Appraisal of Antiophidic Potential of Marine Sponges against Bothrops jararaca and Lachesis muta Venom
Abstract
:1. Introduction
2. Results
2.1. Neutralization of Proteolysis

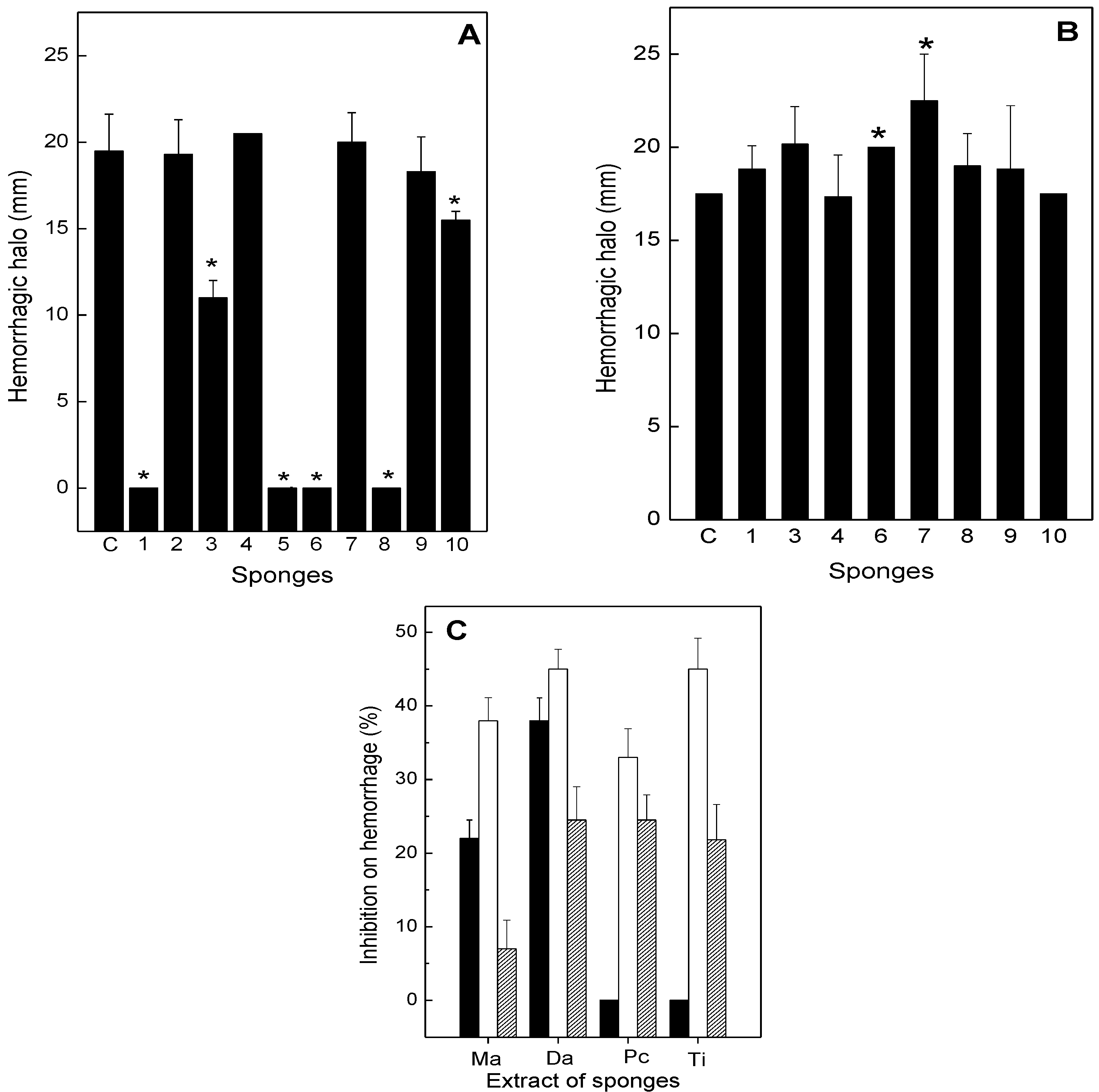
2.2. Neutralization of Hemorrhage
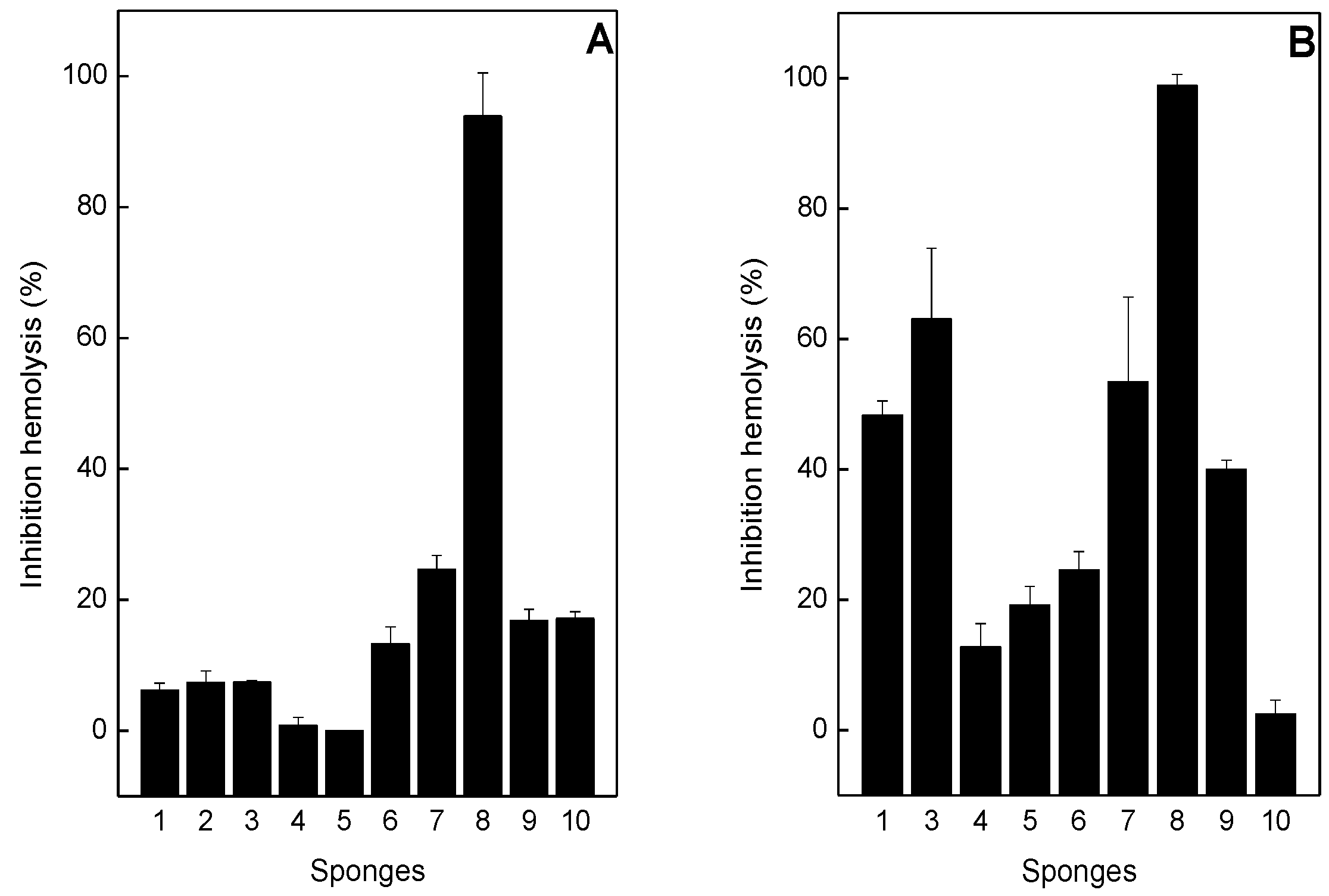
2.3. Neutralization of Hemolysis
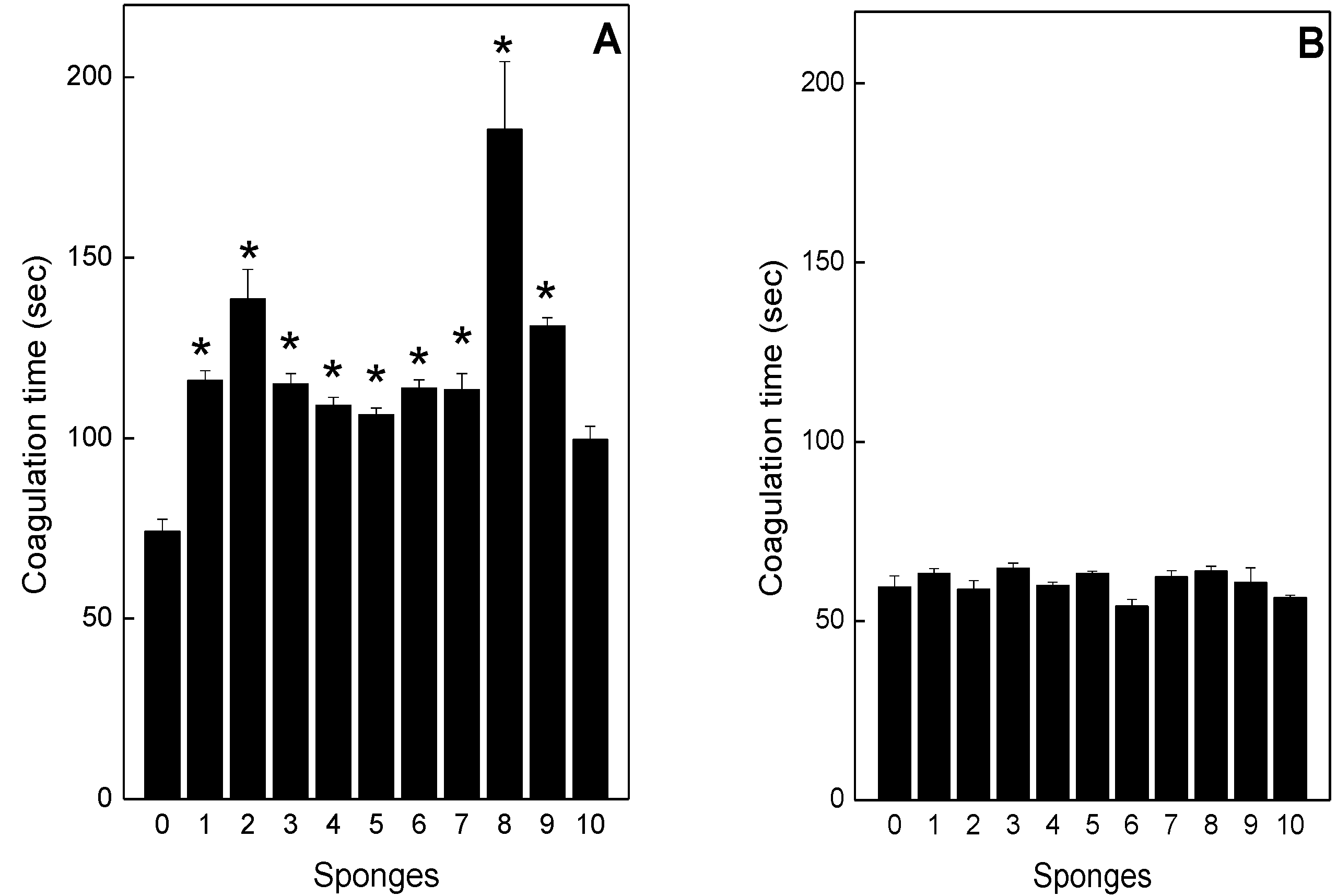
2.4. Neutralization of coagulation
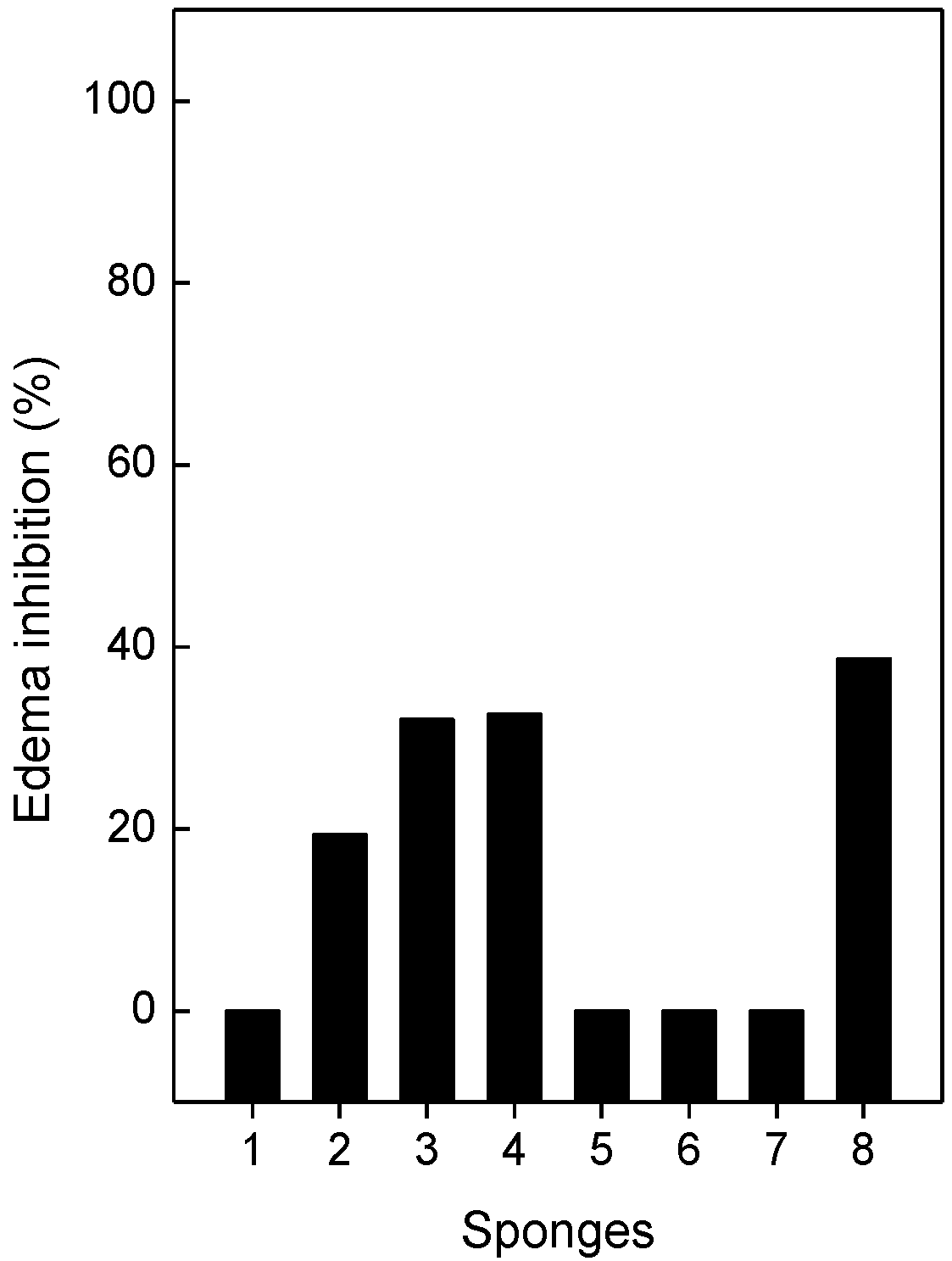
2.5. Neutralization of Edema and Lethality
| Groups | Survival time (min) |
|---|---|
| B. jararaca + NaCl | 126 ± 0.5 |
| B. jararaca + DMSO | 108 ± 0.9 |
| B. jararaca + M. angulosa | 150 ± 0 |
| B. jararaca + T. ignis | 180 ± 0 |
| B. jararaca + A. fulva | 258 ± 3.2 |
| B. jararaca + D. anchorata | 150 ± 0 |
| B. jararaca + A. viridis | 258 ± 3.2 |
| B. jararaca + P. citrine | 372 ± 3.2 |
| B. jararaca + P. janeirensis | 150 ± 0 |
| B. jararaca + H. heliophila | 168 ± 0.5 |
3. Discussion
4. Experimental Section
4.1. Venom and Animals
4.2. Marine Sponge Extracts
4.3. Antiproteolytic Activity
4.4. Antihemorrhagic Activity
4.5. Antihemolytic Activity
4.6. Anticlotting Activity
4.7. Antiedematogenic Activity
4.8. Antilethality Activity
4.9. Statistical Analysis
5. Conclusion
Acknowledgments
Conflicts of interest
References
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar]
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Baldo, C.; Jamora, C.; Yamanouye, N.; Zorn, T.M.; Moura-da-Silva, A.M. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: Tissue distribution and in situ hydrolysis. PLoS Negl. Trop. Dis. 2010, 4, e727. [Google Scholar] [CrossRef]
- Braud, S.; Bon, C.; Wisner, A. Snake venom proteins acting on hemostasis. Biochimie 2000, 82, 851–859. [Google Scholar] [CrossRef]
- Pidde-Queiroz, G.; Furtado Mde, F.; Filgueiras, C.F.; Pessoa, L.A.; Spadafora-Ferreira, M.; Van den Berg, C.W.; Tambourgi, D.V. Human complement activation and anaphylatoxins generation induced by snake venom toxins from Bothrops genus. Mol. Immunol. 2010, 47, 2537–2544. [Google Scholar] [CrossRef]
- Barone, J.M.; Alponti, R.F.; Frezzatti, R.; Zambotti-Villela, L.; Silveira, P.F. Differential efficiency of simvastatin and lipoic acid treatments on Bothrops jararaca envenomation-induced acute kidney injury in mice. Toxicon 2011, 57, 148–156. [Google Scholar] [CrossRef]
- Rucavado, A.; Flores-Sanchez, E.; Franceschi, A.; Magalhaes, A.; Gutierrez, J.M. Characterization of the local tissue damage induced by LHF-II, a metalloproteinase with weak hemorrhagic activity isolated from Lachesis muta muta snake venom. Toxicon 1999, 37, 1297–1312. [Google Scholar] [CrossRef]
- Teixeira Cde, F.; Fernandes, C.M.; Zuliani, J.P.; Zamuner, S.F. Inflammatory effects of snake venom metalloproteinases. Mem. Inst. Oswaldo Cruz 2005, 100, 181–184. [Google Scholar]
- MS (Ministério da Saúde)/FUNASA (Fundação Nacional da Saúde). Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos; MS/ FUNASA: Brasilia, Brazil, 2001.
- Cardoso, J.L.C.; França, F.O.S.; Fan, H.W.; Malaque, C.M.S.; Haddad, V., Jr. Animais Peçonhentos no Brasil. Biologia, Clínica e Terapêutica dos Acidentes; Sarvier/Fapesp: São Paulo, Brazil, 2003; p. 469. [Google Scholar]
- Gutierrez, J.M.; Leon, G.; Burnouf, T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biologicals 2011, 39, 129–142. [Google Scholar] [CrossRef]
- Theakston, R.D.; Fan, H.W.; Warrell, D.A.; Da Silva, W.D.; Ward, S.A.; Higashi, H.G. Use of enzyme immunoassays to compare the effect and assess the dosage regimens of three Brazilian Bothrops antivenoms. The Butantan Institute Antivenom Study Group (BIASG). Am. J. Trop. Med. Hyg. 1992, 47, 593–604. [Google Scholar]
- Isbister, G.K.; White, J.; Currie, B.J.; O’Leary, M.A.; Brown, S.G.; Investigators, A.S.P. Clinical effects and treatment of envenoming by Hoplocephalus spp. snakes in Australia: Australian Snakebite Project (ASP-12). Toxicon 2011, 58, 634–640. [Google Scholar] [CrossRef]
- De Silva, H.A.; Pathmeswaran, A.; Ranasinha, C.D.; Jayamanne, S.; Samarakoon, S.B.; Hittharage, A.; Kalupahana, R.; Ratnatilaka, G.A.; Uluwatthage, W.; Aronson, J.K.; et al. Low-dose adrenaline, promethazine, and hydrocortisone in the prevention of acute adverse reactions to antivenom following snakebite: A randomised, double-blind, placebo-controlled trial. PLoS Med. 2011, 8, e1000435. [Google Scholar] [CrossRef]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B 2004, 803, 41–53. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Kijjoa, A.; Sawangwong, P. Drugs and Cosmetics from the Sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar] [CrossRef]
- Sipkema, D.; Franssen, M.C.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar] [CrossRef]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C 2005, 140, 265–286. [Google Scholar]
- Ohno, O.; Suenaga, K.; Uemura, D. Secondary metabolites with new medicinal functions from marine organisms. Adv. Food Nutr. Res. 2012, 65, 185–193. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Cardenas, P.; Perez, T.; Boury-Esnault, N. Sponge systematics facing new challenges. Adv. Mar. Biol. 2012, 61, 79–209. [Google Scholar] [CrossRef]
- Worheide, G.; Dohrmann, M.; Erpenbeck, D.; Larroux, C.; Maldonado, M.; Voigt, O.; Borchiellini, C.; Lavrov, D.V. Deep phylogeny and evolution of sponges (phylum Porifera). Adv. Mar. Biol. 2012, 61, 1–78. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antiviral Res. 2010, 86, 231–240. [Google Scholar] [CrossRef]
- Xu, J.; Hasegawa, M.; Harada, K.; Kobayashi, H.; Nagai, H.; Namikoshi, M. Melophlins P, Q, R, and S: Four new tetramic acid derivatives, from two Palauan marine sponges of the genus Melophlus. Chem. Pharm. Bull. 2006, 54, 852–854. [Google Scholar] [CrossRef]
- Wright, A.D.; McCluskey, A.; Robertson, M.J.; MacGregor, K.A.; Gordon, C.P.; Guenther, J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela Hooperi. Org. Biomol. Chem. 2011, 9, 400–407. [Google Scholar] [CrossRef]
- De Andrade Moura, L.; Ortiz-Ramirez, F.; Cavalcanti, D.N.; Ribeiro, S.M.; Muricy, G.; Teixeira, V.L.; Fuly, A.L. Evaluation of marine brown algae and sponges from Brazil as anticoagulant and antiplatelet products. Mar. Drugs 2011, 9, 1346–1358. [Google Scholar] [CrossRef]
- Martinez-Poveda, B.; Garcia-Vilas, J.A.; Cardenas, C.; Melgarejo, E.; Quesada, A.R.; Medina, M.A. The brominated compound aeroplysinin-1 inhibits proliferation and the expression of key pro-inflammatory molecules in human endothelial and monocyte cells. PloS One 2013, 8, e55203. [Google Scholar]
- Kim, G.D.; Cheong, O.J.; Bae, S.Y.; Shin, J.; Lee, S.K. 6'-Debromohamacanthin A, a bis (indole) alkaloid, inhibits angiogenesis by targeting the VEGFR2-mediated PI3K/AKT/mTOR signaling pathways. Mar. Drugs 2013, 11, 1087–1103. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponges. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contribution to the study of marine products. XXXII. The nucleosides of sponges. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Fuly, A.L.; Francischetti, I.M.; Zingali, R.B.; Carlini, C.R. Partial purification and some physicochemical properties of phospholipases A2 from the venom of the bushmaster snake (Lachesis muta). Braz. J. Med. Biol. Res. 1993, 26, 459–463. [Google Scholar]
- Gupta, Y.K.; Peshin, S.S. Do herbal medicines have potential for managing snake bite envenomation? Toxicol. Int. 2012, 19, 89–99. [Google Scholar] [CrossRef]
- Williams, D.J.; Jensen, S.D.; Nimorakiotakis, B.; Muller, R.; Winkel, K.D. Antivenom use, premedication and early adverse reactions in the management of snake bites in rural Papua New Guinea. Toxicon 2007, 49, 780–792. [Google Scholar] [CrossRef]
- De Andrade Moura, L.; Bianco, E.M.; Pereira, R.C.; Teixeira, V.L.; Fuly, A.L. Anticoagulation and antiplatelet effects of a dolastane diterpene isolated from the marine brown alga Canistrocarpus cervicornis. J. Thromb. Thrombolysis 2011, 31, 235–240. [Google Scholar] [CrossRef]
- Martz, W. Plants with a reputation against snakebite. Toxicon 1992, 30, 1131–1142. [Google Scholar] [CrossRef]
- Houghton, P.J.; Osibogun, I.M. Flowering plants used against snakebite. J. Ethnopharmacol. 1993, 39, 1–29. [Google Scholar] [CrossRef]
- Sauleau, P.; Martin, M.T.; Dau, M.E.; Youssef, D.T.; Bourguet-Kondracki, M.L. Hyrtiazepine, an azepino-indole-type alkaloid from the Red Sea marine sponge Hyrtios erectus. J. Nat. Prod. 2006, 69, 1676–1679. [Google Scholar] [CrossRef]
- Lombardo, D.; Dennis, E.A. Cobra venom phospholipase A2 inhibition by manoalide. A novel type of phospholipase inhibitor. J. Biol. Chem. 1985, 260, 7234–7240. [Google Scholar]
- De Freitas, J.C.; Blankemeier, L.A.; Jacobs, R.S. In vitro inactivation of the neurotoxic action of beta-bungarotoxin by the marine natural product, manoalide. Experientia 1984, 40, 864–865. [Google Scholar] [CrossRef]
- Fujisawa, D.; Yamazaki, Y.; Lomonte, B.; Morita, T. Catalytically inactive phospholipase A2 homologue binds to vascular endothelial growth factor receptor-2 via a C-terminal loop region. Biochem. J. 2008, 411, 515–522. [Google Scholar] [CrossRef]
- De Paula, R.C.; Castro, H.C.; Rodrigues, C.R.; Melo, P.A.; Fuly, A.L. Structural and pharmacological features of phospholipases A2 from snake venoms. Protein Pep. Lett. 2009, 16, 899–907. [Google Scholar] [CrossRef]
- Fagundes, F.H.; Aparicio, R.; Dos Santos, M.L.; Diz Filho, E.B.; Oliveira, S.C.; Toyama, D.O.; Toyama, M.H. A catalytically inactive Lys49 PLA2 isoform from Bothrops jararacussu venom that stimulates insulin secretion in pancreatic beta cells. Protein Pep. Lett. 2011, 18, 1133–1139. [Google Scholar] [CrossRef]
- Junqueira-de-Azevedo, I.L.; Ching, A.T.; Carvalho, E.; Faria, F.; Nishiyama, M.Y., Jr.; Ho, P.L.; Diniz, M.R. Lachesis muta (Viperidae) cDNAs reveal diverging pit viper molecules and scaffolds typical of cobra (Elapidae) venoms: Implications for snake toxin repertoire evolution. Genetics 2006, 173, 877–889. [Google Scholar] [CrossRef]
- Serrano, S.M.; Shannon, J.D.; Wang, D.; Camargo, A.C.; Fox, J.W. A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: An approach to understanding venom proteomics. Proteomics 2005, 5, 501–510. [Google Scholar] [CrossRef]
- Bastos, J.C.; Kohn, L.K.; Fantinatti-Garboggini, F.; Padilla, M.A.; Flores, E.F.; da Silva, B.P.; De Menezes, C.B.; Arns, C.W. Antiviral activity of Bacillus sp. isolated from the marine sponge Petromica citrina against bovine viral diarrhea virus, a surrogate model of the hepatitis C virus. Viruses 2013, 5, 1219–1230. [Google Scholar] [CrossRef]
- Phelan, R.W.; Barret, M.; Cotter, P.D.; O’Connor, P.M.; Chen, R.; Morrissey, J.P.; Dobson, A.D.; O’Gara, F.; Barbosa, T.M. Subtilomycin: A new lantibiotic from Bacillus subtilis strain MMA7 isolated from the marine sponge Haliclona simulans. Mar. Drugs 2013, 11, 1878–1898. [Google Scholar] [CrossRef]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Marinho, P.R.; Simas, N.K.; Kuster, R.M.; Duarte, R.S.; Fracalanzza, S.E.; Ferreira, D.F.; Romanos, M.T.; Muricy, G.; Giambiagi-Demarval, M.; Laport, M.S. Antibacterial activity and cytotoxicity analysis of halistanol trisulphate from marine sponge Petromica citrina. J. Antimicrob. Chemother. 2012, 67, 2396–2400. [Google Scholar] [CrossRef]
- Garcia, E.S.; Guimaraes, J.A.; Prado, J.L. Purification and characterization of a sulfhydryl-dependent protease from Rhodnius prolixus midgut. Arch. Biochem. Biophys. 1978, 188, 315–322. [Google Scholar] [CrossRef]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–52. [Google Scholar]
- Fuly, A.L.; De Miranda, A.L.; Zingali, R.B.; Guimaraes, J.A. Purification and characterization of a phospholipase A2 isoenzyme isolated from Lachesis muta snake venom. Biochem. Pharmacol. 2002, 63, 1589–1597. [Google Scholar] [CrossRef]
- Yamakawa, M.; Nozani, M.; Hokama, Z. Toxins: Animal, Plant and Microbial; Plenum Press: New York, USA, 1976; pp. 97–120. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faioli, C.N.; Domingos, T.F.S.; De Oliveira, E.C.; Sanchez, E.F.; Ribeiro, S.; Muricy, G.; Fuly, A.L. Appraisal of Antiophidic Potential of Marine Sponges against Bothrops jararaca and Lachesis muta Venom. Toxins 2013, 5, 1799-1813. https://doi.org/10.3390/toxins5101799
Faioli CN, Domingos TFS, De Oliveira EC, Sanchez EF, Ribeiro S, Muricy G, Fuly AL. Appraisal of Antiophidic Potential of Marine Sponges against Bothrops jararaca and Lachesis muta Venom. Toxins. 2013; 5(10):1799-1813. https://doi.org/10.3390/toxins5101799
Chicago/Turabian StyleFaioli, Camila Nunes, Thaisa Francielle Souza Domingos, Eduardo Coriolano De Oliveira, Eládio Flores Sanchez, Suzi Ribeiro, Guilherme Muricy, and Andre Lopes Fuly. 2013. "Appraisal of Antiophidic Potential of Marine Sponges against Bothrops jararaca and Lachesis muta Venom" Toxins 5, no. 10: 1799-1813. https://doi.org/10.3390/toxins5101799
APA StyleFaioli, C. N., Domingos, T. F. S., De Oliveira, E. C., Sanchez, E. F., Ribeiro, S., Muricy, G., & Fuly, A. L. (2013). Appraisal of Antiophidic Potential of Marine Sponges against Bothrops jararaca and Lachesis muta Venom. Toxins, 5(10), 1799-1813. https://doi.org/10.3390/toxins5101799




