Abstract
Fully-stoichiometric SmBaCo2-xMnxO6 oxides (x = 0, 0.5, 1) were obtained through the electrochemical oxidation method performed in 1 M KOH solution from starting materials having close to equilibrium oxygen content. Cycling voltammetry scans allow us to recognize the voltage range (0.3–0.55 V vs. Hg/HgO electrode) for which electrochemical oxidation occurs with high efficiency. In a similarly performed galvanostatic experiment, the value of the stabilized voltage recorded during the oxidation increased with higher Mn content, which seems to relate to the electronic structure of the compounds. Results of the iodometric titration and thermogravimetric analysis prove that the proposed technique allows for an increase in the oxygen content in SmBaCo2-xMnxO5+δ materials to values close to 6 (δ ≈ 1). While the expected significant enhancement of the total conductivity was observed for the oxidized samples, surprisingly, their crystal structure only underwent slight modification. This can be interpreted as due to the unique nature of the oxygen intercalation process at room temperature.
1. Introduction
Nonstoichiometry is well-known to impact the physicochemical properties of ceramic materials, since its change may cause not only modifications in the crystal structure, but also influences the magnetic and transport properties, often having a direct impact on the applicability of particular compounds [1,2,3]. Thus, an extremely important aspect related to nonstoichiometry is the possibility of its strict control. In the case of materials deviating from the stoichiometric composition in the oxygen sublattice, control of the oxygen content can be realized by appropriate heating in atmospheres with controlled total and oxygen partial pressure or by the much less commonly utilized electrochemical oxidation in aqueous solution [4,5,6,7,8,9,10,11,12].
Generally, an effective oxygen incorporation into/release from the crystal structure may be carried out only if sufficiently high mobility of the oxygen anions is ensured. Classically, this can be achieved if high temperatures—above 300 °C, but typically starting from ca. 400–700 °C—are considered. In such conditions, the appropriately adjusted oxygen partial pressure allows a relatively broad extent to control the oxygen content in the considered oxides [4,6]. However, the usefulness of this approach can be limited, which happens if unusually high oxidation states of 3d metals are expected to be present to maintain the electroneutrality of the (usually complex) transition metal-based oxide. Such a situation was observed for SrCoO3-δ, for which even long term oxidation (480 h) conducted at elevated temperature (400 °C) under pO2 as high as 160 MPa does not allow for the preparation of a fully-stoichiometric oxide, but only SrCoO2.97 (δ = 0.03) [5]. On the other hand, stoichiometric SrCoO3 and multiple other fully-oxidized perovskite-type oxides can be successfully obtained by electrochemical oxidation in an alkaline solution [5]. This approach became the subject of intensified studies in the 90s, belonging to the field of soft chemistry methods considered in preparation of oxides having not only the desired content of cations, but controlled oxygen stoichiometry. It was documented that such electrochemical oxidation allows for metastable forms of highly oxidized materials to be obtained even at room temperature, at which the mobility of the oxygen ions in the crystal structure is exceedingly low (even for the best ionic conductors) [4,6,12]. The method itself is based on the obvious (but often neglected) property that according to the Nernst equation, (anodic) polarization η equal to 150 mV leads to increased oxygen activity in the solution, estimated as corresponding to the partial pressure of the oxygen to the order of 109 Pa [7]. With a strong driving force unattainable by other means, electrochemical oxidation allows for the successful and reversible incorporation of oxygen ions into the crystal structure of oxides including the preparation of fully-oxidized compounds.
Practical usage of electrochemical oxidation can be regarded as a relatively popular tool, used mostly in basic studies that create opportunity to systematically evaluate the correlation between oxygen content and physicochemical properties of the ceramic oxides. In this aspect, it has been used up to now to characterize oxides with perovskite and perovskite-related structures (including superconducting La2CuO4±δ) [4,5,6,7,8,11,12]. Until now, however, there have been no reports available that show the application of this method to highly oxidize the A-site cation ordered perovskite-type cobaltites and/or manganites.
The materials that are considered in this paper with the REBaCo2−xMnxO5+δ (RE: rare-earth element) general formula have been relatively well examined ceramics, which have attracted attention due to their excellent transport properties and high catalytic activity in relation to the oxygen reduction reaction [13,14,15,16,17]. As mentioned, REBaCo2−xMnxO5+δ oxides exhibit double perovskite-type crystal structure with a 1:1 layered arrangement of RE-Ba cations present in the A sublattice. Due to the commonly observed high degree of oxygen nonstoichiometry, their chemical formula is generally given as AA’B2O5+δ. The layered A-site ordering results from the large difference between RE3+ and Ba2+ radii, and is typically accompanied by oxygen vacancies, which help to stabilize such structures by lowering the coordination number of smaller RE3+ [18,19].
The possibility of obtaining fully-oxidized REBaCo2−xMnxO6 seems to be intriguing, since crystal structure and other basic physicochemical properties, mainly electronic and ionic components of the electrical conductivity, are strongly linked with the oxygen content [1]. In the case of the electronic charge transport, this is due to the presence of the so-called double exchange (Zener mechanism), the effectiveness of which depends on metal–oxygen–metal (B-O-B) bonds, and as such, requires the presence of oxygen anions [20,21,22]. On the other hand, a high concentration of the oxygen vacancies enhances effective ionic transport at high temperatures, occurring through the vacancy-type mechanism [23].
In this work, SmBaCo2−xMnxO5+δ (x = 0, 0.5 and 1) oxides with the A-site cation-ordered perovskite-type structure were synthesized and examined in terms of crystal structure and electrical conductivity evolution resulting from full oxidation by the electrochemical method. Initially, a sol-gel synthesis route combined with proper annealing conducted in Ar and air atmospheres was used to obtain the desired materials. Thin sinters of SmBaCo2−xMnxO5+δ oxides with the relative density of ca. 80% (i.e., by purpose not fully-densified specimens) were examined by cyclic voltammetry in 1 M KOH solution using a three-electrode setup with a Hg/HgO reference electrode. This enabled us to identify the voltage range in which electrochemical oxidation occurs. The influence of chemical composition on the voltage of galvanostatic oxidation was also analyzed. Finally, fully-stoichiometric SmBaCo2−xMnxO6 oxides were obtained by the properly conducted electrochemical oxidation (galvanostatic mode), and structural properties as well as the electrical conductivity of the samples were studied.
2. Materials and Methods
The considered SmBaCo2−xMnxO5+δ (x = 0, 0.5 and 1) oxides were synthesized with a typical sol-gel method, for which proper amounts of nitrates and two complexing agents, ethylenediaminetetraacetic acid (EDTA) and citric acid, were used (no polymerizing agent was utilized during the synthesis of the materials) [17]. Respective precursors were annealed twice in an Ar (SmBaCo1.5Mn0.5O5+δ and SmBaCoMnO5+δ) or air atmosphere (SmBaCo2O5+δ) at 1200 °C. After the first heating, respective powders were ground in an agate mortar, pelletized, and annealed again. Such a procedure allowed us to obtain pellets with a relative density close to 80%, which is favorable when the efficiency of the electrochemical oxidation is considered, since the process performed in the solution does not proceed efficiently for fully-dense specimens due to the limited area of the solid/liquid interface [5]. Consequently, partially porous, thin pellets, which possessed suitable mechanical strength, were prepared for the electrochemical experiments. Density of the obtained sinters was determined using geometric dimensions, weight, and the theoretical density [g cm−3] determined using GSAS & EXGPUI software (GSAS-I, subversion.xray.aps.anl.gov/trac/EXPGUI) in Rietveld refinements of the unit cell parameters.
Moreover, SmBaCo1.5Mn0.5O5+δ and SmBaCoMnO5+δ samples were subjected to additional heating, conducted at 900 °C in an. air atmosphere for 24 h. Subsequent slow cooling (ca. 2.5 °C min−1) to room temperature (RT) allowed us to obtain the desired materials with a (close to) equilibrium oxygen content [17].
The electrochemical oxidation was performed in 1 M KOH solution using a three-electrode setup with the Hg/HgO reference electrode filled with 1 M KOH (potential versus standard hydrogen electrode - SHE equal to 0.098 V at room temperature). All values of the potential given in this work refer to the mentioned Hg/HgO electrode. The counter electrode was platinum mesh, while the working electrode was prepared from SmBaCo2−xMnxO5+δ rectangular pellets with a thickness close to 0.4 mm and weight equal to ca. 0.2 g wrapped with Pt wire, and having the mentioned relative density close to 80%.
Autolab potentiostat and Nova 2.0 software (Metrohm-Autolab, metrohm-autolab.com) were used for all electrochemical experiments including: (1) cycling voltammetry conducted within a 0–0.6 V potential range with 5 mV s−1 rate and (2) galvanostatic tests performed under constant current equal to 50 μA. During all experiments, a magnetic stirrer was used in order to prevent polarization of the electrode/electrolyte interfaces. Faradic efficiency of the electrochemical oxidation was estimated for all three studied samples. For this purpose, after 15 h of oxidation, the new value of the oxygen content was determined with iodometric titration (as described below). With the known change of the nonstoichiometry and using the Faraday equation, it is possible to estimate the efficiency of the electrochemical process.
The oxygen content of the as-prepared and electrochemically oxidized samples was evaluated with iodometric titration [17]. For this purpose, fine powder (ca. 0.1 g) of the respective material was dissolved in 10 cm3 of 1 M HCl solution. Afterward, an excess of KI (ca. 1 g) and deionized water (ca. 100 cm3) were added, and the sample was placed in a dark place for a few minutes, which allowed for the reduction of Co3+/Mn3+ and Co4+/Mn4+ cations to Co2+/Mn2+. Finally, the formed iodine was titrated with standardized 0.1 M Na2S2O3 solution, and the end-point of the process was detected potentiometrically with an EM40-BNC titrator (Mettler Toledo, mt.com).
For all electrochemically oxidized SmBaCo2−xMnxO6 oxides, two heating/cooling cycles (thermogravimetric measurements) were performed on TA Q5000 IR thermobalance (TA Instruments, tainstruments.com) in synthetic air flow up to 700 °C, with 5 °C min−1 heating/cooling rates. This allowed us to evaluate the oxygen content variation as a function of temperature. Proper measurements were preceded by preheating up to around 200 °C (SmBaCo2O5+δ) or 300 (SmBaCo1.5Mn0.5O5+δ SmBaCoMnO5+δ), aimed at drying the powdered sample and removing the surface-adsorbed species.
Structural characterization was conducted for the as-prepared and electrochemically oxidized samples using x-ray diffraction (XRD) at a 10–110 deg range with CuKα radiation on a Panalytical Empyrean diffractometer equipped with a PIXcel3D detector (Malvern Panalytical, malvernpanalytical.com). Rietveld refinements of the obtained data were performed using GSAS/EXPGUI software (GSAS-I, subversion.xray.aps.anl.gov/trac/EXPGUI) [24,25]. Additional operando XRD experiments during electrochemical oxidation were performed for the selected SmBaCoMnO5+δ oxide using a custom made cell. Constructed as a two-electrode setup (pellet of the active material and Pt counter electrode, both emerged in 1 M KOH) and equipped with a flow pump, the cell was mounted on the diffractometer, with Kapton foil acting as the x-ray window. XRD data were collected every 5 min during galvanostatic polarization (i = 50 μA) performed for ca. 12 h.
Total electrical conductivity was studied on the as-prepared and electrochemically oxidized rectangular pellets with the 4-point direct-current (DC) method in a synthetic air atmosphere in the room temperature (RT)–700 °C temperature range. Data points were collected during slow heating with a ca. 0.35 °C min−1 rate. For this purpose, a Probostat holder (NorECs, norecs.com) combined with Keithley 2000 meter (Tektronix, tek.com) was used. Due to the relatively low density of the samples, in order to derive the propped specific conductivity, Bruggeman effective medium theory correction was implemented [26]. Presented as-prepared SmBaCo1.5Mn0.5O5+δ and SmBaCoMnO5+δ oxide reference data were measured for dense sinters, as reported in our previous works [15,17].
3. Results and Discussion
3.1. Electrochemical Oxidation of SmBaCo1.5Mn0.5O5+δ
The developed synthesis procedure allowed us to obtain sinters with lowered relative density, equal to ca. 80%, which as mentioned, is favorable when the efficiency of electrochemical oxidation is considered [5]. In Figure 1, a typical cycling voltammogram recorded in the mentioned three-electrode setup (with Hg/HgO reference electrode and Pt mesh as a counter electrode) is presented for the selected SmBaCo1.5Mn0.5O5+δ oxide. Its analysis allows for the three main regions corresponding to the following processes to be distinguished [7]: (I) The formation of a double layer on the working electrode/electrolyte interface; (II) the actual electrochemical oxidation (i.e., intercalation of oxygen, which is accompanied by the oxidation of 3d metal elements), covering ca. 0.3–0.55 V voltage range; and (III) the evolution of gaseous oxygen. It must be emphasized that there is no clear border between oxidation of the sample and the oxygen evolution, and it is most likely that both processes take place simultaneously around the 0.5–0.55 V range. Such behavior can be interpreted as resulting from relatively good electrocatalytic properties of the considered layered perovskite [5]. Similar voltammograms were also recorded for the other two SmBaCo2−xMnxO5+δ oxides. Their analysis allowed us to recognize the 0.3–0.55 V region as a suitable one for the electrochemical oxidation of all the materials considered in this work.
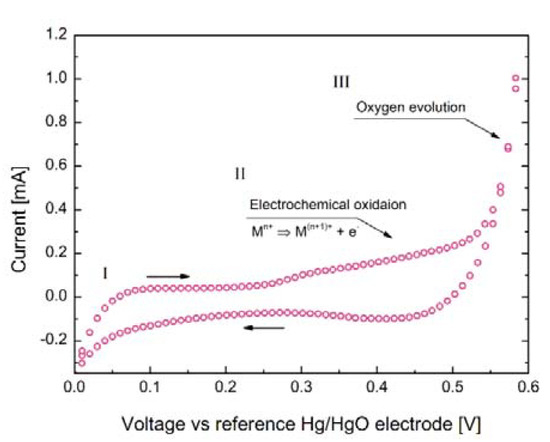
Figure 1.
Cycling voltammogram recorded for SmBaCo1.5Mn0.5O5+δ oxide with a 5 mV s−1 scanning rate. The M symbol represents the 3d metals (i.e., Co and Mn) undergoing the oxidation process.
Typically, electrochemical oxidation can be carried out in potentiostatic or galvanostatic mode [5,9,10]. Since galvanostatic mode also easily allows for the calculation of the faradaic efficiency of the electrochemical process, this approach was selected for the oxidation of all SmBaCo2−xMnxO5+δ layered perovskites. In Figure 2, the voltage changes as a function of time recorded for SmBaCo2O5+δ, SmBaCo1.5Mn0.5O5+δ, and SmBaCoMnO5+δ during galvanostatic polarization (i = 50 μA) in 1 M KOH electrolyte are presented. Please note the indicated initial oxygen content, as derived from iodometric titration [15,17]. There was a visible trend in the recorded curves, according to which increased manganese content in SmBaCo2−xMnxO5+δ caused an increase of the equilibrated value of the voltage at latter stages of the process. Interestingly, it also shortened the voltage stabilization time. The mentioned voltages did not differ strongly, and were equal to 0.482 V for SmBaCo2O5+δ, 0.502 V for SmBaCo1.5Mn0.5O5+δ, and 0.534 V for SmBaCoMnO5+δ. With a low current of 50 µA, the differences cannot be ascribed purely as due to the different ohmic resistance, but rather stem from the intrinsic properties of the oxides. Speculatively, the origin of variation correlates with the electronic structure of the particular compound, as can be supported by the following reasoning. The highest voltage value was recorded for the SmBaCoMnO5+δ oxide with a high Mn content, for which the dominant charge carriers were Co3+/Mn4+ pairs, yielding more localized electronic states [15]. Increasing the concentration of Co3+/Co4+ pairs for the other two samples, which is also accompanied by ongoing delocalization of the electronic states, resulted in the decreased oxidation voltage. In other words, in compounds, in which Co4+ is already present to some degree, further oxidation is somewhat easier.
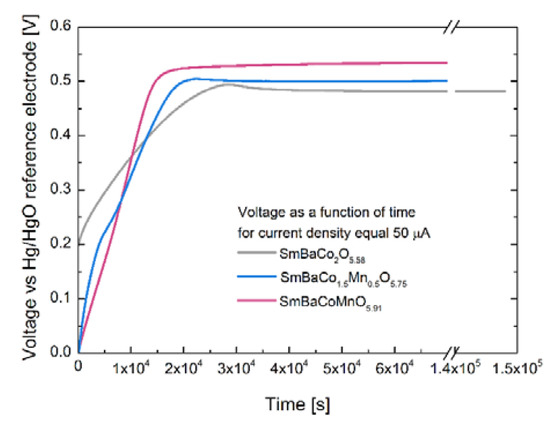
Figure 2.
Voltage as a function of time recorded during the galvanostatic polarization (i = 50 μA) of SmBaCo2O5+δ, SmBaCo1.5Mn0.5O5+δ, and SmBaCoMnO5+δ oxides in the 1 M KOH electrolyte.
Combination of electrochemical oxidation experiments with chemical analysis (iodometric titration) allowed us to determine the approximate time required for the full oxidation of the reported samples as well as to calculate the faradaic efficiency of the respective oxidation processes, which was equal to ca. 77% for SmBaCo2O5+δ, 72% for SmBaCo1.5Mn0.5O5+δ, and 64% for SmBaCoMnO5+δ, remaining relatively high for all compounds. For the purpose of the calculations, it was assumed that the efficiency of the process is constant and does not change with the increasing oxygen content. In that case, since oxygen incorporation and transport are the limiting steps of the process [27,28,29], again, the decreased efficiency (for materials with a higher Mn content) cannot be assigned only to their deteriorated total conductivity [14,30], but rather to the shift of voltage values toward the oxygen evolution reaction, which occurs for samples with higher Mn content (Figure 1). As a result, a larger part of the flowing charge is consumed for the production of gaseous oxygen, and thereby, the efficiency of the electrochemical oxidation decreases. Other hypotheses should also be considered. Since materials with a higher Mn content exhibit increased oxygen content, reduced concentration of the oxygen defects leads to the hindering of the process (through deteriorated ionic transport) and consequent shift of the equilibrium potential toward higher values. In that case, decrease of the faradic efficiency can be associated with the increased oxygen content, not directly with the Co:Mn ratio.
The actual increase of the oxygen content of the electrochemically oxidized materials was proven not only by iodometric titration experiments, but also by thermogravimetric analysis. In Figure 3a–c, two of the heating/cooling cycles recorded in the RT–700 °C range in air atmosphere for the SmBaCo2O5+δ (Figure 3a), SmBaCo1.5Mn0.5O5+δ (Figure 3b), and SmBaCo1.5Mn0.5O5+δ (Figure 3c) oxides are presented. With known absolute oxygen content at RT (determined with iodometric titration), it was possible to calculate the equilibrium oxygen content at all points of both heating/cooling cycles including the initial oxygen content of the electrochemically oxidized materials. Furthermore, most importantly, the calculated values of the initial nonstoichiometry corresponded well with the results determined with iodometric titration (Table 1) and indicated the complete (or almost complete) oxidation of the considered materials with the electrochemical route.
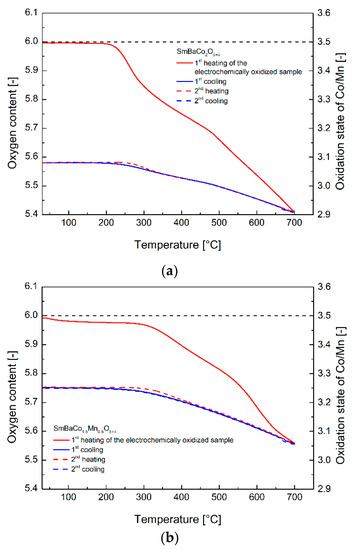
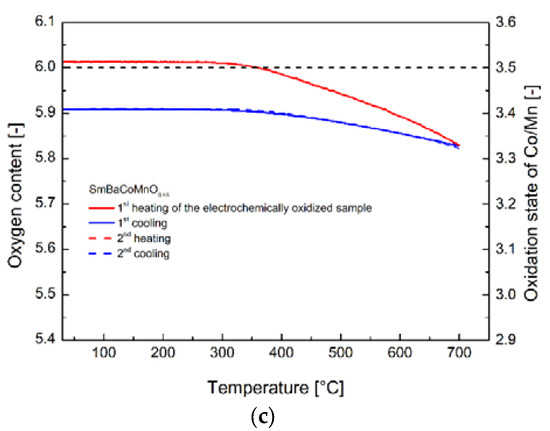
Figure 3.
(a) Oxygen content as a function of temperature recorded in an air atmosphere for the SmBaCo2O5+δ oxide. The two cycles presented were preceded by preheating up to 200 °C with the aim to dry the powdered sample and remove the adsorbed species. (b) Oxygen content as a function of temperature recorded in an air atmosphere for the SmBaCo1.5Mn0.5O5+δ oxide. The two cycles presented were preceded by preheating up to 300 °C with the aim to dry the powdered sample and remove the adsorbed species. (c) Oxygen content as a function of temperature recorded in an air atmosphere for the SmBaCoMnO5+δ oxide. The two cycles presented were preceded by preheating up to 300 °C with the aim to dry the powdered sample and remove the adsorbed species.

Table 1.
Crystal structure parameters at room temperature calculated for SmBaCo2−xMnxO5+δ (x = 0; 0.5 and 1) with equilibrium oxygen content as well as for fully-oxidized materials. Oxygen content was determined with the iodometric titration method.
The oxygen release from the oxidized materials started at around 250–350 °C, with lower temperatures corresponding to the materials with the increased cobalt concentration. In the case of all examined oxides, before reaching 700 °C, all of the non-equilibrium oxygen was removed on heating. Consecutive cooling and the following heating/cooling cycle proceeded according to (near) equilibrium behavior, as reported before for those particular compounds [15,17]. What is interesting, is that in the case of the SmBaCo1.5Mn0.5O5+δ oxide, a noticeable weight change could be observed at relatively low temperatures, even if the sample was initially preheated up to 300 °C for drying and removal of the adsorbed species.
3.2. Physicochemical Properties of the Electrochemically Oxidized SmBaCo2−xMnxO6
3.2.1. Crystal Structure
Structural characterization of the as-prepared and electrochemically oxidized SmBaCo2−xMnxO5+δ materials was carried out with XRD. In Table 1, the values of the refined structural parameters are presented for all the considered oxides. Moreover, the Rietveld refinements of all the XRD data are presented in Figure 4a–c. On the basis of previous works, different space groups were selected for the as-synthesized materials: Pmmm for SmBaCo2O5+δ, P4/mmm for SmBaCo1.5Mn0.5O5+δ, and Cmmm for SmBaCoMnO5+δ. For more details regarding the selection of the crystal structure for compounds with equilibrium oxygen content, see [15,17,31,32].
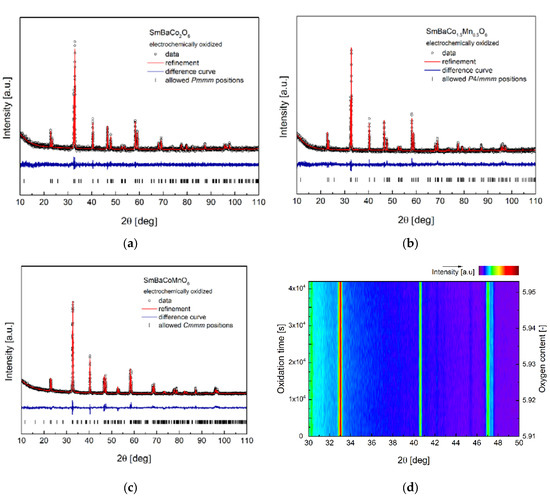
Figure 4.
(a) X-ray diffraction (XRD) data with Rietveld refinement for electrochemically oxidized SmBaCo2O6; (b) XRD data with Rietveld refinement for electrochemically oxidized SmBaCo1.5Mn0.5O6. (c) XRD data with Rietveld refinement for electrochemically oxidized SmBaCoMnO6; (d) Operando XRD data (matrix of points) recorded for SmBaCoMnO5+δ during galvanostatic electrochemical oxidation. Data presented as a function of time and oxygen content calculated on the basis of initial and final oxygen contents measured with iodometric titration (constant effectiveness of the process was assumed).
Analysis of the presented data for the oxidized materials revealed that the introduction of the additional oxygen with the electrochemical method does not affect crystal symmetry, and also does not modify the structural parameters significantly. This can be considered as surprising, since perovskite or similarly-structured oxide samples obtained by quenching and/or annealing at different temperatures and pO2 showed much stronger variations of the crystal structure. Moreover, relatively strong (and monotonic) dependence between the unit cell dimensions and the oxygen content is known to be present [33,34,35]. While it was expected that the increase in the electrochemical oxidation oxygen concentration should cause a noticeable decrease of the unit cell volume of the compounds, this did not happen. Contrary to results obtained for the electrochemical oxidation of simple perovskites [5,6,12], the discussed effect might be related to the preferential, layered placement of the oxygen vacancies in the initial samples. Oxygen, which is intercalated in the Sm-related layers, is not likely to modify the Ba-related sublattice, thus filling out available positions, but only through 2D transfer along the a–b plane direction. This may allow for the oxide to maintain generally unchanged structural parameters, creating a metastable configuration. This can be, to some degree, related to the peculiar structural differences observed between the electrochemically and chemically lithiated (i.e., intercalated with Li) layered oxide samples at room temperature [36,37,38].
Weak impact of the electrochemical intercalation on the crystal structure was also proven with operando XRD studies performed for SmBaCoMnO5+δ oxide during galvanostatic (i = 50 μA) oxidation in a 1 M KOH solution. In Figure 4d, the intensity data for the selected angular range, recorded during ca. 12 h of experiment, are presented. As can be seen, virtually no changes occurred, although iodometric titration performed after the operando experiment revealed increased oxygen content equal to 5.953. While additional operando XRD studies can be considered for other materials and in prolonged experiments, the chemical stability of Kepton foil (acting as the XRD window in the measurement cell) is limited. Additionally, no higher currents can be used due to the polarization effect, which would increase the voltage to the range of the electrolyte decomposition.
It can be summarized that despite a lack of the significant structural changes observed during the electrochemical oxidation, its effectiveness in terms of ability to increase oxygen content, up to fully-stoichiometric compositions, was proven mainly by: (1) thermogravimetric (TG) data recorded from the first heating and titration experiments showed increased oxygen content in the oxidized samples, which cannot only be associated with the surface-adsorbed oxygen; and (2) improved electrical conductivity due to the enhanced B–O–B double exchange of the oxidized samples (please see Section 3.2.2.).
3.2.2. Transport Properties
Electrical conductivity (presumably mixed ionic-electronic one with a much higher electronic component) of all oxidized SmBaCo2−xMnxO6 materials was measured with the 4-probe method in a synthetic air atmosphere in the RT–700 °C temperature range. In Figure 5a–f, the recorded data (recalculated with regard to the size of the rectangular pellets as well as with porosity correction included in [26]) are presented in Arrhenius-type logσT-1000/T, but also in σ-T coordinates. For comparison, values of the total conductivity measured for the dense, as-prepared SmBaCo2−xMnxO5+δ samples are also presented.
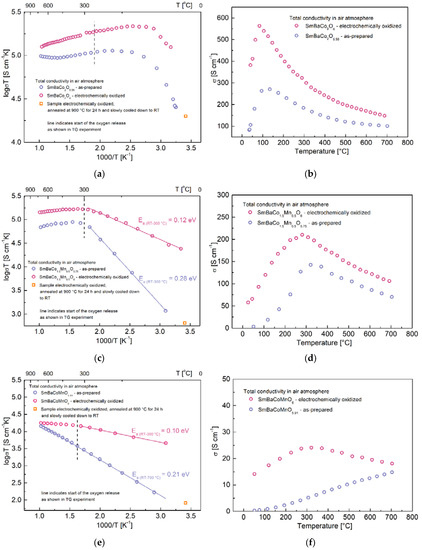
Figure 5.
(a) Total electrical conductivity of SmBaCo2O5.58 and SmBaCo2O6 in air as a function of temperature in Arrhenius-type coordinates. (b) Total electrical conductivity of SmBaCo2O5.58 and SmBaCo2O6 in air as a function of temperature in the σ-T coordinates. (c) Total electrical conductivity of SmBaCo1.5Mn0.5O5.75 and (d) SmBaCo1.5Mn0.5O6 in air as a function of temperature in Arrhenius-type and σ-T coordinates. (e) Total electrical conductivity of SmBaCoMnO5.91 and (f) SmBaCoMnO6 in air as a function of temperature in Arrhenius-type and σ-T coordinates.
Since charge transfer in perovskite and perovskite-related oxides occur through the double-exchange mechanism, depending on the interaction between 3d orbitals of the transition metals and 2p orbitals of oxygen, not only the degree of structural deviation and filling of the 3d states, but also the oxygen content determine its effectiveness as the presence of the oxygen vacancies interrupts the electron transfer paths [22,23,39]. Consequently, it is expected that increased oxygen content should lead to enhancement of the total conductivity, which is the case for all analyzed materials. It must be emphasized that the examined electrochemically oxidized materials can be considered as fully-stoichiometric only at lower temperatures, below around 250–350 °C, depending on the Co:Mn ratio (Figure 3a–c). At higher temperatures oxygen release begins, although it is expected that the process is slower than in TG experiments, which is due to the different geometry of the samples used for the conductivity determination (relatively dense, rectangular sinters), hindering the kinetics of the oxygen release. With regard to the above, in Figure 5a–f, two regions can be distinguished (separated with the dashed line) for the electrochemically oxidized samples: the first corresponds to the fully-stoichiometric SmBaCo2−xMnxO6, and the second to materials with a decreased oxygen content that was lower than 6.0, but higher than the equilibrium one. Moreover, in Figure 5a,c,e, the electrical conductivity values corresponding to the oxidized materials, which were later annealed at 900 °C in air and slowly cooled to room temperature, are also shown. As can be seen, the conductivity decreased to values similar to the as-prepared materials, which also proves that the electrochemical oxidation was successful.
The increased oxygen content in SmBaCo2O5+δ oxides (Figure 5a,b) did not affect the conduction mechanism itself. For both the as-prepared SmBaCo2O5.58 and oxidized SmBaCo2O6, insulator-metal transition was observed close to 100–130 °C, which was previously reported for the material with equilibrium oxygen content [40]. After reaching the transition point, both characteristics were the metallic-type. As expected, in the case of SmBaCo2O6, the transition shifted to a somewhat lower temperature, and the conduction was noticeably improved, especially at lower temperatures. Annealing of the oxidized sample at higher temperatures caused a decrease in the oxygen content, where values approached the equilibrium one, leading to the smaller differences between σtot recorded for both samples. Nevertheless, the observed improvement itself was not as high as expected considering the reported values to the order of 1000–1500 S cm−1 for the chemically oxidized sample [41]. This can be explained due to the fact that no decrease of the unit cell volume upon electrochemical oxidation took place, so the effectiveness of Co–O orbital overlapping remains largely unchanged, not bringing a beneficial contribution to the electronic charge transport process [39].
Introduction of Mn into the Co sublattice leads to a change of the conduction mechanism from metallic-type toward thermally activated, which may be affected at higher temperatures by the oxygen release (Figure 5c–f). In contrast to the SmBaCo2O5+δ oxide, the characteristic recorded at high temperatures did not reflect the insulator-metal transition, but the influence of the oxygen release on the transport properties. Moreover, the increased manganese content in SmBaCo2−xMnxO5+δ led to the deterioration of the transport properties. This was already explained as due to the diversified charge transfer effectiveness between respective ionic pairs, which was as follows: Co4+/Co3+ > Mn4+/Mn3+ > Mn4+/Co3+ [14]. While Co4+/Co3+ couples existed only for SmBaCo2O5+δ, the presence of the more stable Mn4+ ions reduced the concentration of the highly-charged Co4+. Consequently, in this range of concentrations (x), the increased manganese content negatively affected the electrical conductivity by changing the amount of the more effectively conducting species [32]. For more detailed information regarding the impact of Mn content on the conductivity mechanism in the REBaCo2−xMnxO5+δ system, please see [14,15,17].
According to the above, the charge transfer observed for the SmBaCo1.5Mn0.5O5+δ oxide can be described as small polaron hopping, where the mobility is thermally activated. Such a mechanism is affected at high temperatures by delocalization of the charge carriers and release of the oxygen from the crystal structure. It seems that the dominant influence on the conduction curve has the disruption of Zener double exchange, which is reflected by deterioration of the conduction starting at temperatures corresponding to the beginning of the oxygen release [17]. The increased oxygen content (electrochemically introduced) does not affect the conduction mechanism itself, but caused a noticeable decrease in the activation energy in the RT–300 °C range, from 0.28 eV to 0.12 eV. As a result, the highest values of σtot for both samples were recorded around 300 °C, just before oxygen release starts at 150 S cm−1 for the material with equilibrium oxygen content and 220 S cm−1 for the electrochemically oxidized (stoichiometric) material, respectively. It was noticeable that the maximum conductivity for the SmBaCo1.5Mn0.5O6 oxide slightly shifted toward lower temperatures, while the slope of changes at higher temperatures was steeper. Such behavior can be explained by a comparison with the TG curve (Figure 3), where more rapid oxygen release, starting at slightly lower temperatures, was observed during the first heating for the electrochemically oxidized material. At the same time, the used heating rate (0.35 °C min−1) was still not slow enough for the measured pellet to reach the equilibrium oxygen content at 700 °C.
In the case of the as-prepared SmBaCoMnO5.91 material, the whole temperature range slope of the conductivity curve (Figure 5e) was practically unchanged. Since in this sample the oxygen content change associated with the temperature increase was only slight (Figure 3c, second cycle of heating/cooling), it did not affect the thermally activated behavior, which was observed for the previously discussed SmBaCo1.5Mn0.5O5+δ. At the same time, for the electrochemically oxidized SmBaCoMnO6 sample, flattening of the recorded curve (above 300 °C) can be associated with the oxygen release, which was more significant overall than that in the as-prepared material. Additionally, the temperature corresponding to the highest observed σtot shifted from ca. 700 °C for SmBaCoMnO5.91 to 300 °C for SmBaCoMnO6. At 700 °C, the values of the conductivity recorded for both SmBaCoMnO5+δ samples were close, which might be related with the smallest initial change of the oxygen content among the studied series between the as-prepared and oxidized samples (0.09), which was naturally significantly reduced at the highest temperatures. Additionally, the increased oxygen content in this case caused a significant decrease in the activation energy of conductivity at lower temperatures (from 0.21 eV to 0.10 eV, respectively).
Moreover, ionic conduction in all of the considered oxides is also expected to undergo enhancement, which is due to the increased concentration of the oxygen vacancies at higher temperatures (please see TG curves). However, since the contribution of the ionic transport in total conductivity is negligible (transference numbers on the order of 10−4–10−5 [2,14]), this behavior only slightly affected the total conductivity.
4. Conclusions
SmBaCo2−xMnxO5+δ (x = 0; 0.5 and 1) oxides were successfully synthesized using the sol-gel route combined with high-temperature annealing, performed in air or argon atmospheres. It was proven that galvanostatic electrochemical oxidation carried out in the 1 M KOH solution allowed us to induce the incorporation of oxygen into the crystal structure of the considered materials, and consequently, fully-stoichiometric SmBaCo2O6, SmBaCo1.5Mn0.5O6, and SmBaCoMnO6 were obtained, as confirmed by iodometric titration. Faradaic effectiveness of the galvanostatic process occurring at voltages in the order of 0.48–0.53 V decreased with increasing manganese content, which is most likely associated with the increased voltage of the electrochemical oxidation process. XRD studies of the as-synthesized and fully-oxidized materials revealed unexpected, only marginal modification of the crystal structure with varying oxygen content. This is reflected by the not as high as anticipated increase in conduction for the fully-oxidized (metallic) SmBaCo2O6. However, significantly improved transport properties were measured for other two oxidized compounds, especially in the lower temperature range, below that of the excessive oxygen release. Such behavior stems from the increased efficiency of the charge transfer occurring in materials with a higher oxygen content, in which charge transfer is determined by the double exchange mechanism.
Author Contributions
Conceptualization, A.O. and K.Ś.; Methodology, A.O.; Formal analysis, A.O. and K.Ś.; Investigation, A.O. and A.N.; Writing—original draft preparation, A.O.; Writing—review and editing, A.O. and K.Ś.; Supervision, K.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education (MNiSW) on the basis of the decision number 0128/DIA/2016/45.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mizusaki, J. Nonstoichiometry, Diffusion, and electrical properties of perovskite-type oxide electrode materials. Solid State Ionics 1992, 52, 79–91. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ionics 2010, 180, 1620–1625. [Google Scholar] [CrossRef]
- Akahoshi, D.; Ueda, Y. Oxygen Nonstoichiometry, Structures, and Physical Properties of YBaCo2O5+x (0.00 ≤ x ≤ 0.52). J. Solid State Chem. 2001, 156, 355–363. [Google Scholar] [CrossRef]
- Grenier, J.C.; Wattiaux, A.; Monroux, C.; Pouchard, M.; Locquet, J.P. Electrochemical oxygen insertion into La2CuO4-related compounds. Physica C 1994, 235, 79–82. [Google Scholar] [CrossRef]
- Bezdicka, P.; Wattiaux, A.; Grenier, J.C.; Pouchard, M.; Hagenmuller, P. Preparation and Characterization of Fully Stoichiometric SrCoO3 by Electrochemical Oxidation. Z. Annorg. Allg. Chem. 1993, 619, 7–12. [Google Scholar] [CrossRef]
- Grenier, J.C.; Wattiaux, A.; Doumerc, J.P.; Dordor, P.; Fournes, L.; Chaminade, J.P.; Pochard, M. Electrochemical Oxygen Intercalation into Oxide Networks. J. Solid State Chem. 1992, 30, 20–30. [Google Scholar] [CrossRef]
- Grenier, J.C.; Pouchard, M.; Wattiaux, A. Electrochemical synthesis: Oxygen intercalation. Curr. Opin. Solid State. Mater. Sci. 1990, 1, 233–240. [Google Scholar] [CrossRef]
- Grenier, J.C.; Wattiaux, A.; Fournes, L.; Pouchard, M.; Etourneau, J. The Electrochemical Oxidation: A New Way for Preparing Highly Oxidized Ferrites. J. Phys. IV Fr. 1997, 7, 7–10. [Google Scholar] [CrossRef]
- Nemudry, A.; Goldberg, E.L.; Aguirre, M.; Alario-Franco, M.Á. Electrochemical topotactic oxidation of nonstoichiometric perovskites at ambient temperature. Solid State Sci. 2002, 4, 677–690. [Google Scholar] [CrossRef]
- Ceretti, M.; Wahyudi, O.; André, G.; Meven, M.; Villesuzanne, A.; Paulus, W. (Nd/Pr)2NiO4+δ: Reaction Intermediates and Redox Behavior Explored by in Situ Neutron Powder Diffraction during Electrochemical Oxygen Intercalation. Inorg. Chem. 2018, 47, 4657–4666. [Google Scholar] [CrossRef]
- Bezdicka, P.; Foumes, L.; Wattiaux, A.; Grenier, J.C.; Pouchard, M. Mosbauer Characteristics of the Sr2CoFeO6 Perovskite Obtained by Electrochemical Oxidation. Solid State Commun. 1994, 91, 501–505. [Google Scholar] [CrossRef]
- Grenier, J.C.; Wattiaux, A.; Demourgues, A.; Pouchard, M.; Hagenmuller, P. Electrochemical oxidation: A new way for preparing high oxidation states of transition metals. Solid State Ionics 1993, 65, 825–832. [Google Scholar] [CrossRef]
- Guo, W.; Guo, R.; Liu, L.; Cai, G.; Zhang, C.; Wu, C.; Liu, C.; Jiang, H. Thermal and electrochemical properties of layered perovskite PrBaCo2-xMnxO5+d (x = 0.1, 0.2 and 0.3) cathode materials for intermediate temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2015, 40, 12457–12465. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Urones-Garrote, E.; Pérez-Coll, D.; Amador, U.; García-Martín, S. Crystal structure and compositional effects on the electrical and electrochemical properties of GdBaCo2-xMnxO5+δ 0 ≤ x ≤ 2 oxides for use as air electrodes in solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 5452–5460. [Google Scholar] [CrossRef]
- Olszewska, A.; Świerczek, K.; Skubida, W.; Du, Z.; Zhao, H. Versatile Application of Redox Processes for REBaCoMnO5+δ (RE: La, Pr, Nd, Sm, Gd, and Y) Oxides. J. Phys. Chem. C 2019, 123, 48–61. [Google Scholar] [CrossRef]
- Broux, T.; Bahout, M.; Hanlon, J.M.; Hernandez, O.; Paofai, S.; Bernov, A.; Skinner, S.J. High temperature structural stability, electrical properties and chemical reactivity of NdBaCo2-xMnxO5+d (0 ≤ x ≤ 2) for use as cathodes in solid oxide fuel cells. J. Mater. Chem. A 2014, 2, 17015–17023. [Google Scholar] [CrossRef]
- Olszewska, A.; Du, Z.; Świerczek, K.; Zhao, H.; Dabrowski, B. Novel ReBaCo1.5Mn0.5O5+δ (Re: La, Pr, Nd, Sm, Gd and Y) perovskite oxide: Influence of manganese doping on crystal structure, oxygen nonstoichiometry, thermal expansion, transport properties, and application as cathode materials in Solid Oxide Fuel Cells. J. Mater. Chem. A 2018, 6, 13271. [Google Scholar] [CrossRef]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Pilania, G. Effect of cation ordering on oxygen vacancy diffusion pathways in double perovskites. Chem. Mater. 2015, 27, 5020–5026. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Zhou, J.-S. Localized to Itinerant Electronic Transition in Perovskite Oxides; Springer: New York, NY, USA, 2001; pp. 63–129. [Google Scholar]
- Muller, K.; Tool, K. Properties of Perovskites and Other Oxides; World Scientific Publishing Co.: Singapore, 2010; pp. 26–121. [Google Scholar]
- Wolfram, T.; Ellialtioglu, S. Electronic and Optical Properties of d-Band Perovskites; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Tilley, R. Perovskites, Structure-Property Relationships; John Wiley & Sons Ltd.: Chichister, UK, 2016. [Google Scholar]
- Soediono, B. General Structure Analysis System. J. Chem. Inform. Model. 1989, 53, 160. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Dees, D.W.; Claar, T.D.; Fee, D.C. Conductivity of Porous Ni/ZrO2-Y2O3 Cermets. J. Electrochem. Soc. 1987, 134, 1241–2146. [Google Scholar] [CrossRef]
- Wang, L.; Merkle, R.; Mastrikov, Y.A.; Kotomin, E.A.; Maier, J. Oxygen exchange kinetics on solid oxide fuel cell cathode materials—General trends and their mechanistic interpretation. J. Mater. Res. 2012, 27, 2000–2008. [Google Scholar] [CrossRef]
- Riva, M.; Kubicek, M.; Hao, X.; Franceschi, G.; Gerhold, S.; Schmid, M.; Hutter, H.; Fleig, J.; Franchini, C.; Yildiz, B. Diebold, Influence of surface atomic structure demonstrated on oxygen incorporation mechanism at a model perovskite oxide. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Staykov, A.; Téllez, H.; Akbay, T.; Druce, J.; Ishihara, T.; Kilner, J. Oxygen Activation and Dissociation on Transition Metal Free Perovskite Surfaces. Chem. Mater. 2015, 27, 8273–8281. [Google Scholar] [CrossRef]
- Bahout, M.; Pramana, S.; Hanlon, J.; Dorcet, V.; Smith, R.; Paofai, S.; Skinner, S.J. Stability of NdBaCo2-xMnxO5+d (x = 0, 0.5) layered high-temperature in situ neutron powder. J. Mater. Chem. A 2015, 3, 15420–15431. [Google Scholar] [CrossRef]
- Olszewska, A.; Świerczek, K. ReBaCo2-xMnxO5+δ (Re: Rare earth element) layered perovskites for application as cathodes in Solid Oxide Fuel Cells. E3S Web Conf. EDP Sci. 2019, 108, 01020. [Google Scholar] [CrossRef]
- Olszewska, A.; Zhang, Y.; Du, Z.; Marzec, M.; Świerczek, K.; Zhao, H.; Dabrowski, B. Mn-rich SmBaCo0.5Mn1.5O5+d double perovskite cathode material for SOFCs. Int. J. Hydrog. Energy 2019, 44, 27587–27599. [Google Scholar] [CrossRef]
- Klimkowicz, A.; Świerczek, K.; Takasaki, A.; Molenda, J.; Dabrowski, B. Crystal structure and oxygen storage properties of BaLnMn2O5+d (Ln: Pr, Nd, Sm, Gd, Dy, Er and Y) oxides. Mater. Res. Bull. 2015, 65, 116–122. [Google Scholar] [CrossRef]
- Świerczek, K.; Yoshikura, N.; Zheng, K.; Klimkowicz, A. Correlation between crystal and transport properties in LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln-selected lanthanides, Y). Solid State Ionics 2014, 262, 645–649. [Google Scholar] [CrossRef]
- Klimkowicz, A.; Świerczek, K.; Zheng, K.; Wallacher, D.; Takasaki, A. Oxygen release from BaLnMn2O6 (Ln: Pr, Nd, Y) under reducing conditions as studied by neutron diffraction. J. Mater. Sci. 2017, 52, 6476–6485. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kunnikuruvan, S.; Kumar, S.; Markovsky, B.; Aurbach, D.; Dixit, M.; Major, D.T. Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi1-x-yCoxMnyO2 and LiNi1-x-yCoxAlyO2. Chem. Mater. 2020. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Mcbreen, J.; Davidson, I.J.; Whitfield, P.S.; Kargin, I. In situ X-ray absorption study of a layered manganese-chromium oxide- based cathode material. J. Electrochem. Soc. 2002, 149, A176–A184. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Lee, J.F.; Liu, D.G.; Hwang, B.J. In-situ X-ray absorption spectroscopy investigations of a layered LiNi0.65Co0.25Mn0.1O2 cathode material for rechargeable lithium batteries. J. Mater. Chem. 2004, 14, 958–965. [Google Scholar] [CrossRef]
- Galasso, F.S. Structure, Properties and Preparation of Perovskite-Type Compounds; Pergamon Press: Oxford, UK, 1969; pp. 59–121. [Google Scholar]
- Kim, J.-H.; Mogni, L.; Prado, F.; Caneiro, A.; Alonso, J.A.; Manthiram, A. High Temperature Crystal Chemistry and Oxygen Permeation Properties of the Mixed Ionic–Electronic Conductors LnBaCo2O5+d (Ln = Lanthanide). J. Electrochem. Soc. 2009, 156, B1376–B1382. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+d Oxides as Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).