Revisiting the Zintl‒Klemm Concept for ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Tellurides
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Details
2.2. Electronic Structure Computations, Chemical Bonding and Population Analyses
3. Materials and Methods
3.1. Syntheses
3.2. X-Ray Diffractions Studies and Crystal Structure Determinations
3.3. Computational Details
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curtarolo, S.; Hart, G.L.W.; Nardelli, M.B.; Mingo, N.; Sanvito, S.; Levy, O. The high-throughput highway to computational materials design. Nature Mater. 2013, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Nesper, R. Bonding Patterns in Intermetallic Compounds. Angew. Chem. Int. Ed. 1991, 30, 789–817. [Google Scholar] [CrossRef]
- Miller, G.J. The “Coloring Problem” in Solids: How It Affects Structure, Composition and Properties. Eur. J. Inorg. Chem. 1998, 1998, 523–536. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Steinberg, S. Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds. Crystals 2018, 8, 80. [Google Scholar] [CrossRef]
- Fries, K.S.; Steinberg, S. Fermi-Level Characteristics of Potential Chalcogenide Superconductors. Chem. Mater. 2018, 30, 2251–2261. [Google Scholar] [CrossRef]
- Simons, J.; Steinberg, S. Identifying the Origins of Vacancies in the Crystal Structures of Rock Salt-type Chalcogenide Superconductors. ACS Omega 2019, 4, 15721–15728. [Google Scholar] [CrossRef] [PubMed]
- Landrum, G.A.; Dronskowski, R. The Orbital Origins of Magnetism: From Atoms to Molecules to Ferromagnetic Alloys. Angew. Chem. Int. Ed. 2000, 39, 1560–1585. [Google Scholar] [CrossRef]
- Shportko, K.; Kremers, S.; Woda, M.; Lencer, D.; Robertson, J.; Wuttig, M. Resonant bonding in crystalline phase-change materials. Nature Mater. 2008, 7, 653–658. [Google Scholar] [CrossRef]
- Cheng, Y.; Cojocaru-Mirédin, O.; Keutgen, J.; Yu, Y.; Küpers, M.; Schumacher, M.; Golub, P.; Raty, J.-Y.; Dronskowski, R.; Wuttig, M. Understanding the Structure and Properties of Sesqui-Chalcogenides (i.e., V2VI3 or Pn2Ch3 (Pn = Pnictogen, Ch = Chalcogen) Compounds) from a Bonding Perspective. Adv. Mater. 2019, 31, 1904316. [Google Scholar] [CrossRef]
- Toberer, E.S.; Snyder, G.J. Complex thermoelectric materials. Nature Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and Old Concepts in Thermoelectric Materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nature Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Raoux, S.; Welnic, W.; Ielmini, D. Phase Change Materials and Their Applications to Nonvolatile Memories. Chem. Rev. 2010, 110, 240–267. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.Z.; Kane, C.L. Colloquium: Topological Insulators. Rev. Mod. Phys. 2010, 82, 3045–3067. [Google Scholar] [CrossRef]
- Papoian, G.A.; Hoffmann, R. Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl-Klemm Concept to Nonclassical Electron-Rich Networks. Angew. Chem. Int. Ed. 2000, 39, 2408–2448. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Steinberg, S. Revealing the Nature of Bonding in Rare-Earth Transition-Metal Tellurides by Means of Methodes Based on First Principles. Eur. J. Inorg. Chem. 2017, 2017, 3395–3400. [Google Scholar] [CrossRef]
- Göbgen, K.C.; Gladisch, F.C.; Steinberg, S. The Mineral Stützite: A Zintl-Phase or Polar Intermetallic? A Case Study Using Experimental and Quantum-Chemical Techniques. Inorg. Chem. 2018, 57, 412–421. [Google Scholar] [CrossRef]
- Göbgen, K.C.; Fries, K.S.; Gladisch, F.C.; Dronskowski, R.; Steinberg, S. Revealing the Nature of Chemical Bonding in an ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Telluride. Inorganics 2019, 7, 70. [Google Scholar] [CrossRef]
- Corbett, J.D. Exploratory Synthesis: The Fascinating and Diverse Chemistry of Polar Intermetallic Phases. Inorg. Chem. 2010, 49, 13–28. [Google Scholar] [CrossRef]
- Lin, Q.; Miller, G.J. Electron-Poor Polar Intermetallics: Complex Structures, Novel Clusters, and Intriguing Bonding with Pronounced Electron Delocalization. Acc. Chem. Res. 2018, 51, 49–58. [Google Scholar] [CrossRef]
- Ertural, C.; Steinberg, S.; Dronskowski, R. Development of a robust tool to extract Mulliken and Löwdin charges from plane waves and its application to solid-state materials. RSC Adv. 2019, 9, 29821–29830. [Google Scholar] [CrossRef]
- Mitchell, K.; Ibers, J.A. Rare-Earth Transition-Metal Chalcogenides. Chem. Rev. 2002, 102, 1929–1952. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, T.H.; Steinfink, H.; Weiss, E.J. The Phase Equilibria and Crystal Chemistry of the Rare Earth-Group VI Systems. IV. Lanthanum-Tellurium. Inorg. Chem. 1965, 4, 1154–1157. [Google Scholar] [CrossRef]
- Wang, R.; Steinfink, H.; Bradley, W.F. The Crystal Structure of Lanthanum Telluride and of Tellurium-Deficient Neodymium Telluride. Inorg. Chem. 1966, 5, 142–145. [Google Scholar] [CrossRef]
- van der Lee, A.; de Boer, J.L. Redetermination of the structure of hessite, Ag2Te-III. Acta Crystallogr. Sect. C Struct. Chem. 1993, 49, 1444–1446. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.-Y.; Zhang, X.; Kanatzidis, M.G. CsAg5Te3: A new metal-rich telluride with a unique tunnel structure. J. Alloys Compd. 1995, 218, 1–4. [Google Scholar] [CrossRef]
- Stöwe, K. Crystal structure and magnetic properties of CeTe2. J. Alloys Compd. 2000, 307, 101–110. [Google Scholar] [CrossRef]
- Meng, C.-Y.; Chen, H.; Wang, P. Syntheses, Structures, and Physical Properties of CsRE2Ag3Te5 (RE = Pr, Nd, Sm, Gd-Er) and RbRE2Ag3Te5 (RE = Sm, Gd-Dy). Inorg. Chem. 2014, 53, 6893–6903. [Google Scholar] [CrossRef]
- Provino, A.; Steinberg, S.; Smetana, V.; Paramanik, U.; Manfrinetti, P.; Dhar, S.K.; Mudring, A.-V. Gold in the Layered Structures of R3Au7Sn3: From Relativity to Versatility. Cryst. Growth Des. 2016, 16, 5657–5668. [Google Scholar] [CrossRef]
- Steinberg, S.; Dronskowski, R. The Crystal Orbital Hamilton Population (COHP) Method as a Tool to Visualize and Analyze Chemical Bonding in Intermetallic Compounds. Crystals 2018, 8, 225. [Google Scholar] [CrossRef]
- Steinberg, S.; Smetana, V.; Mudring, A.-V. Layered Structures and Disordered Polyanionic Nets in the Cation-Poor Polar Intermetallics CsAu1.4Ga2.8 and CsAu2Ga2.6. Cryst. Growth Des. 2017, 17, 693–700. [Google Scholar] [CrossRef]
- Jansen, M. Homoatomic d10-d10 Interactions: Their Effects on Structure and Chemical and Physical Properties. Angew. Chem. Int. Ed. 1987, 26, 1098–1110. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Janka, O.; Pöttgen, R. Reactive Fluxes. In Handbook of Solid State Chemistry; Dronskowski, R., Kikkawa, S., Stein, A., Eds.; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- WinXPow; Version 2.23; STOE & Cie GmbH: Darmstadt, Germany, 2008.
- Match! Vers. 3.8.0.137; Crystal Impact GbR: Bonn, Germany, 2019.
- SAINT+ and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009.
- XPREP; Version 6.03; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Marsman, M.; Furthmüller, J. Vienna Ab-Initio Simulation Package VASP: The Guide; Computational Materials Physics, Faculty of Physics, Universität Wien: Vienna, Austria, 2014. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter Mater. Phys. 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis As Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Dronskowski, R.; Blöchl, P.E. Crystal Orbital Hamilton Populations (COHP). Energy-Resolved Visualization of Chemical Bonding in Solids Based on Density-Functional Calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréff, A.L.; Dronskowski, R. Analytic Projection from Plane-Wave and PAW Wavefunctions and Application to Chemcial-Bonding Analysis in Solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
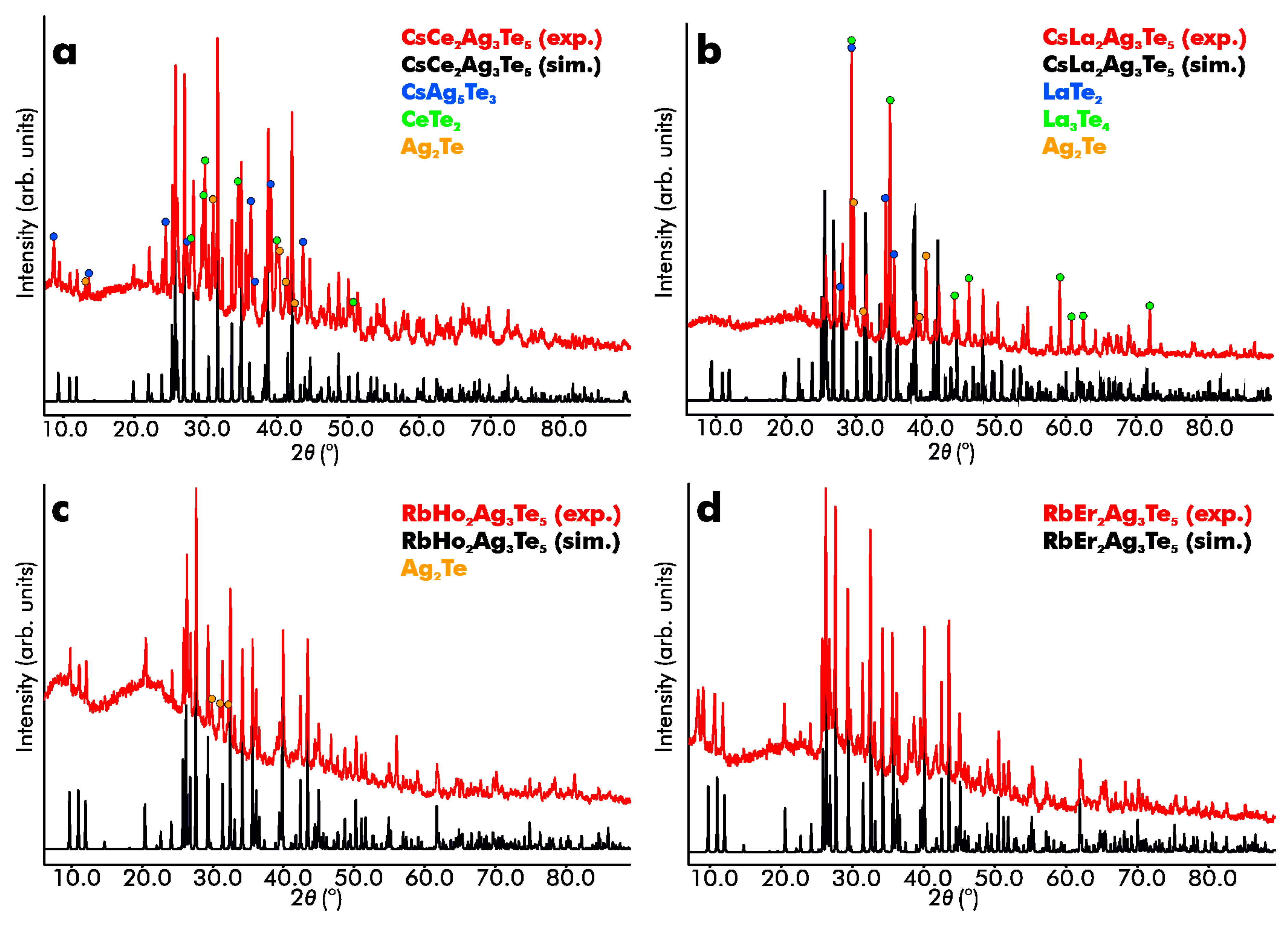
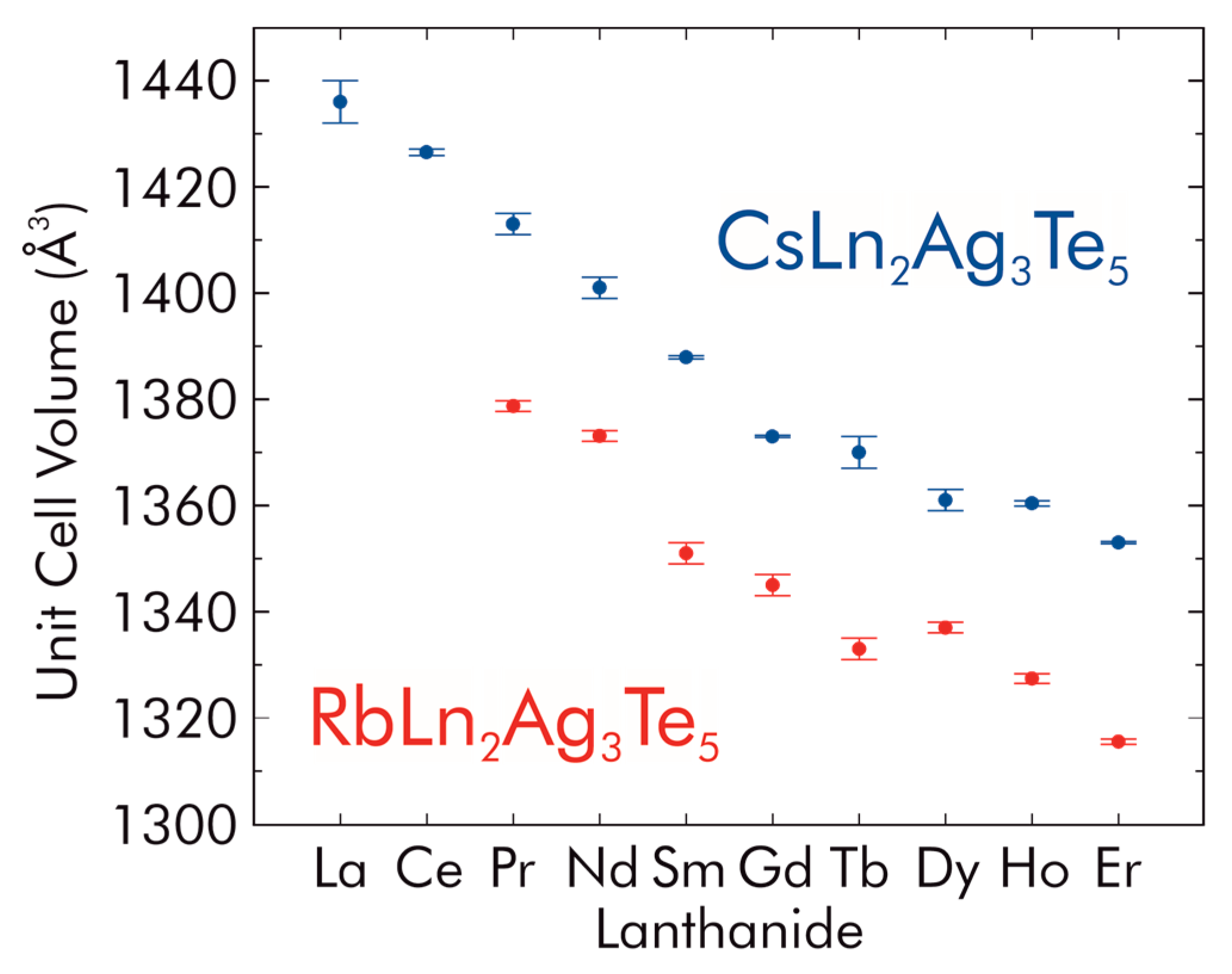
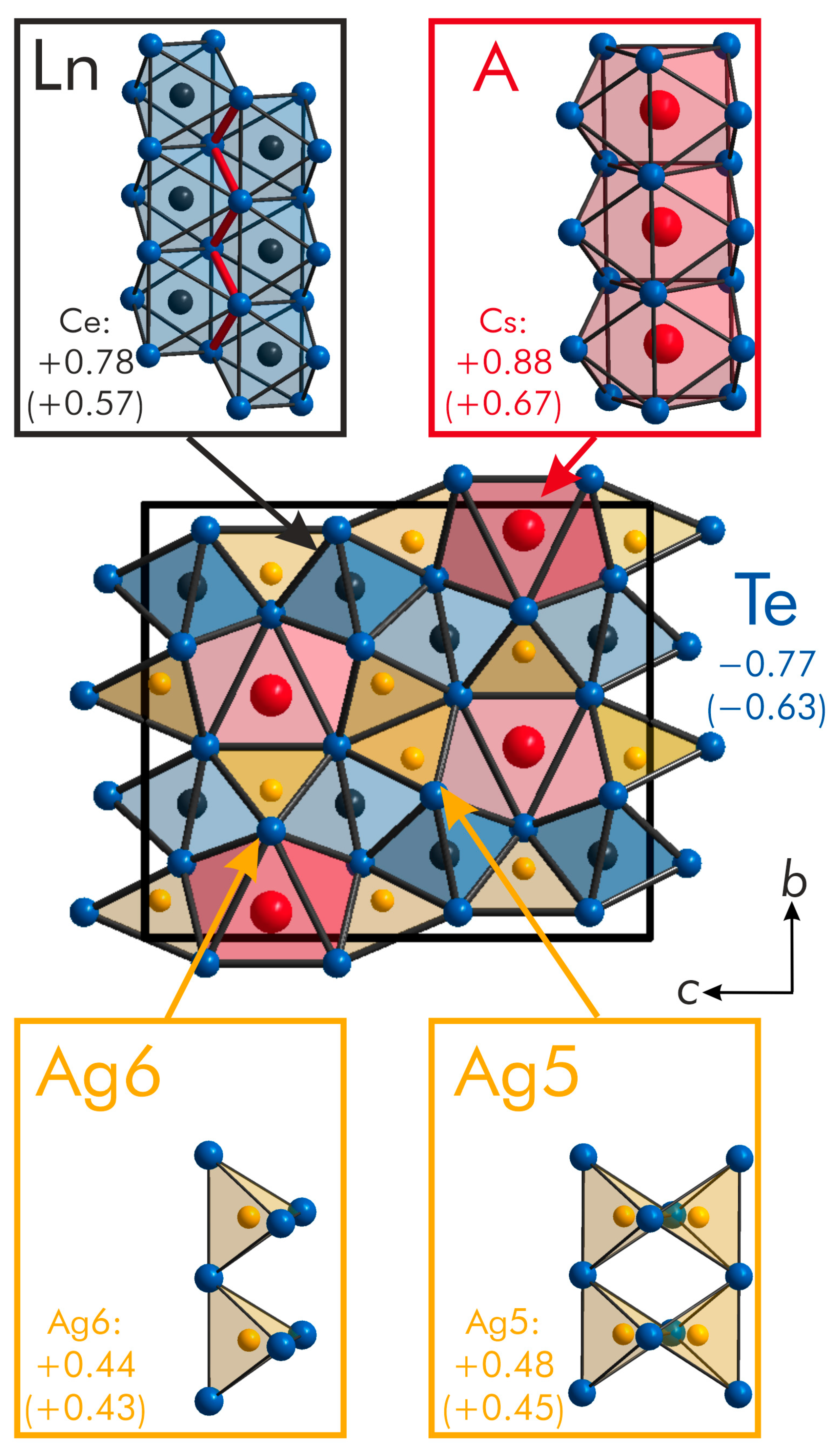

| Formula | RbHo2Ag3Te5 | RbEr2Ag3Te5 | CsLa2Ag3Te5 | CsCe2Ag3Te5 |
|---|---|---|---|---|
| form wt. | 1376.94 | 1381.60 | 1372.34 | 1374.76 |
| space group | Cmcm (no. 63) | |||
| a (Å) | 4.517(2) | 4.493(1) | 4.662(7) | 4.647(1) |
| b (Å) | 16.096(6) | 16.069(4) | 16.21(2) | 16.223(4) |
| c (Å) | 18.256(7) | 18.220(4) | 19.00(3) | 18.921(5) |
| volume (Å3) | 1327.4(9) | 1315.5(5) | 1436(4) | 1426.5(6) |
| Z | 4 | |||
| density (calc.), g/cm3 | 6.890 | 6.976 | 6.348 | 6.401 |
| μ (mm−1) | 30.490 | 31.494 | 22.247 | 22.785 |
| F (000) | 2288 | 2296 | 2280 | 2288 |
| θ range (°) | 2.231−26.563 | 2.236−25.216 | 2.144−25.352 | 2.153−31.006 |
| index ranges | −5 ≤ h ≤ 5 −20 ≤ k ≤ 19 −19 ≤ l ≤ 22 | −5 ≤ h ≤ 5 −18 ≤ k ≤ 18 −21 ≤ l ≤ 13 | −4 ≤ h ≤ 5 −17 ≤ k ≤ 19 −22 ≤ l ≤ 22 | −6 ≤ h ≤ 6 −15 ≤ k ≤ 23 −26 ≤ l ≤ 27 |
| reflections collected | 4259 | 3070 | 4046 | 5769 |
| independent reflections | 809 | 701 | 769 | 1236 |
| refinement method | full-matrix least-squares on F2 | |||
| data/restraints/parameters | 809/0/37 | 701/0/37 | 769/0/37 | 1236/0/37 |
| goodness-of-fit on F2 | 1.16 | 1.13 | 0.89 | 1.06 |
| final R indices [I > 2σ(I)] | R1 = 0.046; wR2 = 0.099 | R1 = 0.057; wR2 = 0.144 | R1 = 0.056; wR2 = 0.129 | R1 = 0.026; wR2 = 0.055 |
| R indices (all data) | R1 = 0.056; wR2 = 0.102 | R1 = 0.061; wR2 = 0.146 | R1 = 0.094; wR2 = 0.145 | R1 = 0.038; wR2 = 0.056 |
| Rint | 0.073 | 0.074 | 0.173 | 0.042 |
| largest difference peak and hole, e−/Å3 | 2.18 and −6.96 | 4.02 and −5.74 | 1.91 and −3.78 | 1.54 and −1.90 |
| Atom | Position | x | y | z | Ueq, Å2 |
|---|---|---|---|---|---|
| RbHo2Ag3Te5 | |||||
| Ho1 | 8f | 0 | 0.1915(1) | 0.4050(1) | 0.0148(3) |
| Te2 | 8f | ½ | 0.0632(1) | 0.3786(1) | 0.0165(3) |
| Te3 | 8f | 0 | 0.1768(1) | 0.5721(1) | 0.0142(3) |
| Te4 | 4c | 0 | 0.2616(1) | ¼ | 0.0161(4) |
| Ag5 | 8f | ½ | 0.0835(1) | 0.5330(1) | 0.0263(4) |
| Ag6 | 4c | ½ | 0.1638(1) | ¼ | 0.0274(5) |
| Rb7 | 4c | 0 | −0.0572(1) | ¼ | 0.0262(6) |
| RbEr2Ag3Te5 | |||||
| Er1 | 8f | 0 | 0.1919(1) | 0.4049(1) | 0.0150(4) |
| Te2 | 8f | ½ | 0.0640(1) | 0.3789(1) | 0.0162(5) |
| Te3 | 8f | 0 | 0.1772(1) | 0.5718(1) | 0.0142(5) |
| Te4 | 4c | 0 | 0.2620(1) | ¼ | 0.0149(5) |
| Ag5 | 8f | ½ | 0.0828(1) | 0.5338(1) | 0.0267(6) |
| Ag6 | 4c | ½ | 0.1638(2) | ¼ | 0.0261(7) |
| Rb7 | 4c | 0 | −0.0569(2) | ¼ | 0.0267(8) |
| CsLa2Ag3Te5 | |||||
| La1 | 8f | 0 | 0.1910(1) | 0.4064(1) | 0.0197(5) |
| Te2 | 8f | ½ | 0.0582(1) | 0.3798(1) | 0.0214(6) |
| Te3 | 8f | 0 | 0.1699(1) | 0.5730(1) | 0.0203(6) |
| Te4 | 4c | 0 | 0.2540(2) | ¼ | 0.0222(7) |
| Ag5 | 8f | ½ | 0.0860(2) | 0.5289(2) | 0.0302(7) |
| Ag6 | 4c | ½ | 0.1598(3) | ¼ | 0.0344(10) |
| Cs7 | 4c | 0 | −0.0596(2) | ¼ | 0.0284(8) |
| CsCe2Ag3Te5 | |||||
| Ce1 | 8f | 0 | 0.1908(1) | 0.4064(1) | 0.0138(1) |
| Te2 | 8f | ½ | 0.0591(1) | 0.3801(1) | 0.0150(1) |
| Te3 | 8f | 0 | 0.1718(1) | 0.5730(1) | 0.0133(1) |
| Te4 | 4c | 0 | 0.2539(1) | ¼ | 0.0156(2) |
| Ag5 | 8f | ½ | 0.0860(1) | 0.5296(1) | 0.0241(2) |
| Ag6 | 4c | ½ | 0.1597(1) | ¼ | 0.0271(2) |
| Cs7 | 4c | 0 | −0.0594(1) | ¼ | 0.0224(2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eickmeier, K.; Fries, K.S.; Gladisch, F.C.; Dronskowski, R.; Steinberg, S. Revisiting the Zintl‒Klemm Concept for ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Tellurides. Crystals 2020, 10, 184. https://doi.org/10.3390/cryst10030184
Eickmeier K, Fries KS, Gladisch FC, Dronskowski R, Steinberg S. Revisiting the Zintl‒Klemm Concept for ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Tellurides. Crystals. 2020; 10(3):184. https://doi.org/10.3390/cryst10030184
Chicago/Turabian StyleEickmeier, Katharina, Kai S. Fries, Fabian C. Gladisch, Richard Dronskowski, and Simon Steinberg. 2020. "Revisiting the Zintl‒Klemm Concept for ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Tellurides" Crystals 10, no. 3: 184. https://doi.org/10.3390/cryst10030184
APA StyleEickmeier, K., Fries, K. S., Gladisch, F. C., Dronskowski, R., & Steinberg, S. (2020). Revisiting the Zintl‒Klemm Concept for ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Tellurides. Crystals, 10(3), 184. https://doi.org/10.3390/cryst10030184