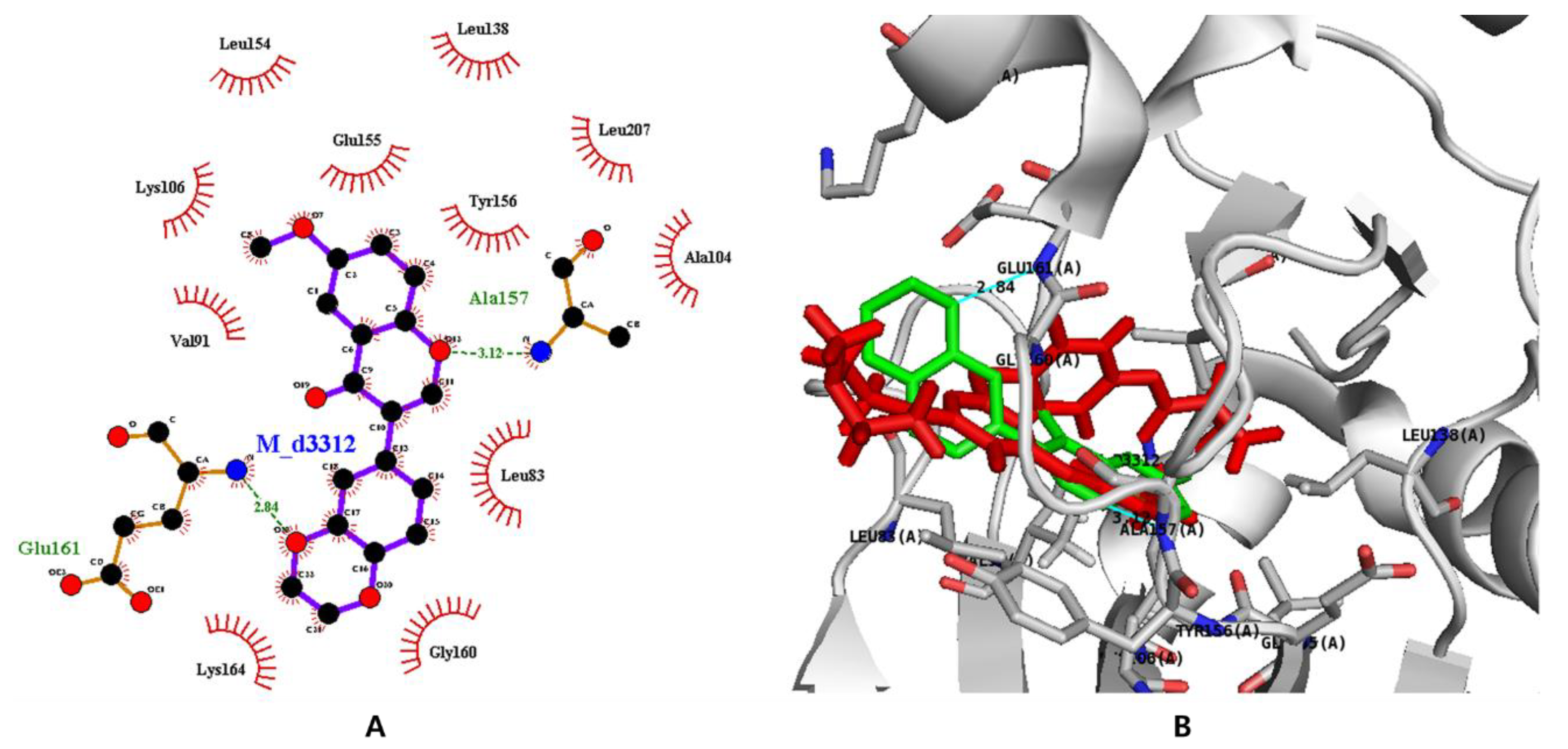
3.7. In Silico Docking with Aurora Kinases
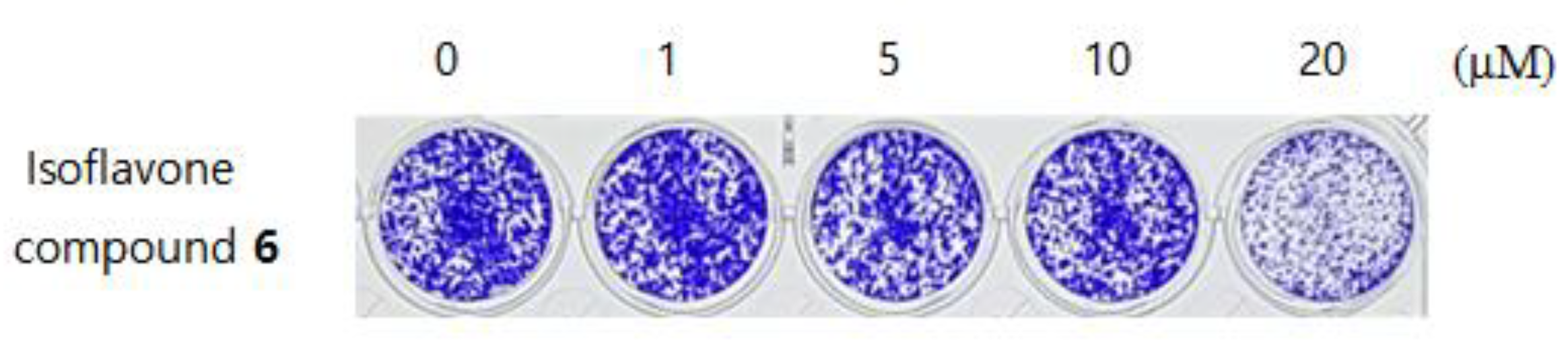
3.8. Anti-Cancer Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Taylor, S.F.; Dupart, P.S.; Arnold, C.L.; Sridhar, J.; Jiang, Q.; Wang, Y.; Skripnikova, E.V.; Zhao, M.; Foroozesh, M. Pyranoflavones: A group of small-molecule probes for exploring the active site cavities of cytochrome P450 enzymes 1A1, 1A2, and 1B1. J. Med. Chem. 2013, 56, 4082–4092. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Furman, C.; Bernier, J.L.; Duriez, P.; Teissier, E.; Cotelle, N. Antioxidant properties of di-tert-butylhydroxylated flavonoids. Free Radic. Biol. Med. 2000, 29, 900–912. [Google Scholar] [CrossRef]
- Park, W.H. MAPK inhibitors differentially affect gallic acid-induced human pulmonary fibroblast cell growth inhibition. Mol. Med. Rep. 2011, 4, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Han, X.Z.; Li, X.; Ren, D.M.; Wang, X.N.; Lou, H.X. Flavonoids from Dracocephalum tanguticum and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Bioorg. Med. Chem. Lett. 2010, 20, 6411–6415. [Google Scholar] [CrossRef]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Barakat, A.; Soliman, A.M.; El-Faham, A.; Ali, M.; Al-Majid, A.M.; Yousuf, S.; Choudhary, M.I. Three multi-components reaction: Synthesis and X-ray single-crystal of hydroacridinone-based hydrazino-s-triazine derivative as a new class of urease inhibitor. Crystals 2020, 10, 14. [Google Scholar] [CrossRef]
- Al-Wabli, R.I.; Al-Ghamdi, A.R.; Aswathy, S.V.; Ghabbour, H.A.; Al-Agamy, M.H.; Hubert Joe, I.H.; Attia, M.I. Synthesis, single crystal X-ray structure, DFT computations, hirshfeld surface analysis and molecular docking simulations on ({[(1E)-1-(1,3-Benzodioxol-5-yl)-3-(1H-imidazol-1-yl) propylidene]amino}oxy)(furan-2-yl)methanone: A new antifungal agent. Crystals 2019, 9, 25. [Google Scholar] [CrossRef]
- Joubert, J. Synthesis, crystal structure, DFT studies, docking, studies, and fluorescent properties of 2-(Adamantan-1-yl)-2H-isoindole-1-carbonitrile. Crystals 2019, 9, 24. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, Y.; Kim, B.S.; Lee, J.; Ahn, S.; Koh, D.; Lim, Y.; Lee, Y.H. Inhibitory effect of synthetic flavone derivatives on pan-aurora kinases: Induction of G2/M cell-cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Sci. 2018, 19, 86. [Google Scholar] [CrossRef]
- Ahn, S.; Ahn, E.; Sung, J.; Koh, D.; Lim, Y.; Park, S. Synthetic polyphenol compounds inhibit β-catenin/Tcf signaling: Structure-activity relationship. J. Ind. Eng. Chem. 2017, 56, 258–269. [Google Scholar] [CrossRef]
- Lee, K.; Lee, D.H.; Jung, Y.J.; Shin, S.Y.; Koh, D.; Lee, Y.H. A methoxyflavanone derivative, 2′,3′,4′-trimethoxy-5,6-naphthoflavanone, inhibits proliferation of HCT116 human colon cancer cells by inducing G2/M cell cycle arrest and apoptosis. Appl. Biol. Chem. 2016, 59, 249–253. [Google Scholar] [CrossRef]
- Sophors, P.; Kim, Y.M.; Seo, G.Y.; Huh, J.S.; Lim, Y.; Koh, D.S.; Cho, M. A synthetic isoflavone, DCMF, promotes human keratinocyte migration by activating Src/FAK signaling pathway. Biochem. Biophys. Res. Commun. 2016, 472, 332–338. [Google Scholar] [CrossRef]
- Ahn, S.; Shin, S.Y.; Jung, Y.; Jung, H.; Kim, B.S.; Koh, D.; Lim, Y. (1) H and (13) C NMR spectral assignments of novel flavonoids bearing benzothiazepine. Magn. Reson. Chem. 2016, 54, 382–390. [Google Scholar] [CrossRef]
- Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta. Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Martin, M.P.; Zhu, J.Y.; Lawrence, H.R.; Pireddu, R.; Luo, Y.; Alam, R.; Ozcan, S.; Sebti, S.M.; Lawrence, N.J.; Schonbrunn, E. A novel mechanism by which small molecule inhibitors induce the DFG flip in Aurora A. ACS Chem. Biol. 2012, 7, 698–706. [Google Scholar] [CrossRef]
- Fancelli, D.; Moll, J.; Varasi, M.; Bravo, R.; Artico, R.; Berta, D.; Bindi, S.; Cameron, A.; Candiani, I.; Cappella, P.; et al. 1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazoles: Identification of a potent Aurora kinase inhibitor with a favorable antitumor kinase inhibition profile. J. Med. Chem. 2006, 49, 7247–7251. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.M.; Santaguida, S.; Musacchio, A.; Knapp, S. Crystal structure of human aurora B in complex with INCENP and VX-680. J. Med. Chem. 2012, 55, 7841–7848. [Google Scholar] [CrossRef]
- Biegasiewicz, K.F.; Gordon, J.S.; Rodriguez, D.A.; Priefer, R. Development of a general approach to the synthesis of a library of isoflavonoid derivatives. Tetrahedron Lett. 2014, 55, 5210–5212. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, Y. Phosphine-free palladium acetate catalyzed Suzuki reaction in water. J. Org. Chem. 2005, 70, 6122–6125. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lim, Y.; Koh, D. Crystal structure of 2-(2,3-di-meth-oxy-naphthalen-1-yl)-3-hy-droxy-6-meth-oxy-4H-chromen-4-one. Acta. Cryst. E Cryst. Commun. 2015, 71, o842–o843. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Lim, Y.; Koh, D. Crystal structure of 2-(3,4-di-meth-oxy-phen-yl)-3-hy-droxy-4H-chromen-4-one. Acta. Cryst. Sect. E Struct. Rep. Online 2014, 70, o999–o1000. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Petersson, G.A.; Allaham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6091. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO Version 3.1.; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jung, K.Y.; Park, J.; Han, Y.S.; Lee, Y.H.; Shin, S.Y.; Lim, Y. Synthesis and biological evaluation of hesperetin derivatives as agents inducing apoptosis. Bioorg. Med. Chem. 2017, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Shin, S.Y.; Ahn, S.; Koh, D.; Lee, Y.H.; Lim, Y. Biological evaluation of 2-pyrazolinyl-1-carbothioamide derivatives against HCT116 human colorectal cancer cell lines and elucidation on QSAR and molecular binding modes. Bioorg. Med. Chem. 2017, 25, 5423–5432. [Google Scholar] [CrossRef]

| Empirical formula | C18H14O5 |
| Formula weight | 310.29 |
| Temperature | 223(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Unit cell dimensions | a = 7.1869(4) Å b = 10.2764(6) Å c = 19.6771(10) Å β = 99.442(2)° |
| Volume | 1433.57(14) Å3 |
| Z | 4 |
| Density (calculated) | 1.438 Mg/m3 |
| Absorption coefficient | 0.106 mm−1 |
| F(000) | 648 |
| Crystal size | 0.190 × 0.150 × 0.100 mm3 |
| Theta range for data collection | 2.098 to 28.296° |
| Index ranges | −9 ≤ h ≤ 9, −13 ≤ k ≤ 13, −26 ≤ l ≤26 |
| Reflections collected | 39243 |
| Independent reflections | 3573 [R(int) = 0.0491] |
| Completeness to theta = 25.242° | 100.00% |
| Refinement method | Full-matrix least-squares on F2 |
| Data / restraints / parameters | 3573 / 0 / 209 |
| Goodness-of-fit on F2 | 1.039 |
| Final R indices [I>2sigma(I)] | R1 = 0.0396, wR2 = 0.0990 |
| R indices (all data) | R1 = 0.0577, wR2 = 0.1083 |
| Largest diff. peak and hole | 0.347 and −0.183 e.Å−3 |
| C(10)-O(3)-C(4)-C(3) | −5.8(2) |
| C(17)-C(14)-O(4)-C(15) | −12.8(2) |
| C(14)-C(17)-O(5)-C(16) | −15.9(2) |
| C(10)-O(3)-C(4)-C(3) | −5.8(2) |
| C(7)-C(2)-C(1)-C(9) | −3.1(2) |
| H(16A)-C(16)-C(15)-H(15A) | 179.1 |
| H(15A)-C(15)-C(16)-H(16B) | −62.3 |
| H(16A)-C(16)-C(15)-H(15B) | −62.5 |
| H(16B)-C(16)-C(15)-H(15B) | 56.1 |
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
|---|---|---|---|---|
| C(13)-H(13)···O(1)#1 | 0.94 | 2.48 | 3.3970(17) | 163.8 |
| C(10)-(10B)···O(3)#2 | 0.97 | 2.55 | 3.4005(19) | 146.9 |
| C(6)-H(6)···O(4)#3 | 0.94 | 2.61 | 3.2639(17) | 127.0 |
| C(16)-(16A)···O(1)#4 | 0.98 | 2.62 | 3.581(2) | 168.2 |
| Dihedral Angle | Experimental | Calculated | |
|---|---|---|---|
| Crystal | Vacuum | DMSO | |
| C(1)-C(9)-C(11)-C(12) | 49.3755 | 42.4779 | 49.5214 |
| C(1)-C(9)-C(11)-C(18) | −134.245 | −138.276 | −131.476 |
| C(8)-C(9)-C(11)-C(12) | −129.908 | −137.454 | −131.062 |
| C(8)-C(9)-C(11)-C(18) | 46.4713 | 41.7922 | 47.9409 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.; Sung, J.; Lee, J.H.; Yoo, M.; Lim, Y.; Shin, S.Y.; Koh, D. Synthesis, Single Crystal X-Ray Structure, Hirshfeld Surface Analysis, DFT Computations, Docking Studies on Aurora Kinases and an Anticancer Property of 3-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-6-methoxy-4H-chromen-4-one. Crystals 2020, 10, 413. https://doi.org/10.3390/cryst10050413
Ahn S, Sung J, Lee JH, Yoo M, Lim Y, Shin SY, Koh D. Synthesis, Single Crystal X-Ray Structure, Hirshfeld Surface Analysis, DFT Computations, Docking Studies on Aurora Kinases and an Anticancer Property of 3-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-6-methoxy-4H-chromen-4-one. Crystals. 2020; 10(5):413. https://doi.org/10.3390/cryst10050413
Chicago/Turabian StyleAhn, Seunghyun, Jiha Sung, Ji Hye Lee, Miri Yoo, Yoongho Lim, Soon Young Shin, and Dongsoo Koh. 2020. "Synthesis, Single Crystal X-Ray Structure, Hirshfeld Surface Analysis, DFT Computations, Docking Studies on Aurora Kinases and an Anticancer Property of 3-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-6-methoxy-4H-chromen-4-one" Crystals 10, no. 5: 413. https://doi.org/10.3390/cryst10050413
APA StyleAhn, S., Sung, J., Lee, J. H., Yoo, M., Lim, Y., Shin, S. Y., & Koh, D. (2020). Synthesis, Single Crystal X-Ray Structure, Hirshfeld Surface Analysis, DFT Computations, Docking Studies on Aurora Kinases and an Anticancer Property of 3-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-6-methoxy-4H-chromen-4-one. Crystals, 10(5), 413. https://doi.org/10.3390/cryst10050413





