Influence of Halogen Substituent on the Self-Assembly and Crystal Packing of Multicomponent Crystals Formed from Ethacridine and Meta-Halobenzoic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Compounds 1–3
2.2. X-ray Measurements and Refinements
3. Results and Discussion
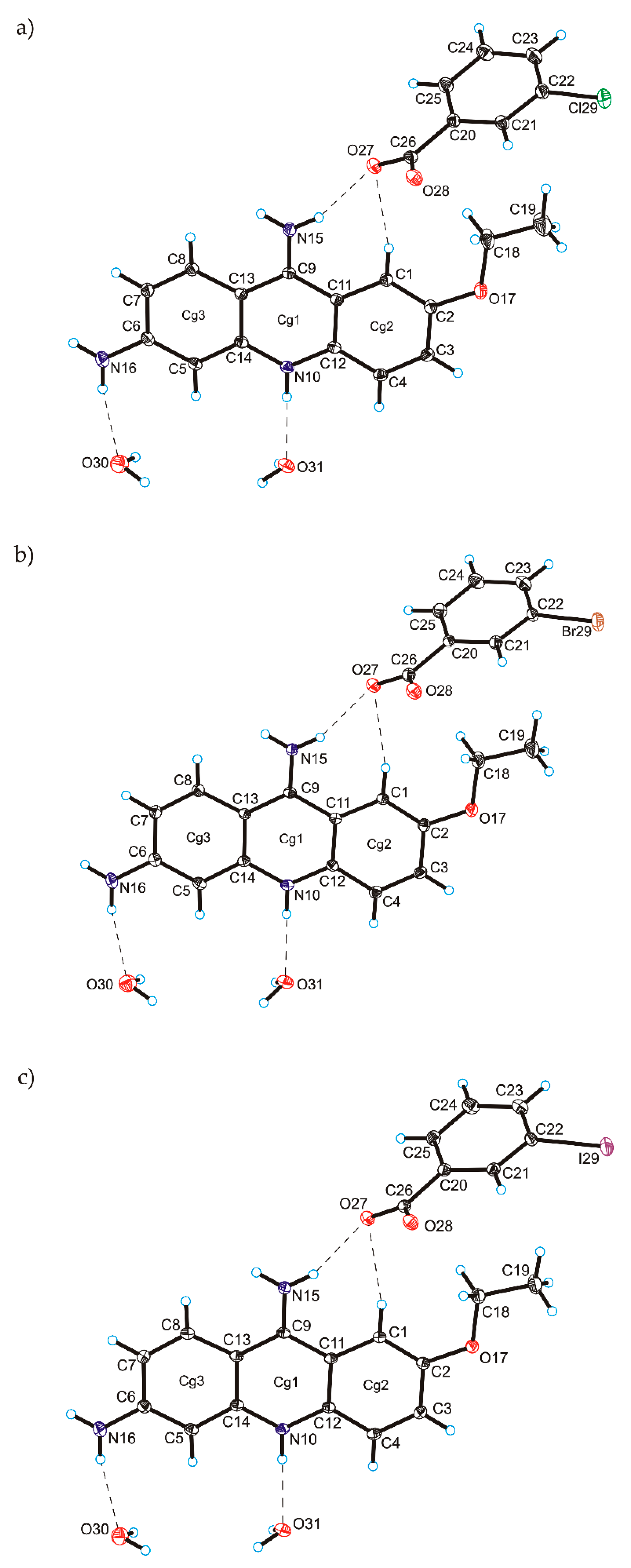
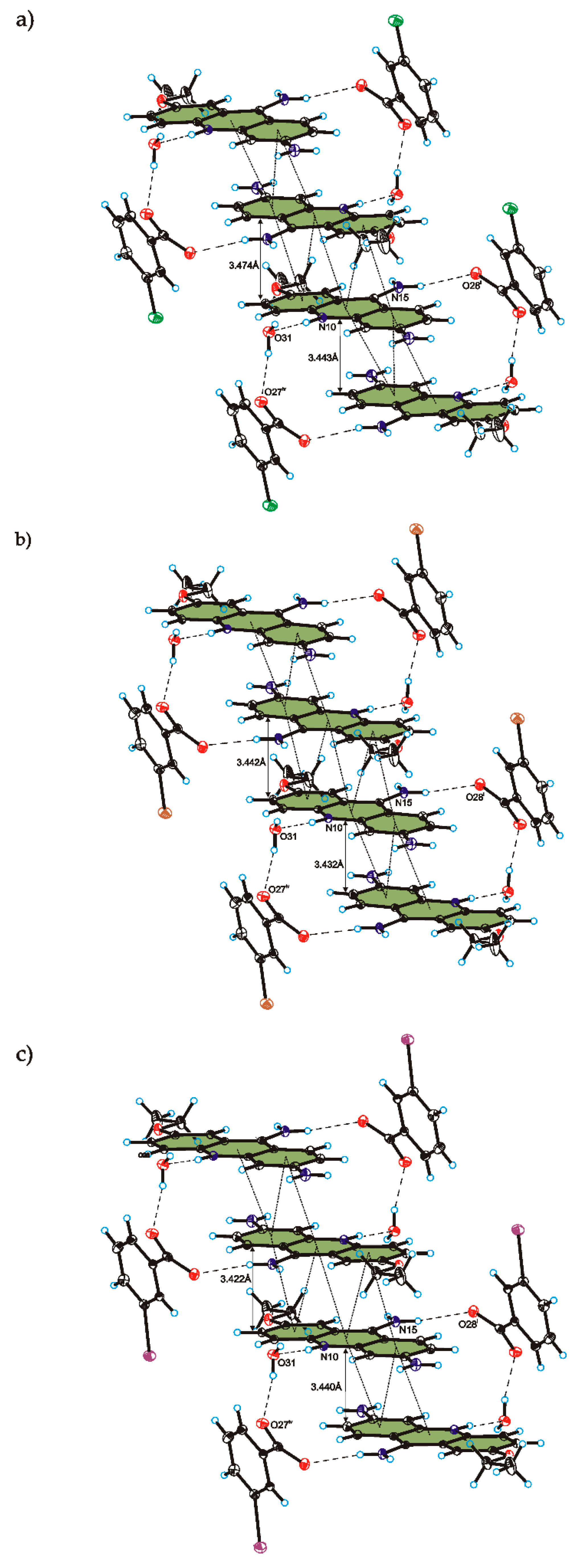
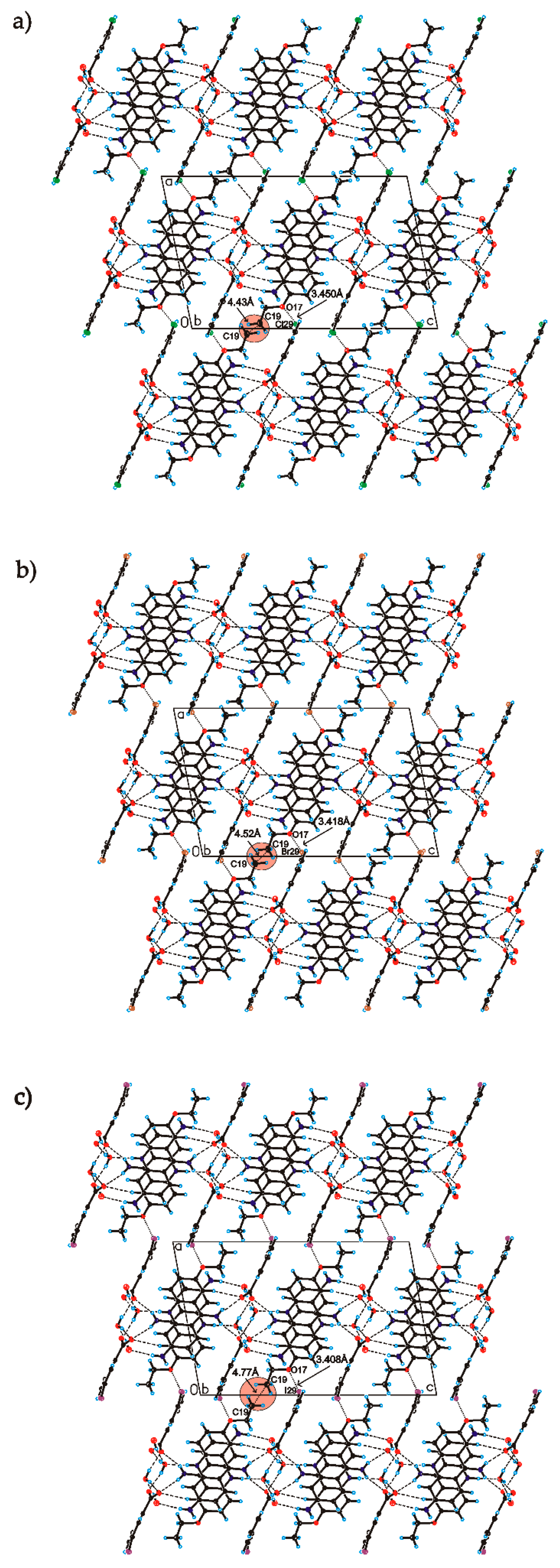
 C
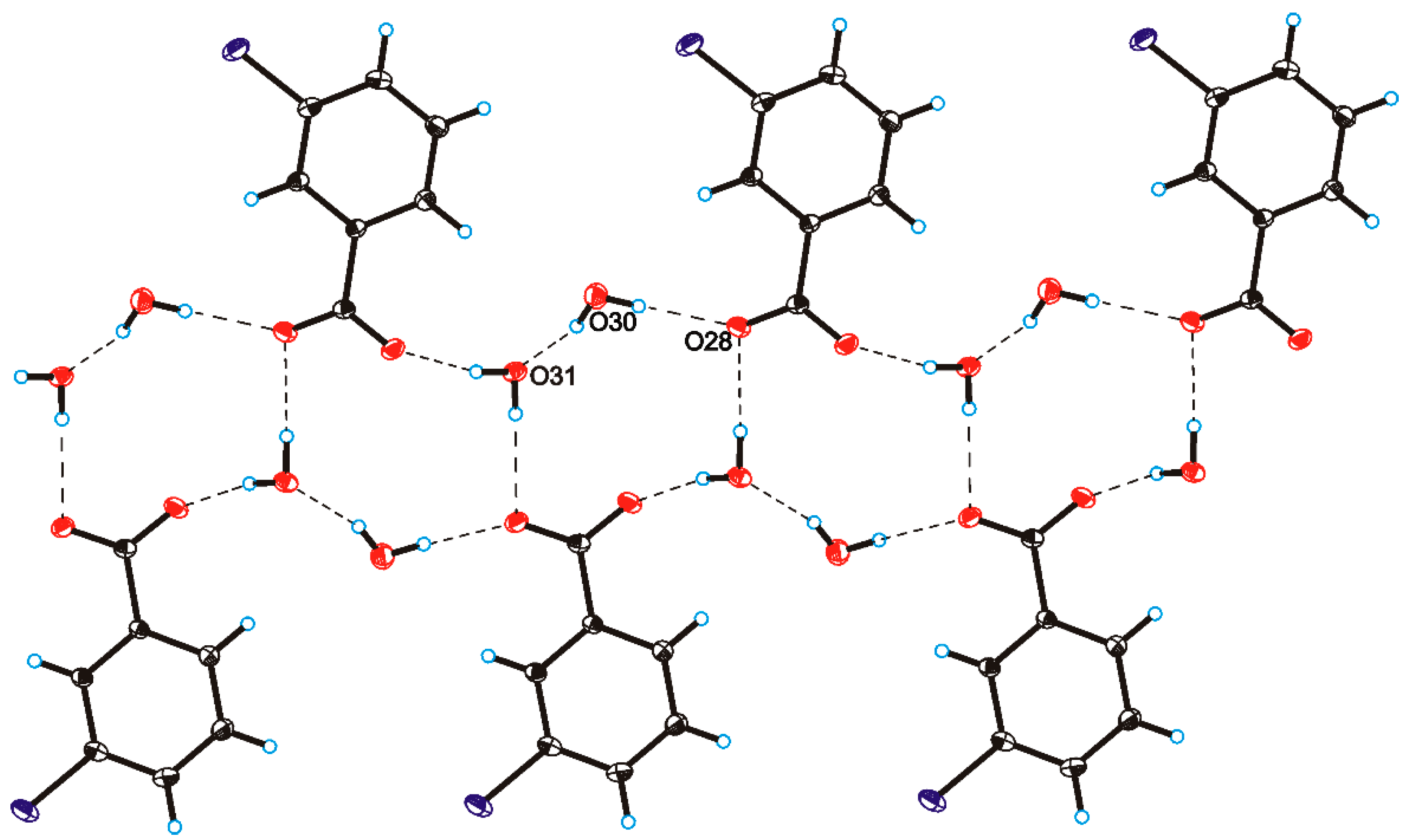
C O)−⋯H–O–H⋯O−⋯H–O–H⋯O–H⋯] and a hydrogen-bonded supramolecular tape motif (Figure 3) [40,41,42,43,44].
O)−⋯H–O–H⋯O−⋯H–O–H⋯O–H⋯] and a hydrogen-bonded supramolecular tape motif (Figure 3) [40,41,42,43,44]. 4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Tech. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharm. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Oie, S.; Kamiya, A. Bacterial contamination of commercially available ethacridine lactate (acrinol) product. J. Hosp. Infect. 1996, 34, 51–58. [Google Scholar] [CrossRef]
- Koelzer, S.C.; Held, H.; Toennes, S.W.; Verhoff, M.A.; Wunder, C. Self-induced illegal abortion with Rivanol®: A medicolegal–toxicological case report. Forensic Sci. Int. 2016, 268, e18–e22. [Google Scholar] [CrossRef]
- Chen, C.; Lin, F.; Wang, X.; Jiang, Y.; Wu, S. Mifepristone combined with ethacridine lactate for the second-trimester pregnancy termination in women with placenta previa and/or prior cesarean deliveries. Arch. Gynecol. Obstet. 2017, 295, 119–124. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.; Schultheiss, N.; Desper, J.; Moore, C. Structural competition between hydrogen bonds and halogen bonds. J. Am. Chem. Soc. 2007, 129, 13772–13773. [Google Scholar] [CrossRef]
- Desiraju, G.R. Designer crystals: Intermolecular interactions, network structures and supramolecular synthons. Chem. Commun. 1997, 16, 1475–1482. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Guru Row, T.N. Charge density analysis of ferulic acid: Robustness of a trifurcated C-H⋯O hydrogen bond. Cryst. Growth Des. 2012, 12, 6083–6091. [Google Scholar] [CrossRef]
- Tothadi, S.; Desiraju, G.R. Designing ternary cocrystals with hydrogen bonds and halogen bonds. Chem. Commun. 2013, 49, 7791–7793. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.-C.; Bläser, D.; Boese, R.; Doughan, B.M.; Haley, M.M. C-H⋯π interactions in ethynylbenzenes: The crystal structures of ethynylbenzene and 1,3,5-triethynylbenzene, and a redetermination of the structure of 1,4-diethynylbenzene. Chem. Comm. 1997, 18, 1703–1704. [Google Scholar] [CrossRef]
- Steiner, T. C-H⋯O hydrogen bonding in crystals. Crystallogr. Rev. 2003, 9, 177–228. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Sinha, A.S.; Chopade, P.D.; Desper, J. Halogen bonding or close packing? Examining the structural landscape in a series of Cu(ii)-acac complexes. Dalton T. 2011, 40, 12160–12168. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Chopade, P.D.; Desper, J. Establishing a hierarchy of halogen bonding by engineering crystals without disorder. Cryst. Growth Des. 2013, 13, 4145–4150. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen bonds in crystal engineering: Like hydrogen bonds yet different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Lommerse, J.P.M.; Stone, A.J.; Taylor, R.; Allen, F.H. The nature and geometry of intermolecular interactions between halogens and oxygen or nitrogen. J. Am. Chem. Soc. 1996, 118, 3108–3116. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Khlobystov, A.N.; Lemenovskii, D.A.; Li, W.-S.; Schröder, M. Crystal engineering: The effects of π-π interactions in copper(I) and silver(I) complexes of 2,7-diazapyrene. Chem. Comm. 1997, 15, 1339–1340. [Google Scholar] [CrossRef]
- Huang, J.; Kertesz, M. Intermolecular covalent π-π bonding interaction indicated by bond distances, energy bands, and magnetism in biphenalenyl biradicaloid molecular crystal. J. Am. Chem. Soc. 2007, 129, 1634–1643. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Shukla, R.; Mohan, T.P.; Vishalakshi, B.; Chopra, D. Experimental and theoretical analysis of lp⋯π intermolecular interactions in derivatives of 1,2,4-triazoles. CrystEngComm 2014, 16, 1702–1713. [Google Scholar] [CrossRef]
- Nelyubina, Y.V.; Barzilovich, P.Y.; Antipin, M.Y.; Aldoshin, S.M.; Lyssenko, K.A. Cation-π and lone pair-π interactions combined in one: The first experimental evidence of (H3O-lp)+⋯π-system binding in a crystal. ChemPhysChem 2011, 12, 2895–2898. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Pro. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef]
- Meyer, F.; Dubois, P. Halogen bonding at work: Recent applications in synthetic chemistry and materials science. CrystEngComm 2013, 15, 3058–3071. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Edit. 2008, 47, 6114–6127. [Google Scholar] [CrossRef]
- Neidle, S.; Aggarwal, A. Nucleic Acid Binding Drugs. The Structure of 3,9-Diamino-7-ethoxyacridine (Rivanol) as the Lactate Monohydrate Salt. Acta Crystallogr. 1982, B38, 2420–2424. [Google Scholar] [CrossRef]
- Fujii, K.; Uekusa, H.; Itoda, N.; Hasegawa, G.; Yonemochi, E.; Terada, K.; Pan, Z.; Harris, K.D.M. Physicochemical understanding of polymorphism and solid-state dehydration/rehydration processes for the pharmaceutical material acrinol, by Ab initio powder X-ray diffraction analysis and other techniques. J. Phys. Chem. C 2010, 114, 580–586. [Google Scholar] [CrossRef]
- Kowalska, K.; Trzybiński, D.; Sikorski, A. Influence of the halogen substituent on the formation of halogen and hydrogen bonding in co-crystal formed from acridine and benzoic acids. CrystEngComm 2015, 17, 7199–7212. [Google Scholar] [CrossRef]
- Sikorski, A.; Trzybiński, D. Synthesis and structural characterization of a cocrystal salt containing acriflavine and 3,5-dinitrobenzoic acid. Tetrahedron Lett. 2014, 55, 2253–2255. [Google Scholar] [CrossRef]
- Sikorski, A.; Trzybiński, D. Networks of intermolecular interactions involving nitro groups in the crystals of three polymorphs of 9-aminoacridinium 2,4-dinitrobenzoate ∙ 2,4-dinitrobenzoic acid. J. Mol. Struct. 2013, 1049, 90–98. [Google Scholar] [CrossRef]
- CrysAlis CCD and CrysAlis RED, Version 1.171.36.24; Oxford Diffraction Ltd.: Yarnton, England, 2012.
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.K. ORTEP II, Report ORNL-5138; Oak Ridge National Laboratory: OakRidge, TN, USA, 1976. [Google Scholar]
- Motherwell, S.; Clegg, S. PLUTO-78, Program for Drawing and Molecular Structure; University of Cambridge: Cambridge, UK, 1978. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Wang, J.-R.; Ye, C.; Mei, X. Structural and physicochemical aspects of hydrochlorothiazide co-crystals. CrystEngComm 2014, 16, 6996–7003. [Google Scholar] [CrossRef]
- Smith, G.; Wermuth, U.D. Bis (guanidinium) 4,5-dichlorophthalate monohydrate. Acta Crystallogr. 2011, E67, o1645. [Google Scholar] [CrossRef]
- Rajam, A.; Thomas Muthiah, P.; Butcher, R.J.; Zeller, M. Crystal structure of 4-amino-5-chloro-2, 6-dimethylpyrimidinium thiophene-2, 5-dicarboxylate. Acta Crystallogr. 2016, E72, 1043–1046. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dixit, K.; Sarma, S.P.; Desiraju, G.R. Aniline–phenol recognition: From solution through supramolecular synthons to cocrystals. IUCr 2014, 1, 228–239. [Google Scholar] [CrossRef]
- Kaur, R.; Gautam, R.; Cherukuvada, S.; Guru Row, T.N. Do carboximide–carboxylic acid combinations form co-crystals? The role of hydroxyl substitution on the formation of co-crystals and eutectics. IUCr J. 2015, 2, 341–351. [Google Scholar] [CrossRef]
- Mukherjee, A. Building upon Supramolecular Synthons: Some Aspects of Crystal Engineering. Cryst. Growth Des. 2015, 15, 3076–3085. [Google Scholar] [CrossRef]
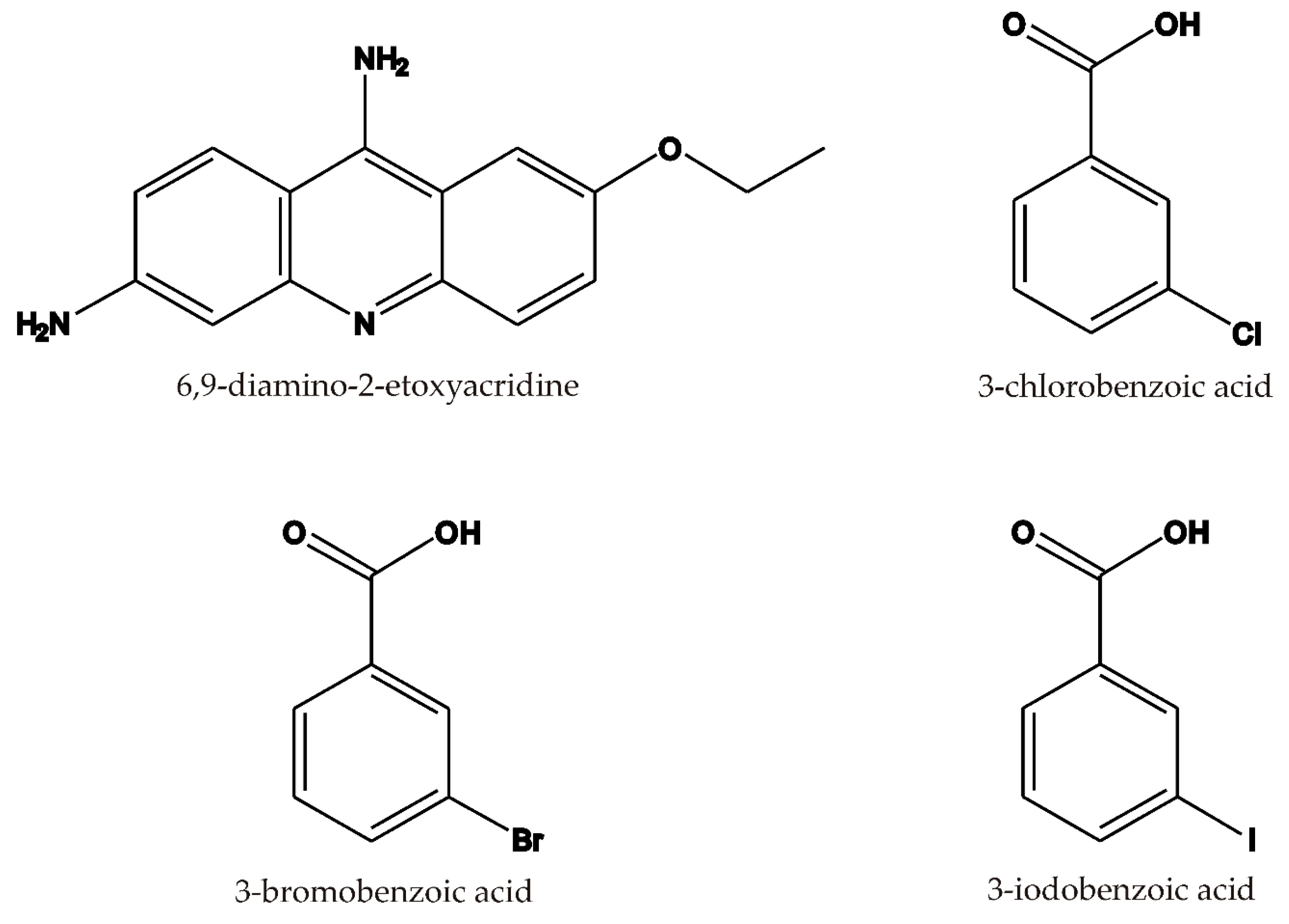
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Chemical formula | C22H24ClN3O5 | C22H24BrN3O5 | C22H24IN3O5 |
| Formula weight/g·mol−1 | 445.89 | 490.35 | 537.34 |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | P21/c | P21/c | P21/c |
| a/Å | 12.941(4) | 13.0933(8) | 13.4621(7) |
| b/Å | 8.401(4) | 8.3893(7) | 8.3911(4) |
| c/Å | 20.580(2) | 20.565(5) | 20.522(3) |
| α/° | 90 | 90 | 90 |
| β/° | 101.475(3) | 101.242(7) | 100.263(5) |
| γ/° | 90 | 90 | 90 |
| V/Å3 | 2192.6(5) | 2215.6(3) | 2281.1(2) |
| Z | 4 | 4 | 4 |
| T/K | 295(2) | 295(2) | 295(2) |
| λMo/Å | 0.71073 | 0.71073 | 0.71073 |
| ρcalc/g·cm−3 | 1.351 | 1.470 | 1.565 |
| F(000) | 936 | 1008 | 1080 |
| µ/mm−1 | 0.213 | 1.894 | 1.441 |
| θ range/° | 3.35-25.00 | 3.17-25.00 | 3.35-25.00 |
| Completness θ/% | 99.7 | 99.8 | 99.8 |
| Reflections collected | 15401 | 14566 | 15803 |
| Reflections unique | 3855 [Rint = 0.0689] | 3893 [Rint = 0.0881] | 3999 [Rint = 0.0562] |
| Data/restraints/parameters | 3855/6/293 | 3893/6/293 | 3999 /6/293 |
| Goodness of fit on F2 | 1.007 | 0.984 | 1.009 |
| Final R1 value (I>2σ(I)) | 0.0593 | 0.0539 | 0.0409 |
| Final wR2 value (I>2σ(I)) | 0.1464 | 0.1010 | 0.0771 |
| Final R1 value (all data) | 0.1114 | 0.1320 | 0.0779 |
| Final wR2 value (all data) | 0.1821 | 0.1263 | 0.0900 |
| CCDC number | 1968938 | 1968940 | 1968939 |
| Compound | D–H⋯A | d(D–H) [Å] | d(H⋯A) [Å] | d(D⋯A) [Å] | ∠D–H⋯A (°) |
|---|---|---|---|---|---|
| 1 | N(10)–H(10)⋯O(31) | 0.86 | 1.95 | 2.810(3) | 178 |
| N(15)–H(15A)⋯O(28)i | 0.86 | 2.16 | 2.957(3) | 155 | |
| N(15) –H(15B)⋯O(27) | 0.86 | 2.13 | 2.960(3) | 161 | |
| N(16)–H(16A)⋯O(30) | 0.86 | 2.17 | 3.016(4) | 168 | |
| O(30)–H(30A)⋯O(31)ii | 0.84(3) | 2.01(3) | 2.824(4) | 163(3) | |
| O(30)–H(30B)⋯O(28)iii | 0.84(3) | 2.00(3) | 2.835(4) | 177(7) | |
| O(31)–H(31A)⋯O(27)iv | 0.84(3) | 1.87(3) | 2.689(3) | 167(4) | |
| O(31)–H(31B)⋯O(28)iii | 0.85(3) | 1.94(3) | 2.775(3) | 169(3) | |
| C(1)–H(1)⋯O(27) | 0.93 | 2.56 | 3.471(4) | 167 | |
| Symmetry code: (i) 1−x,1/2+y,1/2-z; (ii) 1−x, 1/2+y, 3/2−z; (iii) 1−x, 1−y, 1−z; (iv) x, 3/2−y, 1/2+z. | |||||
| 2 | N(10)–H(10)⋯O(31) | 0.86 | 1.95 | 2.813(4) | 177 |
| N(15)–H(15A)⋯O(28)i | 0.86 | 2.15 | 2.950(4) | 156 | |
| N(15)–H(15B)⋯O(27) | 0.86 | 2.13 | 2.954(4) | 160 | |
| N(16)–H(16A)⋯O(30) | 0.86 | 2.18 | 3.026(6) | 168 | |
| O(30)–H(30A)⋯O(31)ii | 0.85(4) | 1.98(4) | 2.811(6) | 167(4) | |
| O(30)–H(30B)⋯O(28)iii | 0.86(3) | 1.98(3) | 2.835(5) | 173(5) | |
| O(31)–H(31A)⋯O(27)iv | 0.85(4) | 1.85(4) | 2.679(5) | 163(5) | |
| O(31)–H(31B)⋯O(28)iii | 0.85(4) | 1.93(4) | 2.763(5) | 169(5) | |
| C(1)–H(1)⋯O(27) | 0.93 | 2.55 | 3.465(5) | 168 | |
| Symmetry code: (i) 1−x, 1/2+y, 1/2−z; (ii) 1−x, 1/2+y, 3/2−z; (iii) 1−x, 1−y, 1−z; (iv) x, 3/2−y,1/2+z. | |||||
| 3 | N(10)–H(10)⋯O(31) | 0.86 | 1.95 | 2.807(4) | 176 |
| N(15)–H(15A)⋯O(28)i | 0.86 | 2.16 | 2.964(4) | 156 | |
| N(15)–H(15B)⋯O(27) | 0.86 | 2.13 | 2.948(4) | 160 | |
| N(16)–H(16A)⋯O(30) | 0.86 | 2.19 | 3.031(5) | 166 | |
| O(30)–H(30A)⋯O(31)ii | 0.84(4) | 2.00(4) | 2.816(5) | 165(4) | |
| O(30)–H(30B)⋯O(28)iii | 0.83(3) | 2.00(3) | 2.830(5) | 172(5) | |
| O(31)–H(31A)⋯O(27)iv | 0.83(4) | 1.87(4) | 2.684(4) | 164(4) | |
| O(31)–H(31B)⋯O(28)iii | 0.83(3) | 1.93(3) | 2.757(4) | 171(3) | |
| C(1)–H(1)⋯O(27) | 0.93 | 2.54 | 3.455(4) | 168 | |
| Symmetry code: (i) 1−x, 1/2+y, 1/2−z; (ii) 1−x, 1/2+y,3/2−z; (iii) 1−x, 1−y, 1−z; (iv) x, 3/2−y, 1/2+z. | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirocki, A.; Sikorski, A. Influence of Halogen Substituent on the Self-Assembly and Crystal Packing of Multicomponent Crystals Formed from Ethacridine and Meta-Halobenzoic Acids. Crystals 2020, 10, 79. https://doi.org/10.3390/cryst10020079
Mirocki A, Sikorski A. Influence of Halogen Substituent on the Self-Assembly and Crystal Packing of Multicomponent Crystals Formed from Ethacridine and Meta-Halobenzoic Acids. Crystals. 2020; 10(2):79. https://doi.org/10.3390/cryst10020079
Chicago/Turabian StyleMirocki, Artur, and Artur Sikorski. 2020. "Influence of Halogen Substituent on the Self-Assembly and Crystal Packing of Multicomponent Crystals Formed from Ethacridine and Meta-Halobenzoic Acids" Crystals 10, no. 2: 79. https://doi.org/10.3390/cryst10020079
APA StyleMirocki, A., & Sikorski, A. (2020). Influence of Halogen Substituent on the Self-Assembly and Crystal Packing of Multicomponent Crystals Formed from Ethacridine and Meta-Halobenzoic Acids. Crystals, 10(2), 79. https://doi.org/10.3390/cryst10020079






