The Methods to Crystallize Anhydrous L-Phenylalanine from Methanol-Water Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Measurement
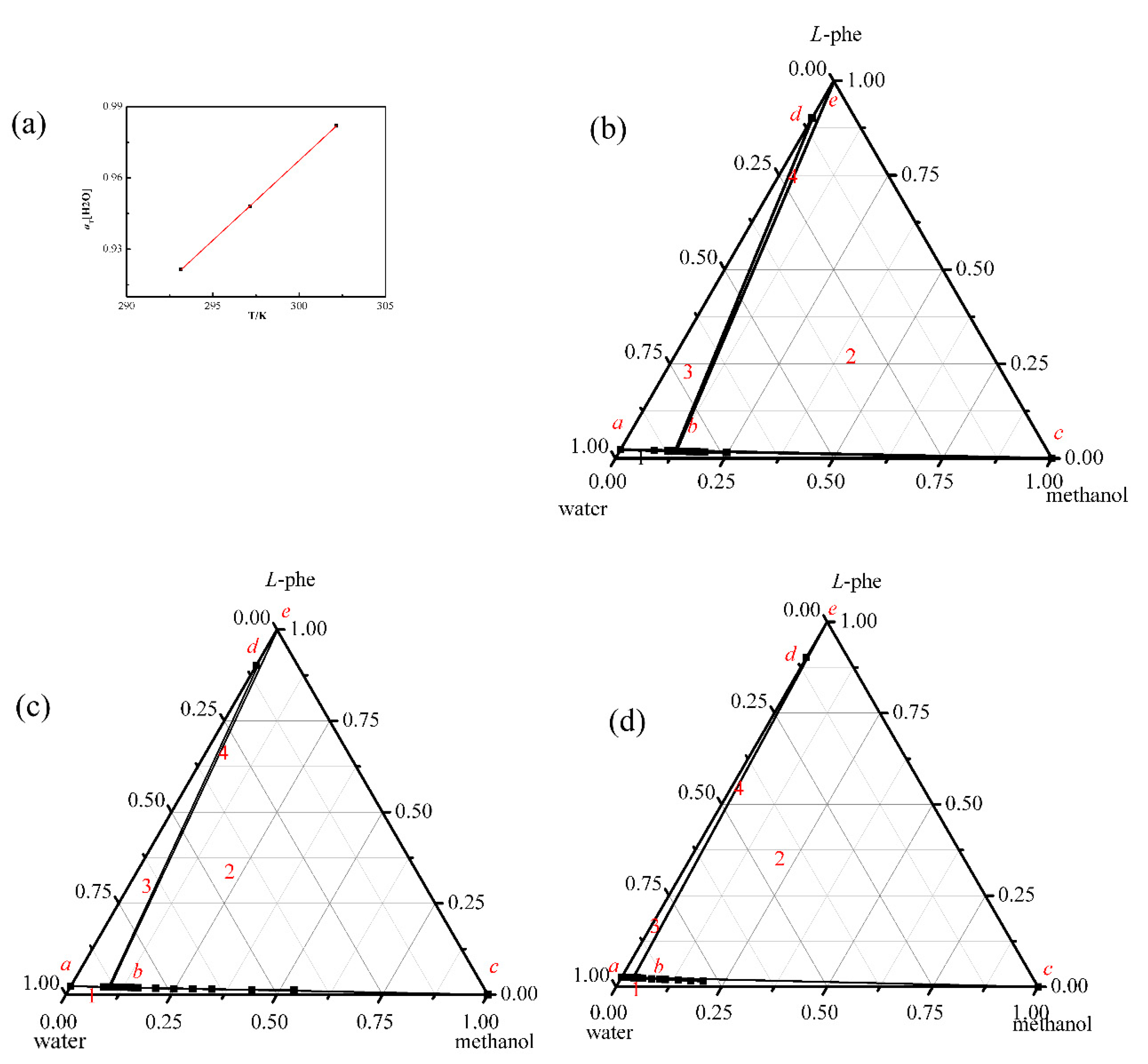
2.3. Transition Water Activity Measurement
2.4. Transformation Controlled by Tailor-Made Additives
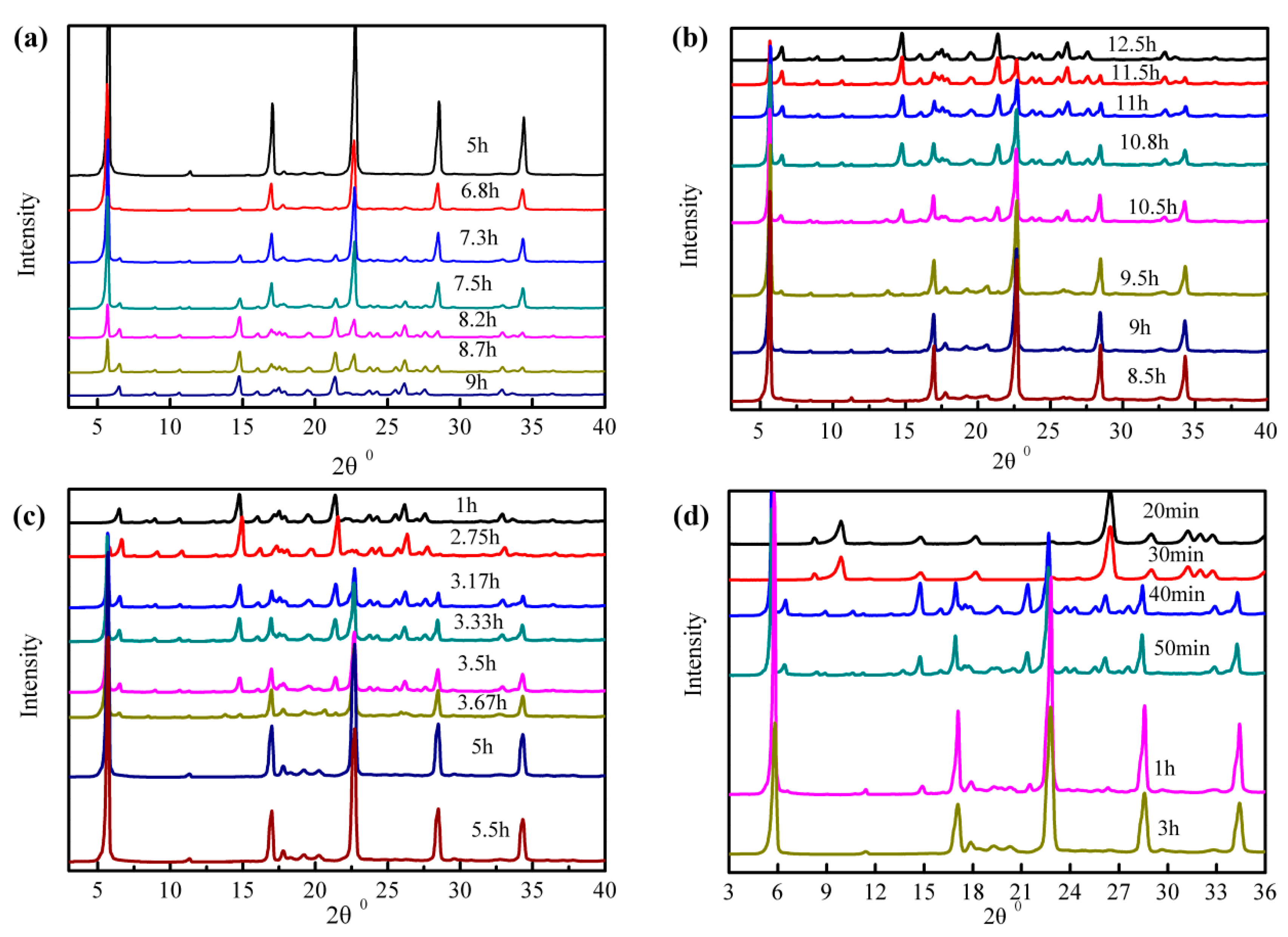
2.5. Methods of Analysis
3. Thermodynamic and Correlating Models
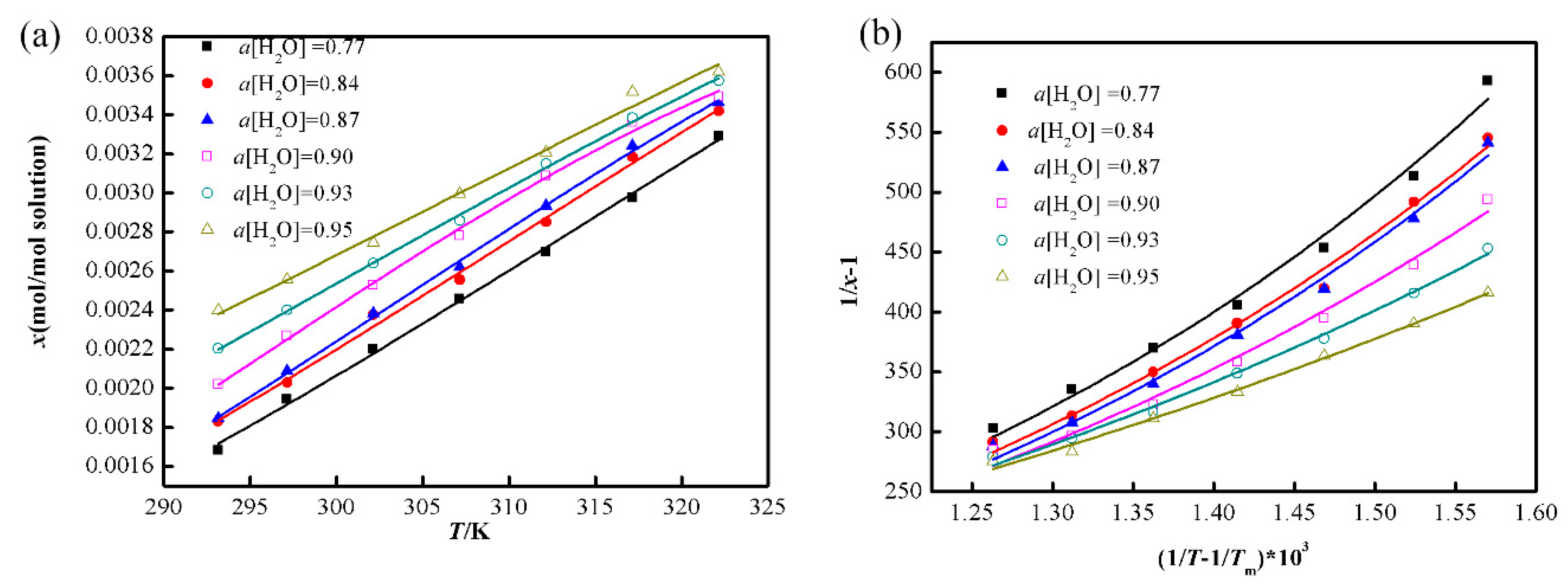
3.1. Correlation of Solubility with Temperature
3.2. Correlation of Water Activity
3.3. Transformation Model
4. Results and Discussion
4.1. Solubility in the Binary Solvent Mixtures
4.2. The Ternary Phase Diagram
4.3. Transformation Kinetics
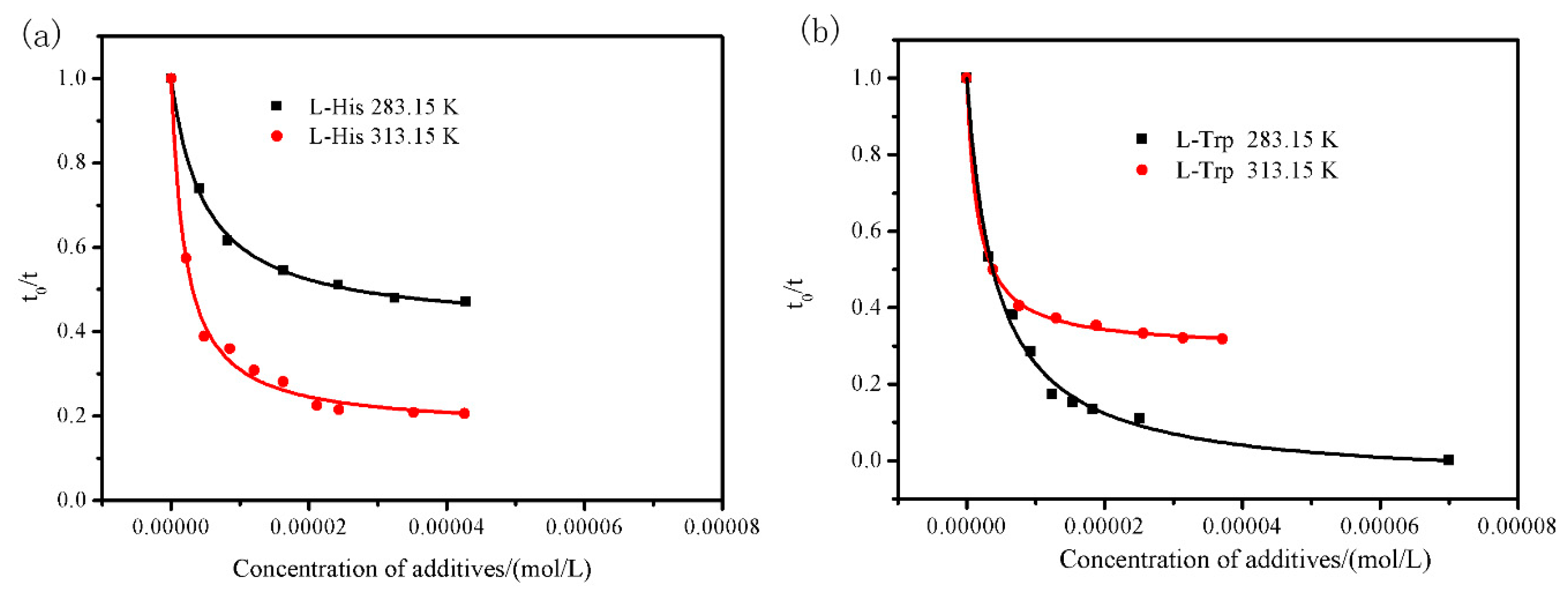
4.4. Transformation Controlling by Additive
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bechtloff, B.; Nordhoff, S.; Ulrich, J. Pseudopolymorphs in industrial use. Cryst. Research. Technol. 2001, 36, 1315–1328. [Google Scholar] [CrossRef]
- Brittain, H.G. Polymorphism and solvatomorphism 2010. J. Pharm. Sci. 2012, 101, 464–484. [Google Scholar] [CrossRef]
- Vippagunta, S.R.; Brittain, H.G.; Grant, D.J. Crystalline solids. Adv. Drug Deliv. Rev. 2001, 48, 3–26. [Google Scholar] [CrossRef]
- Tian, F.; Qu, H.; Zimmermann, A.; Munk, T.; Jørgensen, A.C.; Rantanen, J. Factors affecting crystallization of hydrates. J. Pharm. Pharmacol. 2010, 62, 1534–1546. [Google Scholar] [CrossRef]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef]
- Neglur, R.; Hosten, E.; Aucamp, M.; Liebenberg, W.; Grooff, D. Water and the relationship to the crystal structure stability of azithromycin. J. Therm. Anal. Calorim 2018, 132, 373–384. [Google Scholar] [CrossRef]
- Zhu, H.J.; Yuen, C.M.; Grant, D.J.W. Influence of water activity in organic solvent plus water mixtures on the nature of the crystallizing drug phase. 1. Theophylline. Int. J. Pharm. 1996, 135, 151–160. [Google Scholar] [CrossRef]
- Li, Y.; Chow, P.S.; Tan, R.B.H. Effect of Water Activity on the Transformation between Hydrate and Anhydrate of Carbamazepine. Org. Process Res. Dev. 2008, 12, 264–270. [Google Scholar] [CrossRef]
- Shefter, E.; Higuchi, T. Dissolution Behavior of Crystalline Solvated and nonsolvated forms of some pharmaceuticals. J. Pharm. Sci. 1963, 52, 781–791. [Google Scholar] [CrossRef]
- Liu, C.; Dang, L.; Tong, Y.; Wei, H.Y. Influence of Polymorphs on the Transformation Water Activity of Theophylline. Ind. Eng. Chem. Res. 2013, 52, 14979–14983. [Google Scholar] [CrossRef]
- Cuellar, M.C.; Herreilers, S.N.; Straathof, A.J.J.; Heijnen, J.J.; Wielen, L.A.M.V.D. Limits of operation for the integration of water removal by membranes and crystallization of L-phenylalanine. Ind. Eng. Chem. Res. 2009, 48, 1566–1573. [Google Scholar] [CrossRef]
- Lu, J.; Li, Z.; Jiang, X.L. Solubility of L-phenylalanine in aqueous solutions. J. Chem. Eng. Jpn. 2010, 43, 810–813. [Google Scholar]
- Lu, J.; Lin, Q.; Li, Z.; Rohani, S. Solubility of L-phenylalanine anhydrous and monohydrate forms: Experimental measurements and predictions. J. Chem. Eng. Data 2012, 57, 1492–1498. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Li, Z.; Rohani, S. Characterization and pseudopolymorphism of L-phenylalanine anhydrous and monohydrate forms. Afr. J. Pharm. Pharmacol. 2012, 6, 269–277. [Google Scholar] [CrossRef]
- Li, R.R.; Ye, S.F.; Chen, Y.F. Solubility of sulfachloropyridazine in pure and binary solvent mixtures and investigation of intermolecular interactions. J. Chem. Eng. Data 2018, 63, 2002–2008. [Google Scholar] [CrossRef]
- Chao, Y.; Lo, T.; Luo, N. Selective production of L-aspartic acid and L-Phenylalanine by coupling reactions of aspartase and aminotransferase in Escherichia coli. Enzym. Microb. Technol. 2000, 27, 19–25. [Google Scholar] [CrossRef]
- Xu, H.; Wei, P.; Zhou, H.; Fan, W.P.; Ouyang, P.K. Efficient production of Lphenylalanine catalyzed by a coupled enzymatic system of transaminase and aspartase. Enzym. Microb. Technol. 2003, 33, 537–543. [Google Scholar] [CrossRef]
- Edahiro, J.; Nakamura, M.; Seki, M. Enhanced accumulation of anthocyanin in cultured strawberry cells by repetitive feeding of L-phenylalanine into the medium. J. Biosci. Bioeng. 2005, 99, 43–47. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Li, Y.; Fang, W.Z.; Wang, Q.; Xiao, H.Z.; Dang, L.P. Salting effects on the solubility and transformation kinetics of L-phenylalanine anhydrate/monohydrate in aqueous solutions. Ind. Eng. Chem. Res. 2014, 53, 521–529. [Google Scholar] [CrossRef]
- Mohan, R.; Koo, K.K.; Strege, C.; Myerson, A.S. Effect of additives on the transformation behavior of L-phenylalanine in aqueous solution. Ind. Eng. Chem. Res. 2001, 40, 6111–6117. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cao, X.X.; Ji, S.C.; Lan, P.; Liao, A.P. Solubility and transformation behavior of l-phenylalanine anhydrate with amino acid additives. J. Therm. Anal. Calorim. 2018, 131, 1777–1781. [Google Scholar] [CrossRef]
- Su, J.H.; Qian, C.; Luo, N.Z.; Xiang, X.G.; Xu, Y.M.; Chen, X.Z. Experimental measurement and modeling of the solubility of biotin in six pure solvents at temperatures from 298.15 K to 333.85 K. J. Chem. Eng. Data 2014, 59, 3894–3899. [Google Scholar] [CrossRef]
- Cashell, C.; Corcoran, D.; Hodnett, B.K. Effect of amino acid additives on the crystallization of L-glutamic acid crystal. Cryst. Growth Des. 2005, 5, 593–597. [Google Scholar] [CrossRef]
- Manzurola, E.; Apelblat, A. Solubilities of l-glutamic acid, 3-nitrobenzoic acid, p-toluic acid, calcium-l-lactate, calcium gluconate, magnesium-dl-aspartate, and magnesium-l-lactate in water. J. Chem. Thermodyn. 2002, 34, 1127–1136. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, H.W.; Du, C.B.; Cong, Y.; Zhao, H.K. Solubility determination and modeling for 4, 4-dihydroxydiphenyl sulfone in mixed solvents of (acetone, ethyl acetate, or acetonitrile)+ methanol and acetone + ethanol from (278.15 to 313.15) K. J. Chem. Eng. Data 2016, 61, 3519–3526. [Google Scholar] [CrossRef]
- Yang, P.; Du, S.H.; Qin, Y.J.; Zhao, K.F.; Li, K.L.; Hou, B.H.; Gong, G.B. Determination and correlation of solubility and thermodynamic properties of pyraclostrobin in pure and binary solvents. J. Chem. Thermodyn. 2016, 101, 84–91. [Google Scholar] [CrossRef]
- Yuan, F.H.; Wang, Y.L.; Xiao, L.P.; Huang, Q.Y.; Xu, J.C.; Jiang, C.; Hao, H.X. Solubility of cefoxitin acid in different solvent systems. J. Chem. Thermodyn. 2016, 103, 125–133. [Google Scholar] [CrossRef]
- Zhu, M.H.; Zhang, H.H.; Zhang, K.K.; Yang, Y.Y.; Jiang, S.; Xu, S.J.; Liu, Y.M.; Gong, J.B. Measurement and correlation of solubility of boscalid with thermodynamic analysis in pure and binary solvents from 288.15K to 313.15K. J. Chem. Thermodyn. 2017, 112, 178–187. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, K.J. Solubility of Forms I and II of Clopidogrel Hydrogen Sulfate in Methanol and 2-Propanol Mixture. J. Chem. Eng. Data 2011, 56, 43–47. [Google Scholar] [CrossRef]
- Hong, M.; Xu, L.; Ren, G.; Chen, J.; Qi, M. Solubility of lansoprazole in different solvents. Fluid. Phase. Equilibr. 2012, 331, 18–25. [Google Scholar] [CrossRef]
- Wang, X.F.; Dang, L.; Black, S.; Zhang, X.Y.; Wei, H.Y. How to Crystallize Anhydrous Racemic Tartaric Acid from an Ethanol—Water Solution. Ind. Eng. Chem. Res. 2012, 51, 2789–2796. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-liquid equilibrium. XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Gmehling, J.; Uo, W. Arlt: Vapor-Liquid Equilibrium Data Collection; Dechema: Frankfurt, Germany, 1981; Volume 1. [Google Scholar]
- Kubota, N.; Mullin, J.W.A. kinetic model for crystal growth from aqueous solution in the presence of impurity. J. Cryst. Growth 1995, 152, 203–208. [Google Scholar] [CrossRef]
- Davey, R.J.; Mullin, J.W. Growth of the 100 faces of ammonium dihydrogen phosphate crystals in the presence of ionic species. J. Cryst. Growth 1974, 26, 45–51. [Google Scholar] [CrossRef]
- Martins, P.M.; Rocha, F.; Damas, A.M.; Rein, P. Unsteady-state inhibition of crystal growth caused by solution impurities. CrystEngComm 2011, 13, 1103–1110. [Google Scholar] [CrossRef]

| T/K | xexp | xcal,Apel | xcal,λh | T/K | xexp | xcal,Apel | xcal,λh | xexp | xcal,Apel | xcal,λh | xexp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a[H2O] = 0.77 | a[H2O] = 0.90 | a[H2O] = 0 | |||||||||
| 322.15 | 3.29 | 3.27 | 3.37 | 322.15 | 3.49 | 3.52 | 3.67 | 322.15 | 0.947 | 3.52 | 3.67 |
| 317.15 | 2.97 | 3.00 | 3.02 | 317.15 | 3.36 | 3.32 | 3.34 | 317.15 | 0.841 | 3.32 | 3.34 |
| 312.15 | 2.70 | 2.72 | 2.71 | 312.15 | 3.09 | 3.08 | 3.03 | 312.15 | 0.760 | 3.08 | 3.03 |
| 307.15 | 2.46 | 2.45 | 2.42 | 307.15 | 2.78 | 2.82 | 2.75 | 307.15 | 0.693 | 2.82 | 2.75 |
| 302.15 | 2.20 | 2.18 | 2.15 | 302.15 | 2.53 | 2.54 | 2.49 | 302.15 | 0.579 | 2.54 | 2.49 |
| 297.15 | 1.94 | 1.92 | 1.91 | 297.15 | 2.27 | 2.25 | 2.24 | 297.15 | 0.515 | 2.25 | 2.24 |
| 293.15 | 1.68 | 1.72 | 1.73 | 293.15 | 2.02 | 2.02 | 2.06 | 293.15 | 0.423 | 2.02 | 2.06 |
| a[H2O] = 0.84 | a[H2O] = 0.93 | a[H2O] = 1 | |||||||||
| 322.15 | 3.42 | 3.43 | 3.52 | 322.15 | 3.57 | 3.59 | 3.66 | 322.15 | 3.79 | 3.59 | 3.66 |
| 317.15 | 3.18 | 3.15 | 3.17 | 317.15 | 3.38 | 3.36 | 3.38 | 317.15 | 3.68 | 3.36 | 3.38 |
| 312.15 | 2.85 | 2.87 | 2.85 | 312.15 | 3.15 | 3.13 | 3.11 | 312.15 | 3.45 | 3.13 | 3.11 |
| 307.15 | 2.55 | 2.59 | 2.56 | 307.15 | 2.86 | 2.89 | 2.85 | 307.15 | 3.21 | 2.89 | 2.85 |
| 302.15 | 2.38 | 2.32 | 2.29 | 302.15 | 2.64 | 2.64 | 2.62 | 302.15 | 3.07 | 2.64 | 2.62 |
| 297.15 | 2.03 | 2.05 | 2.04 | 297.15 | 2.40 | 2.40 | 2.39 | 297.15 | 2.76 | 2.40 | 2.39 |
| 293.15 | 1.83 | 1.84 | 1.85 | 293.15 | 2.20 | 2.20 | 2.23 | 293.15 | 2.57 | 2.20 | 2.23 |
| a[H2O] = 0.87 | a[H2O] = 0.95 | ||||||||||
| 322.15 | 3.46 | 3.47 | 3.60 | 322.15 | 3.62 | 3.66 | 3.70 | ||||
| 317.15 | 3.24 | 3.21 | 3.24 | 317.15 | 3.52 | 3.44 | 3.45 | ||||
| 312.15 | 2.93 | 2.94 | 2.91 | 312.15 | 3.21 | 3.22 | 3.20 | ||||
| 307.15 | 2.62 | 2.65 | 2.60 | 307.15 | 2.99 | 3.00 | 2.98 | ||||
| 302.15 | 2.38 | 2.36 | 2.32 | 302.15 | 2.74 | 2.78 | 2.76 | ||||
| 297.15 | 2.09 | 2.08 | 2.07 | 297.15 | 2.56 | 2.55 | 2.56 | ||||
| 293.15 | 1.84 | 1.85 | 1.88 | 293.15 | 2.40 | 2.38 | 2.40 | ||||
| Equation Model | Parameter | 0 | 0.77 | 0.84 | 0.87 | 0.90 | 0.93 | 0.95 | 1 |
|---|---|---|---|---|---|---|---|---|---|
| Apelblat | A | 322.8 | 169.60 | 178.46 | 246.33 | 315.16 | 149.99 | 80.57 | 223.12 |
| B | −17198.80 | −9805.76 | −10150.70 | −13260.89 | −16202.01 | −8473.50 | −5137.36 | −11537.24 | |
| C | −47.73 | −25.09 | −26.43 | −36.51 | −46.84 | −22.39 | −12.16 | −33.40 | |
| R2 | 0.9928 | 0.9970 | 0.9949 | 0.9983 | 0.9926 | 0.9981 | 0.9903 | 0.9932 | |
| ARD | 0.017 | 0.010 | 0.010 | 0.0062 | 0.0074 | 0.0045 | 0.0082 | 0.0071 | |
| 105RMSD | 1.19 | 3.49 | 1.67 | 2.63 | 1.86 | 3.15 | 2.37 | 2.85 | |
| λh | λ | 0.025 | 0.042 | 0.038 | 0.041 | 0.028 | 0.018 | 0.012 | 0.010 |
| 10−3h | 103.04 | 48.74 | 50.96 | 48.55 | 60.38 | 78.15 | 96.40 | 100.32 | |
| R2 | 0.9795 | 0.9889 | 0.9908 | 0.9913 | 0.9865 | 0.9950 | 0.9945 | 0.9783 | |
| ARD | 0.015 | 0.018 | 0.013 | 0.015 | 0.019 | 0.0095 | 0.0080 | 0.030 | |
| 105RMSD | 2.55 | 4.85 | 5.22 | 5.88 | 7.45 | 4.02 | 4.15 | 6.38 |
| Water Activity | Solubility 103x | Stable Crystal | Water Activity | Solubility 103x | Stable Crystal | Water Activity | Solubility 103x | Stable Crystal |
|---|---|---|---|---|---|---|---|---|
| 293.15 K | 297.15 K | 302.15 K | ||||||
| 0.837 | 1.89 | A | 0.586 | 1.79 | A | 0.837 | 2.38 | A |
| 0.875 | 2.03 | A | 0.683 | 1.81 | A | 0.869 | 2.38 | A |
| 0.887 | 2.07 | A | 0.766 | 1.93 | A | 0.899 | 2.53 | A |
| 0.899 | 2.11 | A | 0.803 | 1.97 | A | 0.927 | 2.64 | A |
| 0.910 | 2.16 | A | 0.837 | 2.03 | A | 0.937 | 2.67 | A |
| 0.921 | 2.27 | A + M | 0.869 | 2.08 | A | 0.953 | 2.74 | A |
| 0.932 | 2.29 | M | 0.899 | 2.27 | A | 0.968 | 2.83 | A |
| 0.953 | 2.40 | M | 0.905 | 2.30 | A | 0.972 | 2.88 | A |
| 0.910 | 2.34 | A | 0.977 | 2.92 | A | |||
| 0.916 | 2.36 | A | 0.982 | 2.89 | A + M | |||
| 0.921 | 2.38 | A | 0.986 | 2.94 | M | |||
| 0.927 | 2.40 | A | 0.996 | 2.98 | M | |||
| 0.932 | 2.47 | A | 1 | 3.01 | M | |||
| 0.943 | 2.50 | A | ||||||
| 0.948 | 2.37 | A + M | ||||||
| 0.953 | 2.46 | M | ||||||
| 1 | 2.57 | M | ||||||
| Additive Concentration ×105/(mol/ml) | Transformation Time/h | Additive Concentration ×105/(mol/ml) | Transformation Time/h |
|---|---|---|---|
| L-His | |||
| 283.15 K | 313.15 K | ||
| 0 | 4 | 0 | 9 |
| 0.41 | 5.42 | 0.48 | 23.17 |
| 0.82 | 6.50 | 0.85 | 25 |
| 1.63 | 7.33 | 1.21 | 29.17 |
| 2.43 | 7.83 | 1.63 | 32 |
| 3.25 | 8.33 | 2.11 | 40 |
| 4.27 | 8.50 | 2.44 | 41.83 |
| L-Trp | |||
| 283.15 K | 313.15 K | ||
| 0 | 4 | 0 | 9 |
| 0.31 | 7.50 | 0.38 | 18 |
| 0.67 | 10.5 | 0.76 | 22.2 |
| 0.93 | 14 | 1.30 | 24.17 |
| 1.24 | 23 | 1.88 | 25.5 |
| 1.54 | 26.17 | 2.56 | 27 |
| 1.83 | 29.83 | 3.14 | 28 |
| 2.51 | 36.17 | 3.71 | 28.33 |
| Additive Type | Temperature | Transformation Behavior | K | e | R2 | Additive Concentration/(mol/ml) | Results |
|---|---|---|---|---|---|---|---|
| L-Trp | 283.15K | from A to M | 2.38 × 105 | 1.06 | 0.994 | 7.00 × 10−5 | Fully inhibited |
| 313.15K | from M to A | 6.34 × 105 | 0.71 | 0.999 | partially inhibited | ||
| L-His | 283.15K | from A to M | 2.04 × 105 | 0.59 | 0.998 | partially inhibited | |
| 313.15K | from M to A | 4.88 × 105 | 0.83 | 0.993 | partially inhibited |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Ji, S.; Ben, Y.; Kuang, W.; Liao, A.; Lan, P.; Zhang, J. The Methods to Crystallize Anhydrous L-Phenylalanine from Methanol-Water Solution. Crystals 2020, 10, 60. https://doi.org/10.3390/cryst10020060
Cao X, Ji S, Ben Y, Kuang W, Liao A, Lan P, Zhang J. The Methods to Crystallize Anhydrous L-Phenylalanine from Methanol-Water Solution. Crystals. 2020; 10(2):60. https://doi.org/10.3390/cryst10020060
Chicago/Turabian StyleCao, Xiaoxue, Shaochang Ji, Yumei Ben, Wenjie Kuang, Anping Liao, Ping Lan, and Jinyan Zhang. 2020. "The Methods to Crystallize Anhydrous L-Phenylalanine from Methanol-Water Solution" Crystals 10, no. 2: 60. https://doi.org/10.3390/cryst10020060
APA StyleCao, X., Ji, S., Ben, Y., Kuang, W., Liao, A., Lan, P., & Zhang, J. (2020). The Methods to Crystallize Anhydrous L-Phenylalanine from Methanol-Water Solution. Crystals, 10(2), 60. https://doi.org/10.3390/cryst10020060




