Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins
Abstract
:1. Introduction—Definition and Incidence
2. Objective
| Class | Criteria | Level of Evidence | Recommendation |
|---|---|---|---|
| I | Prospective, randomized, controlled, outcome masked, representative population with criteria A–E * | A: Two or more Class I studies | Established as effective, ineffective, or harmful |
| II | Prospective, matched cohort, representative population, masked outcome and meets A–E * OR RCT with one criteria in A–E * lacking | B: At least one Class I or two Class II | Probably effective, ineffective, or harmful and recommended |
| III | Controlled trial **, representative population, outcome independent of patient treatment | C: At least one Class II | Possibly effective, ineffective or harmful, may be used at discretion of clinician |
| IV | Uncontrolled study, case series, case report or expert opinion. | U | Data inadequate or conflicting |
3. Method
4. Treatments
4.1. Oral (Table 2)
Level of Evidence
| Author and year | Agent | Associated illness | N | Study design | Class | Outcome measured | Findings | Side effects |
|---|---|---|---|---|---|---|---|---|
| Camp-Bruno et al. 1989 [23] | BZ (mean dose 3.8 mg) | DD | 20 | DB, PBOC, CO | III | Efficacy and incidence of side-effects at 1-week, baseline, 2-week PBO and 2-week BZ conditions | A significant decrease in drooling during the BZ condition relative to PBO was demonstrated and conservative response rates ranged up to 65%–70% | Minor problems (dry mouth) eliminated by small dose adjustments. More serious cholinergic side-effects which resolved in 24–48 h required discontinuations of the drug in three patients. |
| Mier et al. 2000 [24] | GLYC | DD | 39 | DB, PBOC, CO, Dose-ranging | III | Parent and investigator evaluation of change in sialorrhea and adverse effects | GLYC in doses of 0.10 mg/kg per dose is effective at controlling sialorrhea | Even at low doses, 20% of children may exhibit adverse effects severe enough to require drug discontinuation. |
| Thomsen et al. 2007 [25] | Sublingual Ipratropium Bromide Spray (1–2 sprays up to 4 times a day) | PD | 17 | Randomized, DB, PBOC, CO | II | Objective measure of weight of saliva production | Ipratopium bromide spray had no significant effect on weight of saliva produced. | No significant adverse side effects |
| Mato et al. 2010 [26] | Topical Scopolamine | Handicapped * | 30 | prospective, randomized, DB, PBOC, CO | II | Severity of drooling was quantified using a modified Thomas-Stonell and Greenberg visual scale simplified into three grades: 1 = dry; 2 = mild/moderate; 3 = severe/fulsome. The frequency of drooling was estimated using the number of bibs used each day. | Significant drooling reduction (p < 0.005) in the scopolamine group in the 1 and 2 week controls (69% and 80% respectively ≤ grade 3). The mean number of bibs/day decreased during the scopolamine phase from 6/day at baseline to 3/day at the 2 week control. | 4 patients (13.3%) dropped out because of scopolamine side effects and minor adverse reactions were observed in three other patients |
| Arbrouw et al. 2010 [20] | GLYC 1 mg 3 times daily | PD | 23 | 4-week, randomized, DB, PBOC, CO | II | Sialorrhea was scored on a daily basis by the patients or a caregiver with a sialorrhea scoring scale ranging from 1 (no sialorrhea) to 9 (profuse sialorrhea). | Mean sialorrhea score improved from 4.6 (1.7) with PBO to 3.8 (1.6) with GLYC (p = 0.011). 9 patients (39.1%) with GLYC had a clinically relevant improvement of at least 30% vs. 1 patient (4.3%) with PBO (p = 0.021). | No significant differences in adverse events between GLYC and PBO treatment. |
| Liang et al. 2010 [19] | GLYC and Biperiden | CI | 13 | 12-week, randomized, DB, CO, fixed-dose | III | Sialorrhea and global cognitive function were assessed by using DRS and MMSE respectively | At 1 week, both drugs improved CIS compared with baseline (biperiden: p = 0.005; GLYC: p = 0.002). DRS score was lower than baseline in 4 weeks with biperiden (p = 0.003) and also with GLYC at 4 weeks (p = 0.002). MMSE scores with either drug did not differ from baseline at one week or 4 weeks (p = 0.437, p = 0.76). Patients treated with biperiden had significantly reduced MMSE scores after 1 week (p = 0.049). | 2 adverse events were reported by the patients during both treatment phases. 1 patient complained of constipation, and the other complained of inner unrest |
| Lloret et. al. 2011 [21] | Intra-oral Tropicamide films | PD | 19 | DB, randomized, PBOC, two-phased, Latin-square CO study | II | VAS and saliva amount by cotton rolls. | 1 mg of tropicamide resulted in significant VAS score decrease and reduction in saliva volume (27%, 33%, and 20% respective for 0.3, 1 and 3 mg) when compared to PBO | No adverse events were reported |
4.2. Topical (Table 2)
Level of Evidence
4.3. Surgical
5. Botulinum Toxin Treatment (Table 3)
| Author/year | Assoc. illness | N | Class | Agent/dose | Glands injected | Primary outcome | Result | Side effects |
|---|---|---|---|---|---|---|---|---|
| Lipp et al. 2003 [28] | 12 ALS, 12 PD, 4 MSA, 4 CBD | 32 | II | A/Abo 18.75, 37.5, or 75 MU | B/L PG | Weight of dental rolls every 4 weeks during a 24-week period | Significant decrease of sialorrhea during the study period measured by dental rolls when compared with PBO group p < 0.05 only in the 75 U group | None reported |
| Mancini 2003 [33] | 14 PD, 6 MSA | 20 | II | 450 U A/Abo | B/L PG and B/L SMG | Treatment efficacy and safety were assessed at baseline, 1 week and 3 months after A/Abo injections using clinical scales (DS and DF) and side effect surveillance | After treatment, the average secretion of saliva in the A/Abo group was significantly lower than in the PBO group as appraised by clinical measurements (p = 0.005); no treatment difference at 3 months | None |
| Lagalla et al. 2006 [34] | PD | 32 | II | A/Ona50 U per PG, | B/L PG | DSFS, VAS-FD and SD, UPDRS-ADL item scores for drooling and swallowing at baseline and 1 month after treatment. Saliva reduction (weight of dental rolls). GIS was also applied | Subjects treated with A/Ona experienced a reduction in both drooling frequency and familial and social disability, as well as in saliva production (p < 0.0001) | Mild transient swallowing difficulty in 1 pt |
| Lin et al. 2008 [16] | CP | 13 | III | Ona/A 2U/kg body weight | C/L PG and SMG | DSFS, saliva weight, and DQ | Significant difference in DSFS at 2, 4, 6, 8, 12 week post injection, saliva weight at 6, 12 week after injection, and DQ 2, 6, 8, 10 week after injection all significant at p < 0.05. Saliva weight significant to longest follow up of 22 weeks. | Not stated in text |
| Alrefai et al. 2009 [35] | CP | 24 | III | 100 U A/Abo in the first visit and 140 U at the second visit 4 months later regardless of effect | B/L PG and SMG | DF and DS were performed at the time of injection, at 1 month, and at baseline prior to the second injection. A second set of injections of either 140 U of A/Abo or PBO was given 4 months later, and the same rating scales were used using Fisher’s Exact Test | Scores of the median frequency (p = 0.034) and severity (p = 0.026) of drooling were reduced in the treatment group. | Minor and transient increase in drooling after Injection in 2 pts * |
| Pei-Hsuan Wu et al. 2011 [36] | CP | 20 | I | A/Ona body weight titrated | B/L PG and SMG | Subjective drooling scales, salivary flow rate, and oral health (salivary compositions and cariogenic bacterial counts) at 1 and 3 months | Decrease in salivary flow rate was significantly higher in the A/Ona group at the 1-month (p = 0.037) and 3-month (p = 0.041) follow up compared to control | No reported adverse effects |
| Author/year | Method used to locate injection site | Method of injection | Number of sites injected | Use of anesthesia/type |
|---|---|---|---|---|
| Lipp et al. 2003 [28] | Anatomic landmarks | 30-gauge, 25-mm needle were used to inject each parotid gland. (one in gland mass (0.3 mL), one above masseter 0.2 mL) | 2 per parotid | Not stated |
| Mancini 2003 [33] | Ultrasound | Through a 26-gauge syringe, 0.65 mL of solution in each parotid gland and 0.35 mL of solution in each submandibular gland. | Not clear, but assumed 1 injection per gland on both sides | Not stated, however patients complained of painful injections |
| Lagalla et al. 2006 [34] | Anatomic landmarks | 27-gauge needle penetrating to adepth of 1–1.5 cm into the preauricular portion of parotid gland, behind the angle of the ascending mandibular ramus, and then into the inferoposterior portion of the gland, lying just before the mastoid process. | 3 per parotid | Not states |
| Lin et al. 2008 [16] | Ultrasound | Not stated in paper | Not stated in paper | Not stated |
| Alrefai et al. 2009 [35] | Anatomic landmarks | Each side was injected with a 10 mm (30 G) needle into the paotid gland with 50 U. | 2 per parotid | Not used |
| Pei-Hsuan Wu et al. 2011 [36] | Ultrasound | 30-gauge needle to the bilateral parotid and submandibular glands with concentration of 10 U/0.1 mL. | 4 (1 injection in both parotids and submandibular glands) | Not stated |
| Author/year | Assoc. illness | N | Class | Agent/dose | Glands injected | Primary outcome | Result | Side effects |
|---|---|---|---|---|---|---|---|---|
| Jongerius et al. 2004 [32] | CP | 45 | III | A/Ona by weight vs. transdermal scopolamine | B/L SMG | DQ, Teacher Drooling Scale (TDS) and VAS | Drooling decreased with both scopolamine and A/Ona injection; however greatest reductions were achieved 2 to 8 weeks after A/Ona injection. 61.5% of patients responded to BoNT injections. Statistical significance for DQ was stated at (p < 0.05). DQ had a response rate of 53% for scopolamine and 48.7% for A/Ona | 71% had moderate to severe side effects for scopolamine. Only minimal and incidental side effects were reported for BoNT. |
| Wilken et al. 2008 [31] | CP or NDD | 30 | III | 100 /kg of B/Rima or 80 MU of A/Ona | B/L PG and SMG | Parent questionnaire and TDS | Four weeks after the first injection 29 patients responded with a reduction of TDS score to 1 or 2 rated in the parent’ s questionnaire. | Intermittent problems with swallowing due to viscous saliva (5), unilateral parotitis (1). |
| Guibaldi et al. 2011 [40] | ALS (15) and PD (12) | 27 | III | 250 U A/Abo or 2,500 U B/Rima | B/L PG and SMG | Magnitude of change in saliva production determined by weighing five cotton rolls after retaining for 5 minutes in the mouth. | B/Rima showed improvement in subjective and objective measured with a shorter latency for improvement onset when compared to A/Abo (p = 0.002). Mean benefit of duration was 75 days for A/Abo and 93 days for B/Rima. | Change in saliva thickness |
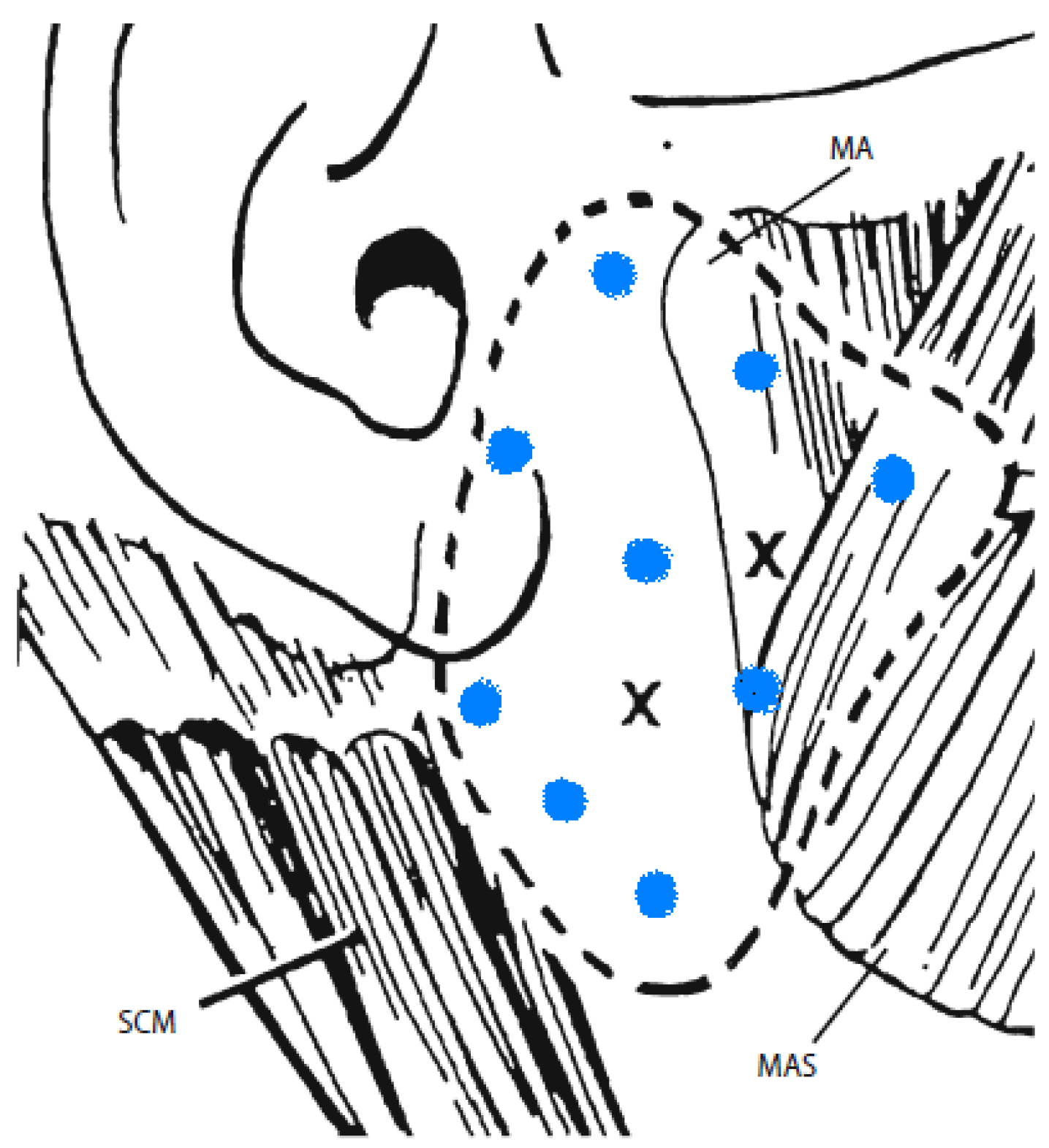
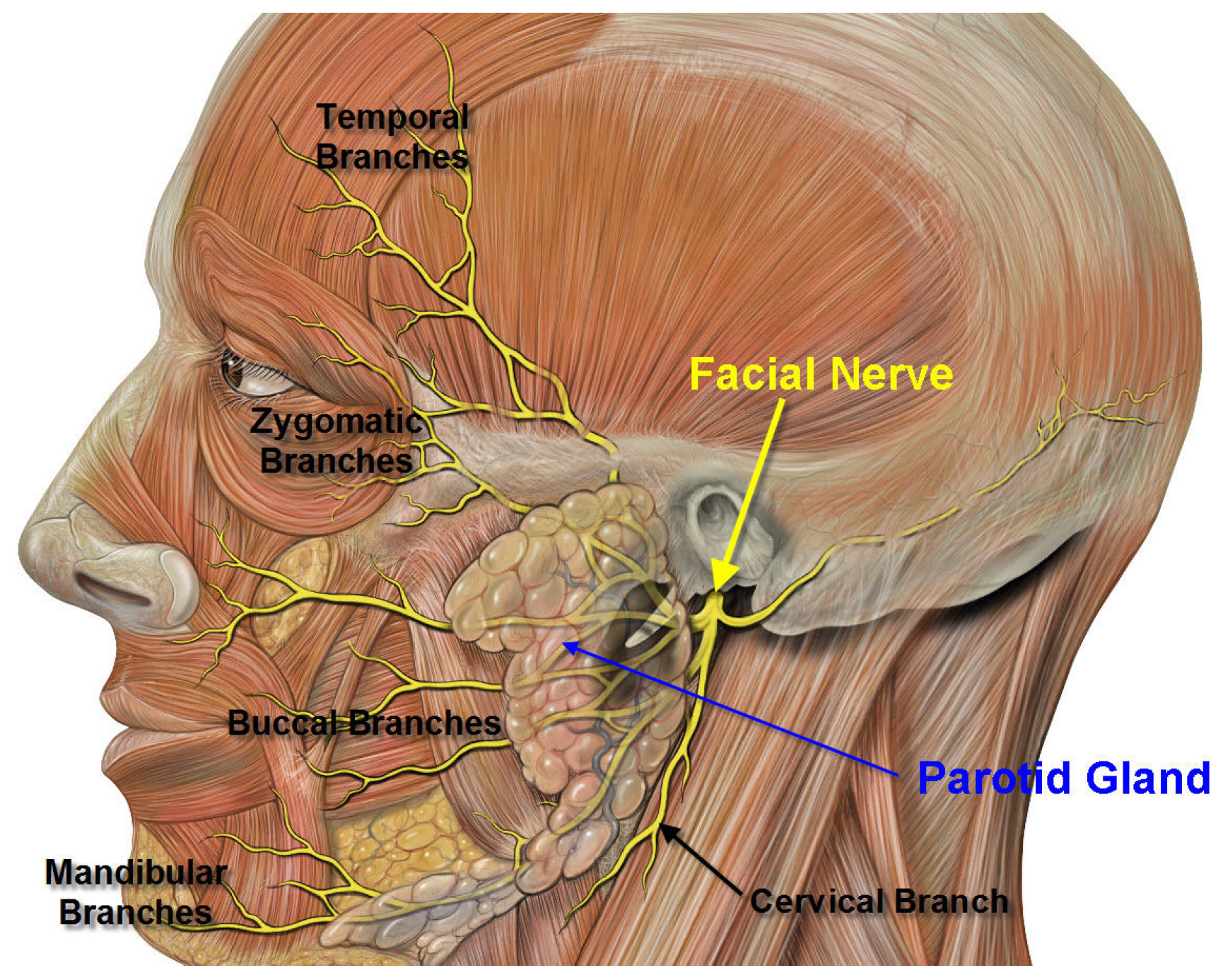
6. Commentary on Studies
| Author/year | Assoc. illness | N | Class | Agent/dose | Glands injected | Primary outcome | Result | Side effects |
|---|---|---|---|---|---|---|---|---|
| Ondo 2004 [37] | PD | 16 | II | B/Rima 2500 U (1000 U in each PG) | PG and SMG | UPDRS, Drooling Rating Scale, DSFS, VAS, GIS at baseline and one month, drooling and dysphagia questionnaires | Improvement on the VAS (p <0.001), GIC (p < 0.005), Drooling Rating Scale (p < 0.05), and DFSS (p < 0.001). There was no change in UPDRS, head posture, or Dysphagia Scale | Dry mouth (3), worsened gait (2), diarrhea (1), neck pain (1) in B/Rima group |
| Jackson et al. 2009 [38] | ALS | 20 | I | B/Rima 2500 U (500 U in PG, 750 U in SMG) | B/L PG and SMG | GIC by the subject 8 weeks after the injection. | GIC of 82% at 2 weeks compared to 38% of those who received PBO (p < 0.05). This significant effect was sustained at 4 weeks. At 12 weeks, 50% of patients who received B/Rima continued to report improvement compared to 14% of those who received PBO. | No adverse side effects |
| Lagalla 2009 [29] | PD | 36 | II | 4000 U B/Rima | PG | DSFS, VAS-FD and VAS-SD, UPDRS-ADL scores for drooling and swallowing at baseline and one month after treatment. Objective saliva reduction (saliva production over five minutes by weighing dental rolls), GIS | One month after injections, B/Rima group showed improvement in almost all subjective outcomes. Two-way analysis of variance gave a significant time × treatment effect, F-value being 52.5 (p < 0.0001) for DS-FS, 23.2 (p < 0.0001) for VAS-FD, 29 (p < 0.0001) for VAS-SD, and 28.9 (p < 0.0001) for UPDRS-ADL drooling item score, with benefits in B/Rima group lasting 19.2 +/− 6.3 weeks | Transient dysphagia worsening which resolved in 2 weeks in B/Rima group (3) |
| Chinnap-ongse et al. 2012 [39] | ALS and PD | 54 | I | B/Rima 1500, 2500 or 3500 U for PG and 250 U for the SMG | PG and SMG | Safety/tolerability, as assessed by adverse events | At 4 weeks postinjection, Drooling Frequency and Severity Scale scores significantly improved vs. PBO in a dose-related manner (p < 0.0001 and p < 0.0001 respectively). Unstimulated salivary flow rates significantly decreased in all active groups vs. PBO (p ≤ 0.0009). | GI-related events more frequently in the active groups (dry mouth most common) |
| Author/year | Method used to locate injection site | Method of injection | Number of sites injected | Use of anesthesia/type |
|---|---|---|---|---|
| Ondo et al. 2004 [37] | Anatomical landmarks | All injection sites were localized with anatomic markers and injected with a 29-gauge tuberculin syringe at a depth of 0.5 inch. Two vertically placed locations just dorsal to the palpated masseter muscle (parotid gland) were injected as well as one location just anterior and medial to the genu of the mandible (submandibular gland). | 2 places in both parotids and 1 site in both submandibular glands. | Not stated |
| Jackson et al. 2009 [38] | EMG—For the parotid, absence of motor unit potential was used to confirm placement. For the submandibular when insertional motor unit activity was observed (indicating myohyoid/ hyoglossus/digastric), the needle was withdrawn slightly until muscle activity was absent deep in the submandibular triangle. | Each parotid gland was injected at two sites with 0.1 cc of study medication (total of 500 U/gland) directing the needle toward the tail of the parotid, between the sternocleidomastoid muscle and the angle of the mandible. Each submandibular gland was injected at two sites with 0.15 cc of study medication (total of 750 U/gland), placing the needle percutaneously in the submandibular triangle. | Each parotid gland was injected at two sites, Each submandibular gland was injected at two sites | Not stated. |
| Lagalla et al. 2009 [29] | Anatomical landmarks | 0.8 mL of drug into each pre-auricular portion of the parotid gland. The injections were performed using a 1 mL syringe with 27-gauge needle penetrating to a depth of 1–1.5 cm into two sites, behind the angle of the ascending mandibular ramus and into the infero-posterior portion of the gland, just before the mastoid process. | 2 sites in each parotid | Not stated |
| Chinnapongse et al. 2012 [39] | Anatomical landmarks | Not described | Not described | Not stated |
| Author/year | Method used to locate injection site | Method of injection | Number of sites injected |
|---|---|---|---|
| Jongerius et al. 2004 [32] | Ultrasound | Injected bilaterally in the submandibular glands using a 25-G needle * after general anesthesia | 3 per submandibular gland |
| Wilken et al. 2008 [31] | Ultrasound | Injected into parotid (one in front of the isthmus and the other one below) and submandibular using 27G needle *after local anesthesia with 20% Emla in 5 pts. | 3 site per side: 2 in the parotid and one in the submandibular gland |
| Guibaldi et al. 2011 [40] | Ultrasound | Not clearly stated | parotid gland (two sites per gland) and each submandibular gland (one site per gland) |
| Author & year | Focus of review | Conclusions | Comments |
|---|---|---|---|
| Reddihough et al. 2010 [41] | Adult and children with different etiologies | Study suggests established evidence (Level A) for both BoNT-A and BoNT-B | Conclusions based on randomized controlled trials, systematic reviews, AAN criteria but for some studies class rating does not accord with AAN rating (example Alrefai et al. is rated class I; rated class III by AAN search members). |
| Lim et al. 2010 [42] | Discusses the use of botulinum toxin in neurologic practice | Suggests level B evidence for BoNTs in sialorrhea (probably effective) | Conclusion based solely on two class II studies, and it is unclear which class II studies were used |
| Dand et al. 2010 [43] | Reviews the available treatments for sialorrhea (pharmacologic and non-pharmacologic) | BoNT when injected into the parotid gland may improve quality of life up to 4 months, injection into salivary ducts not recommended. | No assessment of level of evidence was cited |
| Habek et al. 2010 [44] | Botulinum toxin in the management of MS | BoNTs should be used with caution in MS since no blinded or controlled studies exist. | There are no controlled studies to date on the application in MS patients with sialorrhea. |
| Young et al. 2011 [45] | Cochrane review on individuals with MND/ ALS: BoNT, radiotherapy. | Suggested use of BoNT-B for treatment of sialorrhea in clinical practice due to better efficacy. | For BoNT efficacy only double-blind study (Jakson et al.2009) was cited and many open studies. |
| Seppi et al. 2011 [46] | An update on the efficacy of treatments for non-motor symptoms of PD based on EBM methodology using RCTs. | BoNTs A and B: established efficacy in sialorrhea. Glyccopyrolate: efficiency beyond one week is not established. Ipratropium bromide spray: insufficient data | Different used for assessment of efficacy than AAN criteria are referenced. |
| Squires et al. 2012 [47] | Adults with different neurological conditions | Pharmacologic intervention is effective but short lived. Evidence is strongest for BoNTs | Conclusions/recommendations for clinical practice brief without applying evidence based assessment criteria. |
| Rodwell et al. 2012 [48] | Systematic Review of Efficacy of BoNT in children with cerebral palsy | Data from 6 RCT suggest efficacy of BoNTs in sialorrhea. More data on adverse effects are needed. | Review is limited to children with cerebral palsy. |
| Intiso et al. 2012 [49] | Review focused of BoNT use in neurohabilitation for sialorrhea (ALS, PD and CP). | BoNTs and B are both effective in reducing drooling. Type B: more effective, shorter latency; more side effects. Duration of effect: comparable. | Does not describe levels of evidence and is not limited to highest level of evidence studies |
| Walshe et al. 2012 [50] | Interventions to treat drooling in children with CP | Unable to reach conclusion on efficacy /safety of BoNTs or pharmaceutical interventions in CP. | Looks only at the pediatric population |
7. Conclusions
8. Technical Note from Senior Author
Conflicts of Interest
References
- Garnock-Jones, K.P. Glycopyrrolate oral solution: For chronic, severe drooling in pediatric patients with neurologic conditions. Paediatric. Drugs 2012, 14, 263–269. [Google Scholar] [CrossRef]
- Hamdy, S.; Aziz, Q.; Rothwell, J.C.; Hobson, A.; Barlow, J.; Thompson, D.G. Cranial nerve modulation of human cortical swallowing motor pathways. Am. J. Physiol. 1997, 272, G802–G808. [Google Scholar]
- Johnson, H.; Scott, A. 6 Saliva Management. In Dysphagia: Foundation, Theory and Practice; Cichero, J.A.Y., Murdoch, B.E., Eds.; John Wiley & Sons Ltd: West Sussex, UK, 2006; p. 126. [Google Scholar]
- Volonte, M.A.; Porta, M.; Comi, G. Clinical assessment of dysphagia in early phases of parkinson’s disease. Neurol. Sci. 2002, 23, S121–S122. [Google Scholar]
- Glickman, S.; Deaney, C.N. Treatment of relative sialorrhoea with botulinum toxin type a: Description and rationale for an injection procedure with case report. Eur. J. Neurol. 2001, 8, 567–571. [Google Scholar] [CrossRef]
- Hung, C.C.; Fu, P.K.; Wang, H.Y.; Chan, C.H.; Lan, T.H. Treatment effects of traditional chinese medicines suoquan pill and wuling powder on clozapine-induced hypersalivation in patients with schizophrenia: Study protocol of a randomized, placebo-controlled trial. J. Chin. Integr. Med. 2011, 9, 495–502. [Google Scholar] [CrossRef]
- Scully, C.; Limeres, J.; Gleeson, M.; Tomas, I.; Diz, P. Drooling. J. Oral Pathol. Med. 2009, 38, 321–327. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Bui, D.T. Anatomy, function, and evaluation of the salivary glands. Salivary Glands Disorders 2007, 1, 1–16. [Google Scholar] [CrossRef]
- Hollinshead, W.H. Anatomy for Surgeons; Hoeber Medical Division, Harper and Row: New York, NY, USA, 1968; pp. 551–556. [Google Scholar]
- Myer, C.M., 3rd. Sialorrhea. Pediatr. Clin. North Am. 1989, 36, 1495–1500. [Google Scholar]
- Garrett, J.R.; Proctor, G.B. Control of Salivation. In The Scientific Basis of Eating: Taste and Smell, Salivation, Mastication and Swallowing and Their Dysfunctions; Frontiers of Oral Biology; Linden, D., Roger, W.A., Eds.; Karger: Basel, Switzerland, 1998; pp. 135–155. [Google Scholar]
- Dodds, W.J. Physiology of swallowing. Dysphagia 1989, 3, 171–178. [Google Scholar] [CrossRef]
- Senner, J.E.; Logemann, J.; Zecker, S.; Gaebler-Spira, D. Drooling, saliva production, and swallowing in cerebral palsy. Dev. Med Child Neurol. 2004, 46, 801–806. [Google Scholar]
- Lakraj, A.-A.D.; Moghimi, N.; Jabbari, B. Hyperhidrosis: Anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins 2013, 5, 821–840. [Google Scholar] [CrossRef]
- French, J.; Gronseth, G. Lost in a jungle of evidence: We need a compass. Neurology 2008, 71, 1634–1638. [Google Scholar]
- Lin, Y.C.; Shieh, J.Y.; Cheng, M.L.; Yang, P.Y. Botulinum toxin type a for control of drooling in asian patients with cerebral palsy. Neurology 2008, 70, 316–318. [Google Scholar] [CrossRef]
- Fairhurst, C.B.; Cockerill, H. Management of drooling in children. Arch. Dis. Child. Educ. Pract. Ed. 2011, 96, 25–30. [Google Scholar] [CrossRef]
- Borg, M.; Hirst, F. The role of radiation therapy in the management of sialorrhea. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 1113–1119. [Google Scholar] [CrossRef]
- Liang, C.S.; Ho, P.S.; Shen, L.J.; Lee, W.K.; Yang, F.W.; Chiang, K.T. Comparison of the efficacy and impact on cognition of glycopyrrolate and biperiden for clozapine-induced sialorrhea in schizophrenic patients: A randomized, double-blind, crossover study. Schizophr. Res. 2010, 119, 138–144. [Google Scholar] [CrossRef]
- Arbouw, M.E.; Movig, K.L.; Koopmann, M.; Poels, P.J.; Guchelaar, H.J.; Egberts, T.C.; Neef, C.; van Vugt, J.P. Glycopyrrolate for sialorrhea in parkinson disease: A randomized, double-blind, crossover trial. Neurology 2010, 74, 1203–1207. [Google Scholar] [CrossRef]
- Lloret, S.P.; Nano, G.; Carrosella, A.; Gamzu, E.; Merello, M. A double-blind, placebo-controlled, randomized, crossover pilot study of the safety and efficacy of multiple doses of intra-oral tropicamide films for the short-term relief of sialorrhea symptoms in parkinson's disease patients. J. Neurol. Sci. 2011, 310, 248–250. [Google Scholar] [CrossRef]
- Vashishta, R.; Nguyen, S.A.; White, D.R.; Gillespie, M.B. Botulinum toxin for the treatment of sialorrhea: A meta-analysis. Otolaryngol. Head Neck Surg. 2013, 148, 191–196. [Google Scholar] [CrossRef]
- Camp-Bruno, J.A.; Winsberg, B.G.; Green-Parsons, A.R.; Abrams, J.P. Efficacy of benztropine therapy for drooling. Dev. Med. Child Neurol. 1989, 31, 309–319. [Google Scholar]
- Mier, R.J.; Bachrach, S.J.; Lakin, R.C.; Barker, T.; Childs, J.; Moran, M. Treatment of sialorrhea with glycopyrrolate: A double-blind, dose-ranging study. Arch. Pediatr. Adolesc. Med. 2000, 154, 1214–1218. [Google Scholar]
- Thomsen, T.R.; Galpern, W.R.; Asante, A.; Arenovich, T.; Fox, S.H. Ipratropium bromide spray as treatment for sialorrhea in parkinson’s disease. Mov. Disord. 2007, 22, 2268–2273. [Google Scholar]
- Mato, A.; Limeres, J.; Tomas, I.; Munoz, M.; Abuin, C.; Feijoo, J.F.; Diz, P. Management of drooling in disabled patients with scopolamine patches. Br. J. Clin. Pharmacol. 2010, 69, 684–688. [Google Scholar] [CrossRef]
- Crysdale, W.S. Management options for the drooling patient. Ear Nose Throat J. 1989, 68, 820, 825–826, 829–830. [Google Scholar]
- Pal, P.K.; Calne, D.B.; Calne, S.; Tsui, J.K. Botulinum toxin a as treatment for drooling saliva in pd. Neurology 2000, 54, 244–247. [Google Scholar] [CrossRef]
- Lagalla, G.; Millevolte, M.; Capecci, M.; Provinciali, L.; Ceravolo, M.G. Long-lasting benefits of botulinum toxin type b in parkinson’s disease-related drooling. J. Neurol. 2009, 256, 563–567. [Google Scholar] [CrossRef]
- Froedtert Hospital, Wisconsin. Available online: http://www.froedtert.com/HealthResources/ReadingRoom/HealthBlogs/Reflections/HalfofWhatWeTeachYou.htm (accessed on 2 April 2013).
- Wilken, B.; Aslami, B.; Backes, H. Successful treatment of drooling in children with neurological disorders with botulinum toxin a or b. Neuropediatrics 2008, 39, 200–204. [Google Scholar] [CrossRef]
- Jongerius, P.H.; van den Hoogen, F.J.; van Limbeek, J.; Gabreels, F.J.; van Hulst, K.; Rotteveel, J.J. Effect of botulinum toxin in the treatment of drooling: A controlled clinical trial. Pediatrics 2004, 114, 620–627. [Google Scholar] [CrossRef]
- Lipp, A.; Trottenberg, T.; Schink, T.; Kupsch, A.; Arnold, G. A randomized trial of botulinum toxin a for treatment of drooling. Neurology 2003, 61, 1279–1281. [Google Scholar] [CrossRef]
- Mancini, F.; Zangaglia, R.; Cristina, S.; Sommaruga, M.G.; Martignoni, E.; Nappi, G.; Pacchetti, C. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type a in the treatment of drooling in parkinsonism. Mov. Disord. 2003, 18, 685–688. [Google Scholar] [CrossRef]
- Lagalla, G.; Millevolte, M.; Capecci, M.; Provinciali, L.; Ceravolo, M.G. Botulinum toxin type a for drooling in parkinson's disease: A double-blind, randomized, placebo-controlled study. Mov. Disord. 2006, 21, 704–707. [Google Scholar] [CrossRef]
- Alrefai, A.H.; Aburahma, S.K.; Khader, Y.S. Treatment of sialorrhea in children with cerebral palsy: A double-blind placebo controlled trial. Clin. Neurol. Neurosurg. 2009, 111, 79–82. [Google Scholar] [CrossRef]
- Ondo, W.G.; Hunter, C.; Moore, W. A double-blind placebo-controlled trial of botulinum toxin b for sialorrhea in parkinson’s disease. Neurology 2004, 62, 37–40. [Google Scholar] [CrossRef]
- Jackson, C.E.; Gronseth, G.; Rosenfeld, J.; Barohn, R.J.; Dubinsky, R.; Simpson, C.B.; McVey, A.; Kittrell, P.P.; King, R.; Herbelin, L. Randomized double-blind study of botulinum toxin type b for sialorrhea in als patients. Muscle Nerve 2009, 39, 137–143. [Google Scholar] [CrossRef]
- Chinnapongse, R.; Gullo, K.; Nemeth, P.; Zhang, Y.; Griggs, L. Safety and efficacy of botulinum toxin type b for treatment of sialorrhea in parkinson's disease: A prospective double-blind trial. Mov. Disord. 2012, 27, 219–226. [Google Scholar] [CrossRef]
- Guidubaldi, A.; Fasano, A.; Ialongo, T.; Piano, C.; Pompili, M.; Masciana, R.; Siciliani, L.; Sabatelli, M.; Bentivoglio, A.R. Botulinum toxin a versus b in sialorrhea: A prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or parkinson’s disease. Mov. Disord. 2011, 26, 313–319. [Google Scholar] [CrossRef]
- Reddihough, D.; Erasmus, C.E.; Johnson, H.; McKellar, G.M.; Jongerius, P.H. Botulinum toxin assessment, intervention and aftercare for paediatric and adult drooling: International consensus statement. Eur. J. Neurol. 2010, 17, 109–121. [Google Scholar] [CrossRef]
- Lim, E.C.; Seet, R.C. Use of botulinum toxin in the neurology clinic. Nat. Rev. Neurol. 2010, 6, 624–636. [Google Scholar] [CrossRef]
- Dand, P.; Sakel, M. The management of drooling in motor neurone disease. Int. J. Palliat. Nurs. 2010, 16, 560–564. [Google Scholar]
- Habek, M.; Karni, A.; Balash, Y.; Gurevich, T. The place of the botulinum toxin in the management of multiple sclerosis. Clin. Neurol. Neurosurg. 2010, 112, 592–596. [Google Scholar] [CrossRef]
- Young, C.A.; Ellis, C.; Johnson, J.; Sathasivam, S.; Pih, N. Treatment for sialorrhea (excessive saliva) in people with motor neuron disease/amyotrophic lateral sclerosis. Cochrane Database Syst Rev. 2011. May 11 (5):CD006981. [Google Scholar] [CrossRef]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The movement disorder society evidence-based medicine review update: Treatments for the non-motor symptoms of parkinson’s disease. Mov. Disord. 2011, 26, S42–S80. [Google Scholar] [CrossRef]
- Squires, N.; Wills, A.; Rowson, J. The management of drooling in adults with neurological conditions. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 171–176. [Google Scholar] [CrossRef]
- Rodwell, K.; Edwards, P.; Ware, R.S.; Boyd, R. Salivary gland botulinum toxin injections for drooling in children with cerebral palsy and neurodevelopmental disability: A systematic review. Dev. Med. Child Neurol. 2012, 54, 977–987. [Google Scholar] [CrossRef]
- Intiso, D.; Basciani, M. Botulinum toxin use in neuro-rehabilitation to treat obstetrical plexus palsy and sialorrhea following neurological diseases: A review. NeuroRehabilitation 2012, 31, 117–129. [Google Scholar]
- Walshe, M.; Smith, M.; Pennington, L. Interventions for drooling in children with cerebral palsy. Cochrane Database Syst. Rev. 2012, 11. [Google Scholar] [CrossRef]
- Wu, K.P.; Ke, J.Y.; Chen, C.Y.; Chen, C.L.; Chou, M.Y.; Pei, Y.C. Botulinum toxin type a on oral health in treating sialorrhea in children with cerebral palsy: A randomized, double-blind, placebo-controlled study. J. Child Neurol. 2011, 26, 838–843. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lakraj, A.A.; Moghimi, N.; Jabbari, B. Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins 2013, 5, 1010-1031. https://doi.org/10.3390/toxins5051010
Lakraj AA, Moghimi N, Jabbari B. Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins. 2013; 5(5):1010-1031. https://doi.org/10.3390/toxins5051010
Chicago/Turabian StyleLakraj, Amanda Amrita, Narges Moghimi, and Bahman Jabbari. 2013. "Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins" Toxins 5, no. 5: 1010-1031. https://doi.org/10.3390/toxins5051010
APA StyleLakraj, A. A., Moghimi, N., & Jabbari, B. (2013). Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins, 5(5), 1010-1031. https://doi.org/10.3390/toxins5051010





