A Novel Phenolic Foam-Derived Magnetic Carbon Foam Treated as Adsorbent for Rhodamine B: Characterization and Adsorption Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Carbon Foam
2.3. Adsorption Experiment
2.3.1. The Adsorption Capacity (mg/g) and Efficiency η (%) Values of the Magnetic Adsorbent
2.3.2. Effect of Adsorbent Dosage
2.3.3. Effect of Contact Time
2.3.4. Effect of pH
2.4. Characterization
3. Results and Discussion
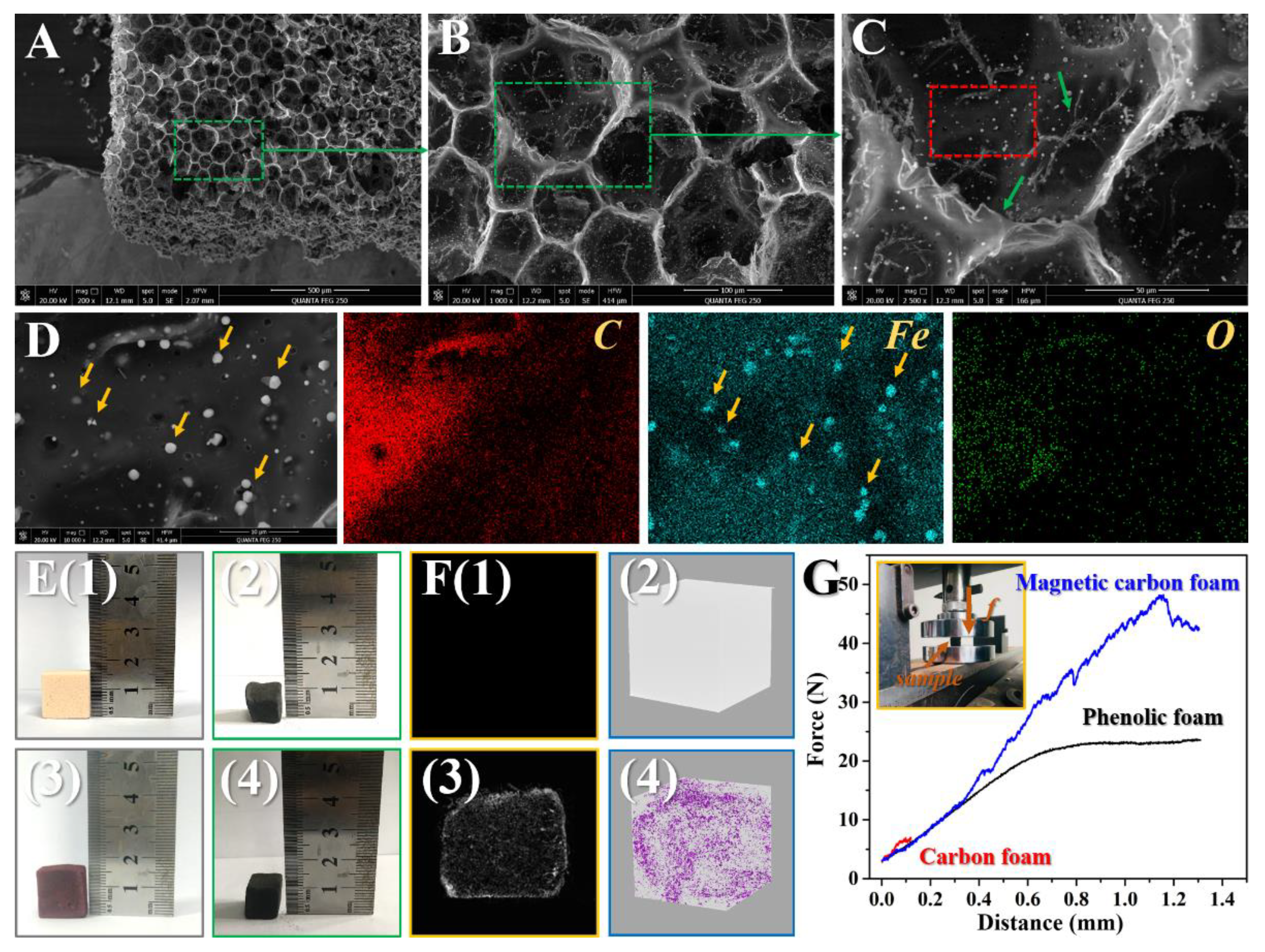
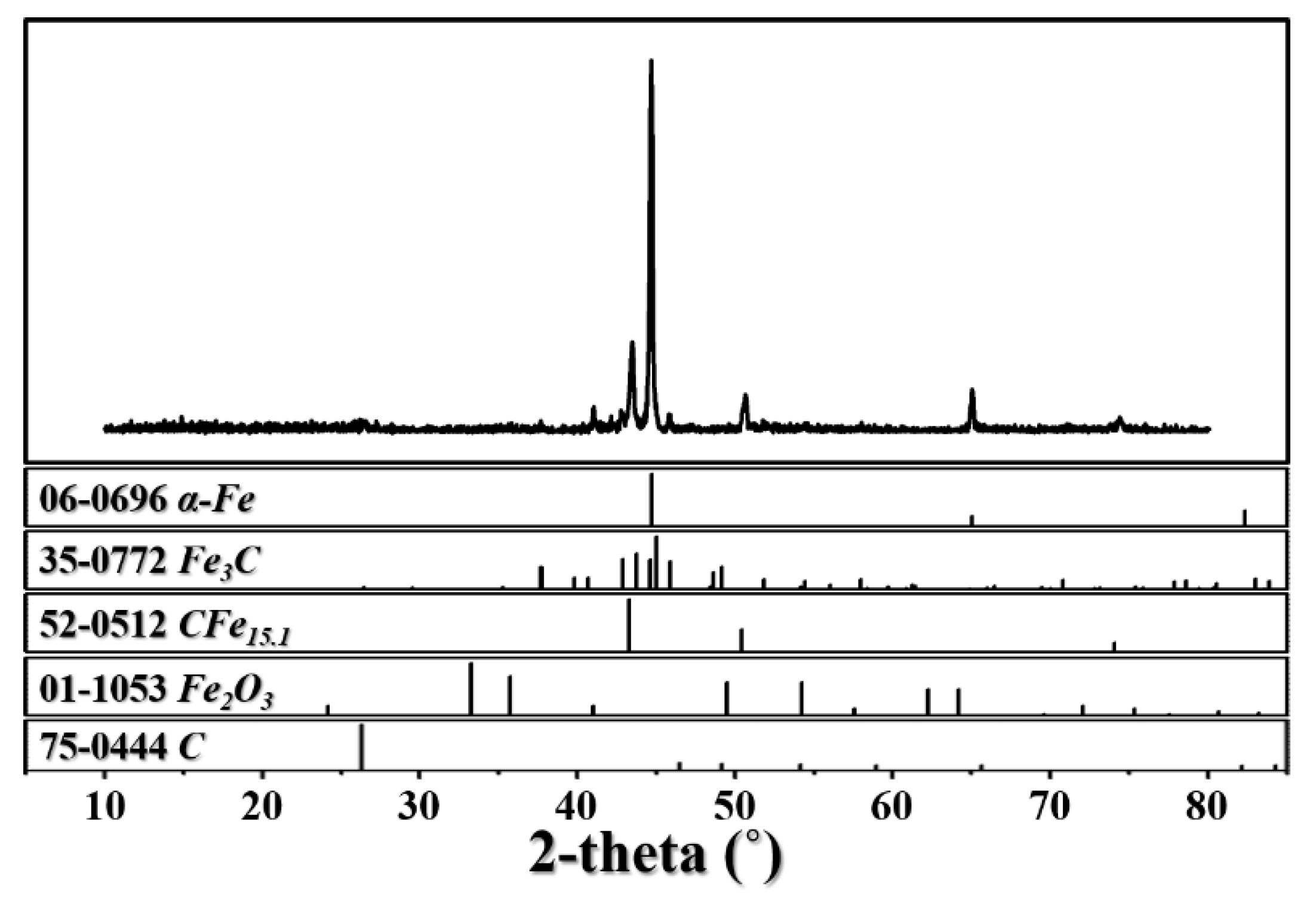
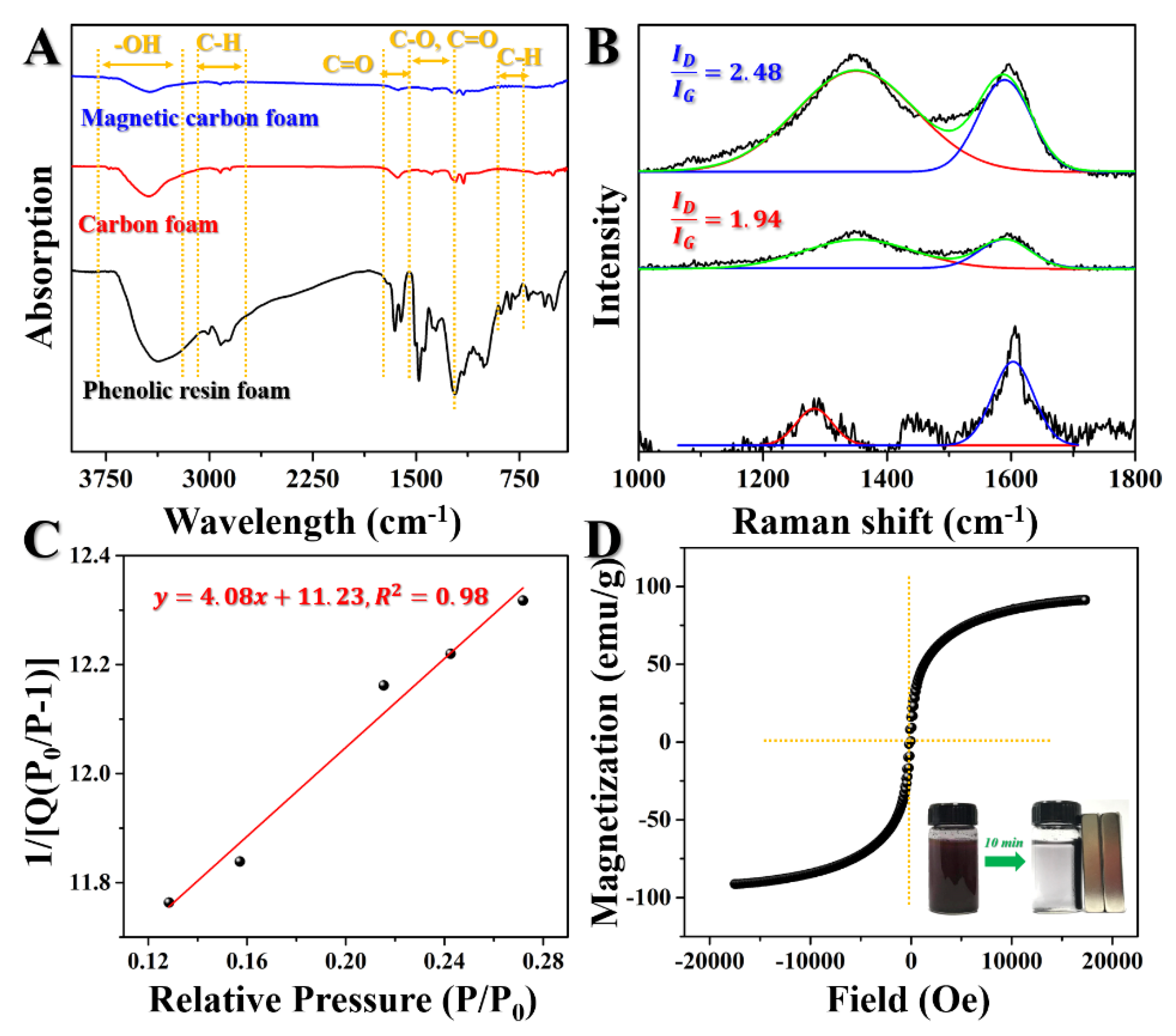
3.1. Characterization of the Samples
3.2. Adsorption Investigations
3.2.1. Effect of Adsorbent Dosage
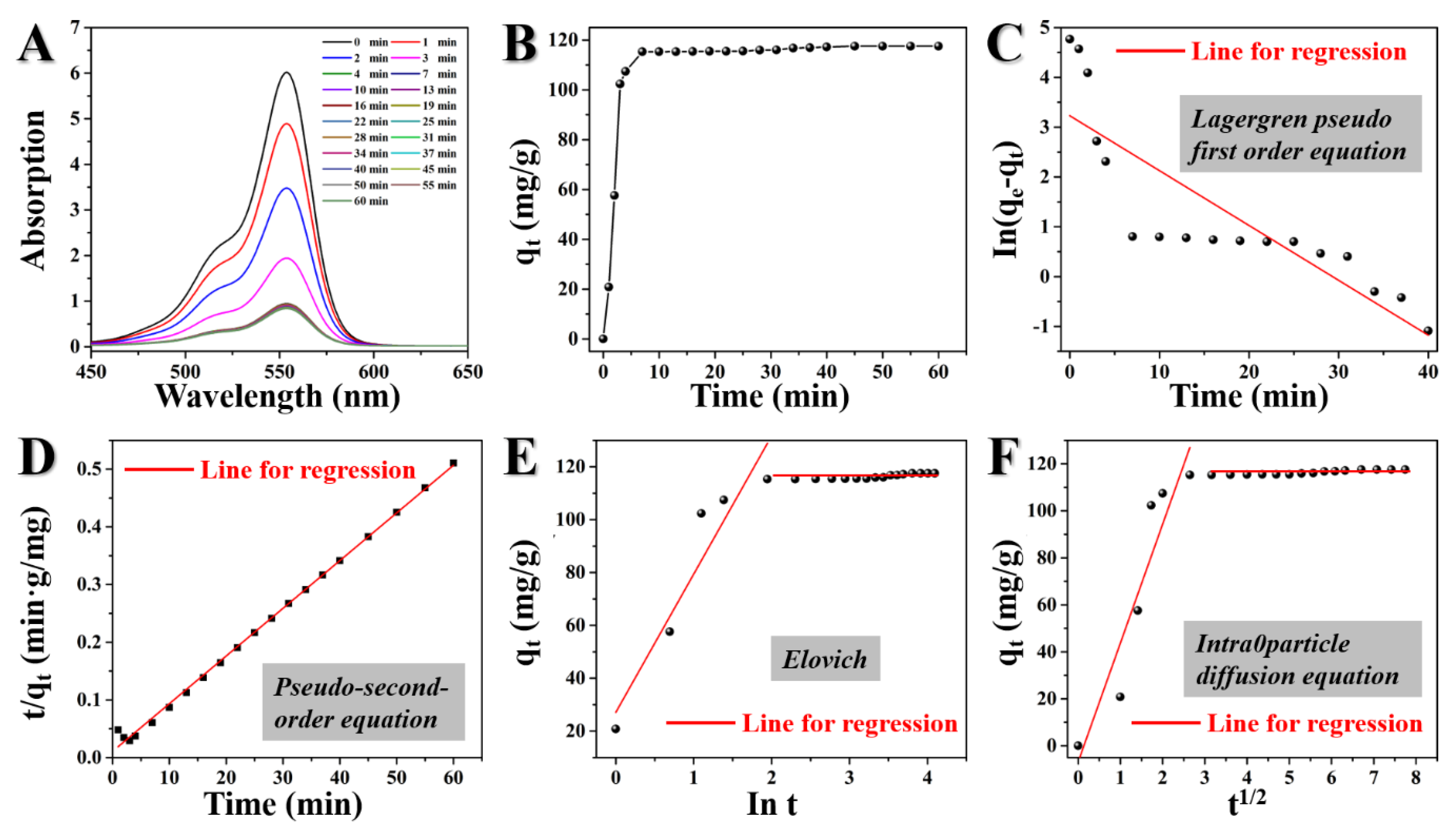
3.2.2. Adsorption Kinetics
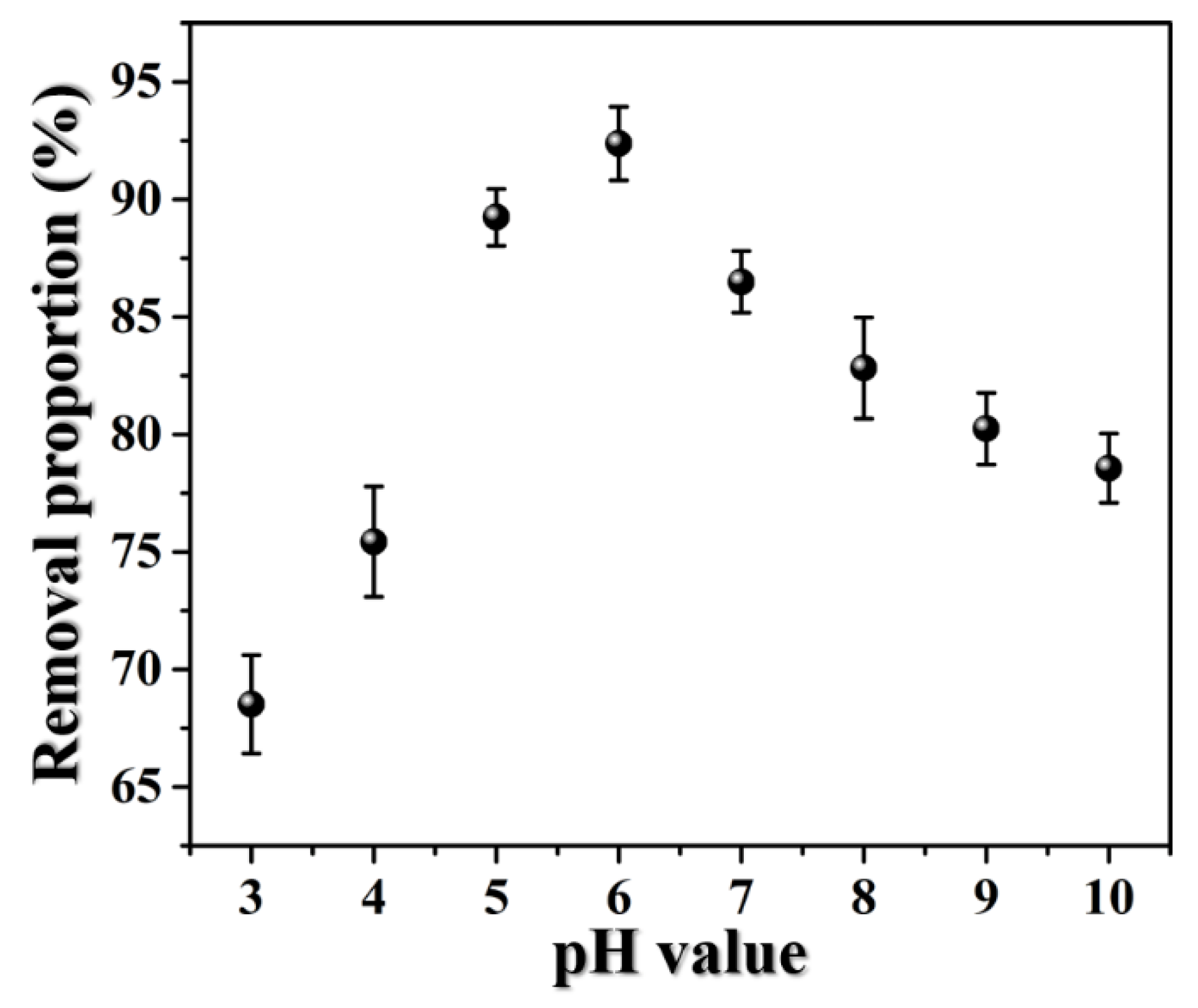
3.2.3. pH Effect on the Adsorption of RhB
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiwaan, H.A.; Atwee, T.M.; Azab, E.A.; El-Bindary, A.A. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide. J. Mol. Struct. 2020, 1200, 127115. [Google Scholar] [CrossRef]
- Li, C.; Lai, K.; Zhang, Y.; Pei, L.; Huang, Y. Use of Surface-enhanced Raman Spectroscopy for the Test of Residuals of Prohibited and Restricted Drugs in Fish Muscle. Acta Chim. Sin. 2013, 71, 221. [Google Scholar] [CrossRef][Green Version]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—A review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Luna Quinto, M.; Khan, S.; Picasso, G.; Taboada Sotomayor, M.D.P. Synthesis, characterization, and evaluation of a selective molecularly imprinted polymer for quantification of the textile dye acid violet 19 in real water samples. J. Hazard. Mater. 2020, 384, 121374. [Google Scholar] [CrossRef]
- Soyekwo, F.; Liu, C.; Wen, H.; Hu, Y. Construction of an electroneutral zinc incorporated polymer network nanocomposite membrane with enhanced selectivity for salt/dye separation. Chem. Eng. J. 2020, 380, 122560. [Google Scholar] [CrossRef]
- Ding, J.; Pan, Y.; Li, L.; Liu, H.; Zhang, Q.; Gao, G.; Pan, B. Synergetic adsorption and electrochemical classified recycling of Cr(VI) and dyes in synthetic dyeing wastewater. Chem. Eng. J. 2020, 384, 123232. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Shen, L.; Yu, Y.; Hu, L.; Zhou, Y. Performance of different macrophytes in the decontamination of and electricity generation from swine wastewater via an integrated constructed wetland-microbial fuel cell process. J. Environ. Sci. 2020, 89, 252–263. [Google Scholar] [CrossRef]
- Naik, A.P.; Mittal, H.; Wadi, V.S.; Sane, L.; Raj, A.; Alhassan, S.M.; Al Alili, A.; Bhosale, S.V.; Morajkar, P.P. Super porous TiO2 photocatalyst: Tailoring the agglomerate porosity into robust structural mesoporosity with enhanced surface area for efficient remediation of azo dye polluted waste water. J. Environ. Manag. 2020, 258, 110029. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khan, M.I.; Rabat, N.E.; Zulfiqar, M.; Man, Z.; Siame, J.; Azizli, K.A. A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. J. Environ. Manag. 2018, 224, 327–339. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Simchi, A.; Shahriyari Far, H. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2020, 81, 405–414. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.; Zhang, M.; Zhang, M. Fabrication and application of hierarchical porous carbon for the adsorption of bulky dyes. Microporous Mesoporous Mater. 2019, 290, 109651. [Google Scholar] [CrossRef]
- Lou, Z.; Yuan, C.; Zhang, Y.; Li, Y.; Cai, J.; Yang, L.; Wang, W.; Han, H.; Zou, J. Synthesis of porous carbon matrix with inlaid Fe3C/Fe3O4 micro-particles as an effective electromagnetic wave absorber from natural wood shavings. J. Alloys Compd. 2019, 775, 800–809. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, H.; Chen, C.; Yang, S.; Chen, L.; Alsaedi, A.; Hayat, T. Enhanced removal of uranium(VI) from aqueous solution by a novel Mg-MOF-74-derived porous MgO/carbon adsorbent. J. Colloid Interface Sci. 2019, 537, A1–A10. [Google Scholar] [CrossRef]
- Lou, Z.; Li, R.; Wang, P.; Zhang, Y.; Chen, B.; Huang, C.; Wang, C.; Han, H.; Li, Y. Phenolic foam-derived magnetic carbon foams (MCFs) with tunable electromagnetic wave absorption behavior. Chem. Eng. J. 2019, 123571. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the High Activity of Fe–N–C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe–Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Nie, Y.; Yang, M.; Qu, J. Mechanism of Catalytic Ozonation in Fe2O3/Al2O3@SBA-15 Aqueous Suspension for Destruction of Ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.-S.; Qin, Y.-H.; Niu, D.-F.; Tian, J.-W.; Zhang, X.-S.; Zhou, X.-G.; Sun, S.-G.; Yuan, W.-K. Effect of carbon nanofiber surface functional groups on oxygen reduction in alkaline solution. J. Power Sources 2013, 225, 192–199. [Google Scholar] [CrossRef]
- Edwards, E.R.; Antunes, E.F.; Botelho, E.C.; Baldan, M.R.; Corat, E.J. Evaluation of residual iron in carbon nanotubes purified by acid treatments. Appl. Surf. Sci. 2011, 258, 641–648. [Google Scholar] [CrossRef]
- Ismail, H.M.; Cadenhead, D.A.; Zaki, M.I. Surface Reactivity of Iron Oxide Pigmentary Powders toward Atmospheric Components: XPS and Gravimetry of Oxygen and Water Vapor Adsorption. J. Colloid Interface Sci. 1996, 183, 320–328. [Google Scholar] [CrossRef]
- Liu, B.; Yao, H.; Daniels, R.A.; Song, W.; Zheng, H.; Jin, L.; Suib, S.L.; He, J. A facile synthesis of Fe3C@mesoporous carbon nitride nanospheres with superior electrocatalytic activity. Nanoscale 2016, 8, 5441–5445. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yan, C.; Zhang, Y.; Gu, N. Shape Evolution of “Multibranched” Mn–Zn Ferrite Nanostructures with High Performance: A Transformation of Nanocrystals into Nanoclusters. Chem. Mater. 2013, 25, 3702–3709. [Google Scholar] [CrossRef]
- Shao, Y.; Guizani, C.; Grosseau, P.; Chaussy, D.; Beneventi, D. Biocarbons from microfibrillated cellulose/lignosulfonate precursors: A study of electrical conductivity development during slow pyrolysis. Carbon 2018, 129, 357–366. [Google Scholar] [CrossRef]
- Han, J.; Jun, B.-M.; Heo, J.; Lee, G.; Yoon, Y.; Park, C.M. Highly efficient organic dye removal from waters by magnetically recoverable La2O2CO3/ZnFe2O4-reduced graphene oxide nanohybrid. Ceram. Int. 2019, 45, 19247–19256. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Liao, J. Facile synthesis of reduced-graphene-oxide/rare-earth-metal-oxide aerogels as a highly efficient adsorbent for Rhodamine-B. Appl. Surf. Sci. 2020, 504, 144377. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Dewage, N.B.; Karunanayake, A.G.; Farmer, E.L.; Perez, F.; Hassan, E.B.; Mlsna, T.E.; Pittman, C.U. Rhodamine B Adsorptive Removal and Photocatalytic Degradation on MIL-53-Fe MOF/Magnetic Magnetite/Biochar Composites. J. Inorg. Organomet. Polym. 2020, 30, 214–229. [Google Scholar] [CrossRef]
- Li, Y.; Yin, X.; Huang, X.; Tian, J.; Wu, W.; Liu, X. The novel and facile preparation of 2DMoS2@C composites for dye adsorption application. Appl. Surf. Sci. 2019, 495, 143626. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem. Eng. J. 2019, 375, 122003. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Li, X.; Shao, W.-J.; Kou, Y.-L.; Yang, H.-P.; Zhang, D.-J. Fabrication of 3D Porous Polyvinyl Alcohol/Sodium Alginate/Graphene Oxide Spherical Composites for the Adsorption of Methylene Blue. J. Nanosci. Nanotechnol. 2020, 20, 2205–2213. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Qiu, W.; Wang, P.-P.; Liu, Y.-L.; Zou, J.; Wang, L.; Ma, J. Mechanism study about the adsorption of Pb(II) and Cd(II) with iron-trimesic metal-organic frameworks. Chem. Eng. J. 2020, 385, 123507. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.F.; Li, J.; Liu, Y.X. Fabrication, Characterization and Photocatalytic Activity of TiO2/Cellulose Composite Aerogel. KEM 2014, 609–610, 542–546. [Google Scholar] [CrossRef]
- Vargas, A.M.M.; Cazetta, A.L.; Kunita, M.H.; Silva, T.L.; Almeida, V.C. Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): Study of adsorption isotherms and kinetic models. Chem. Eng. J. 2011, 168, 722–730. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; An, C.; Zhao, S.; Chen, X.; Zhang, P. Adsorption of anionic azo dyes from aqueous solution on cationic gemini surfactant-modified flax shives: Synchrotron infrared, optimization and modeling studies. J. Clean. Prod. 2018, 172, 1986–1997. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Lahtinen, M.; Sillanpää, M. Preparation and characterization of a novel chitosan/Al2O3/magnetite nanoparticles composite adsorbent for kinetic, thermodynamic and isotherm studies of Methyl Orange adsorption. Chem. Eng. J. 2015, 259, 1–10. [Google Scholar] [CrossRef]


| Rhodamine B C0(mg/L): 100, qe(exp.) (mg/g): 117.527 | |||
|---|---|---|---|
| Kinetic Models and Parameters | Lagergren pseudo-first order equation: ln(qe − qt) = lnqe − k1t (1) | ||
| k1 (min−1): 0.110 | R2: 0.715 | ||
| Pseudo-second-order: t/qt = 1/k2qe2 + t/qe , h = k2qe2 (2) | |||
| k2 (g·mg−1·min−1): 0.00643 | h (mg·g−1·min−1): 94.073 | R2: 0.997 | |
| Elovich: qt = 1/β·ln(αβ) + 1/β ln(t) (3) | |||
| α (mg·g−1·min2): 87.933 | β (g·mg−1·min): 0.0191 | R2: 0.870 | |
| Intra-particle diffusion equation: qt = k3·t1/2 + C (4) | |||
| k3 (mg·g−1·min−1/2): 50.574 | C: −6.857 | R2: 0.854 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Q.; Li, R.; Lou, Z.; Li, Y. A Novel Phenolic Foam-Derived Magnetic Carbon Foam Treated as Adsorbent for Rhodamine B: Characterization and Adsorption Kinetics. Crystals 2020, 10, 159. https://doi.org/10.3390/cryst10030159
Zhang Y, Wang Q, Li R, Lou Z, Li Y. A Novel Phenolic Foam-Derived Magnetic Carbon Foam Treated as Adsorbent for Rhodamine B: Characterization and Adsorption Kinetics. Crystals. 2020; 10(3):159. https://doi.org/10.3390/cryst10030159
Chicago/Turabian StyleZhang, Yao, Qiuyi Wang, Ru Li, Zhichao Lou, and Yanjun Li. 2020. "A Novel Phenolic Foam-Derived Magnetic Carbon Foam Treated as Adsorbent for Rhodamine B: Characterization and Adsorption Kinetics" Crystals 10, no. 3: 159. https://doi.org/10.3390/cryst10030159
APA StyleZhang, Y., Wang, Q., Li, R., Lou, Z., & Li, Y. (2020). A Novel Phenolic Foam-Derived Magnetic Carbon Foam Treated as Adsorbent for Rhodamine B: Characterization and Adsorption Kinetics. Crystals, 10(3), 159. https://doi.org/10.3390/cryst10030159





