Ricin Toxicity to Intestinal Cells Leads to Multiple Cell Death Pathways Mediated by Oxidative Stress
Abstract
1. Introduction
2. Results
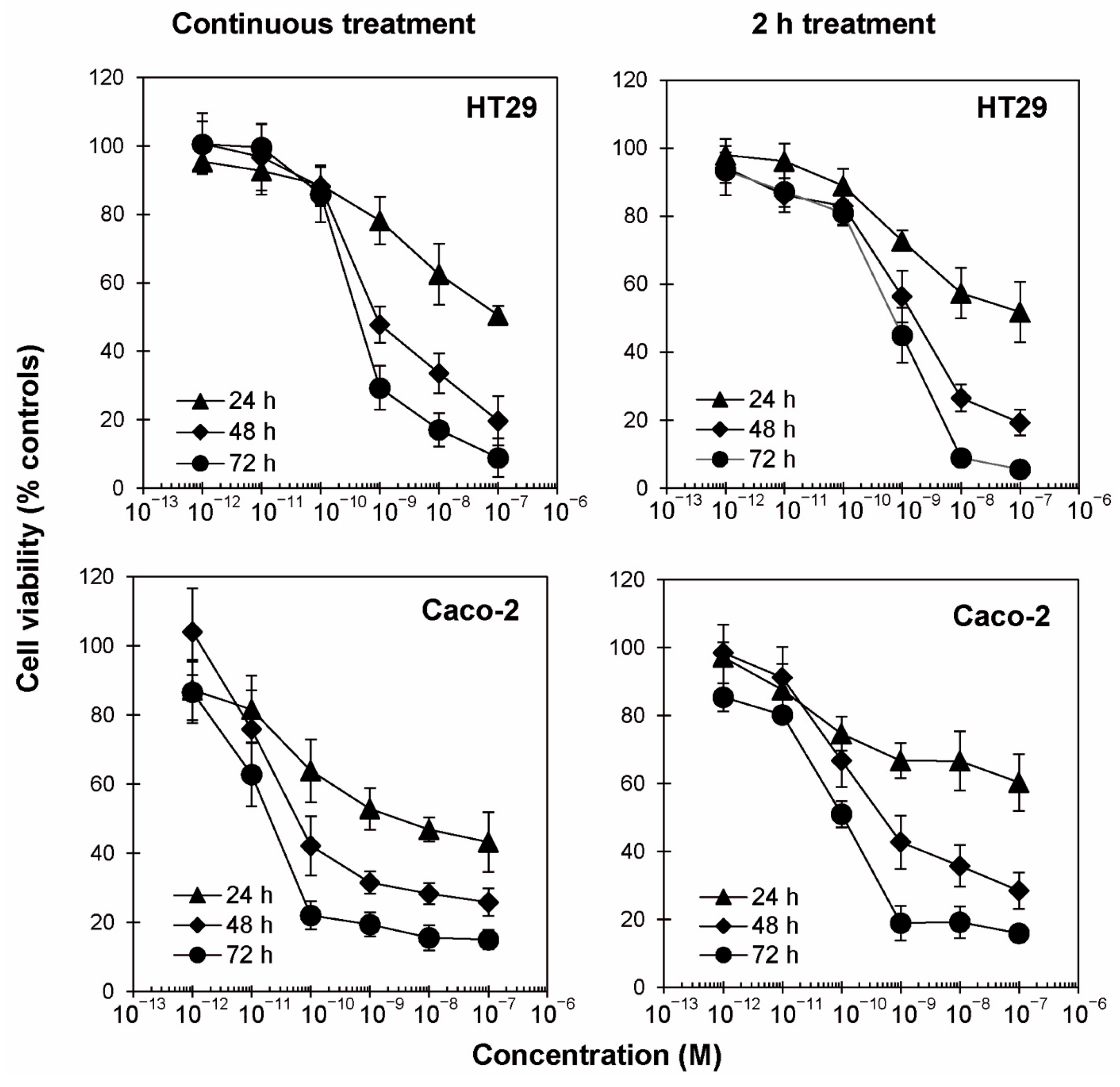
2.1. Cytotoxic Effects of Ricin on Colon Adenocarcinoma Cells
2.2. Effects of Ricin on Barrier Integrity in Caco-2 Monoculture and Caco-2/HT29 Co-Culture Cell Models
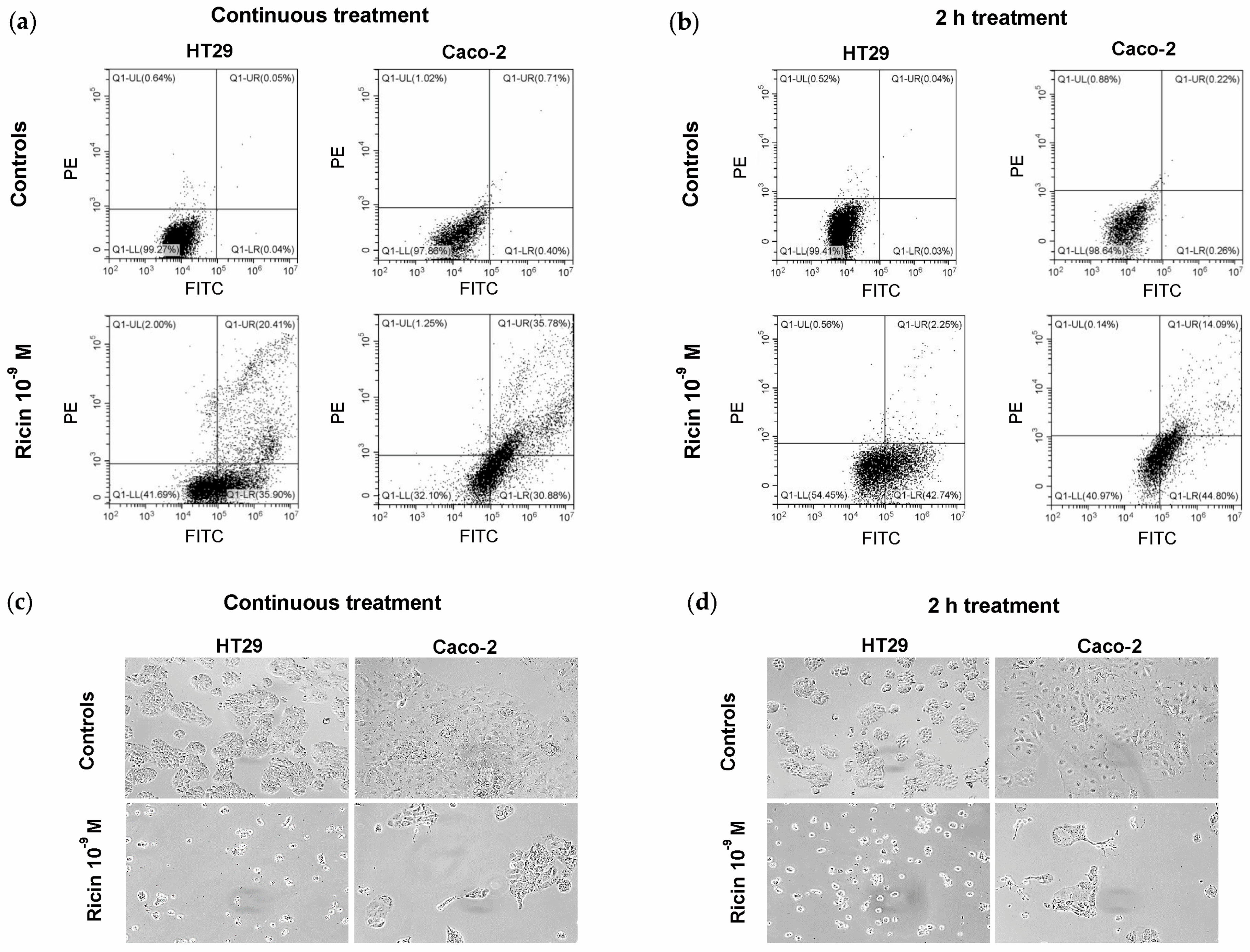
2.3. Evaluation of Cell Death Induced by Ricin in HT29 and Caco-2 Cells
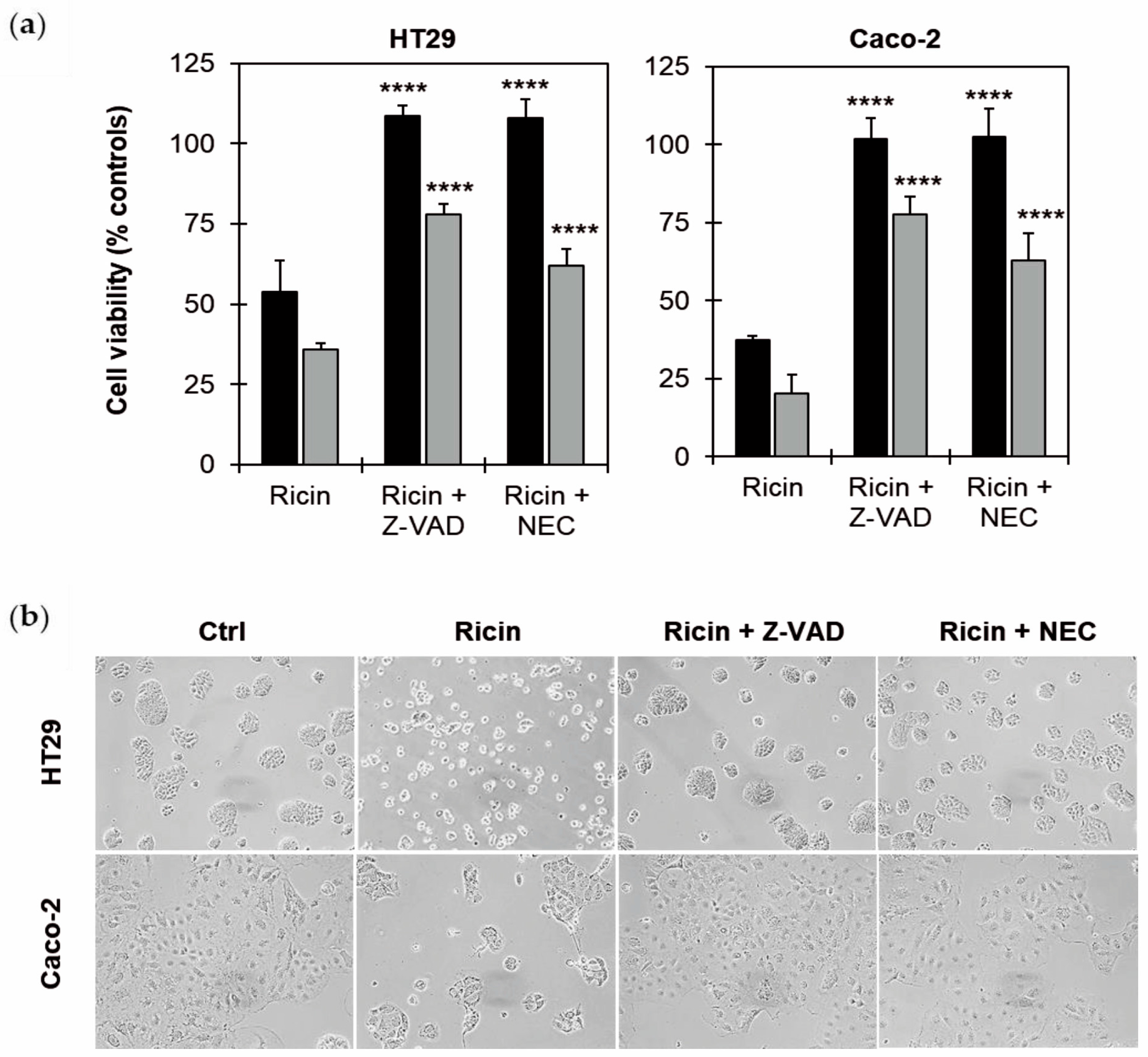
2.4. Effects of Cell Death Inhibitors on Ricin Cytotoxicity in HT29 and Caco-2 Cells
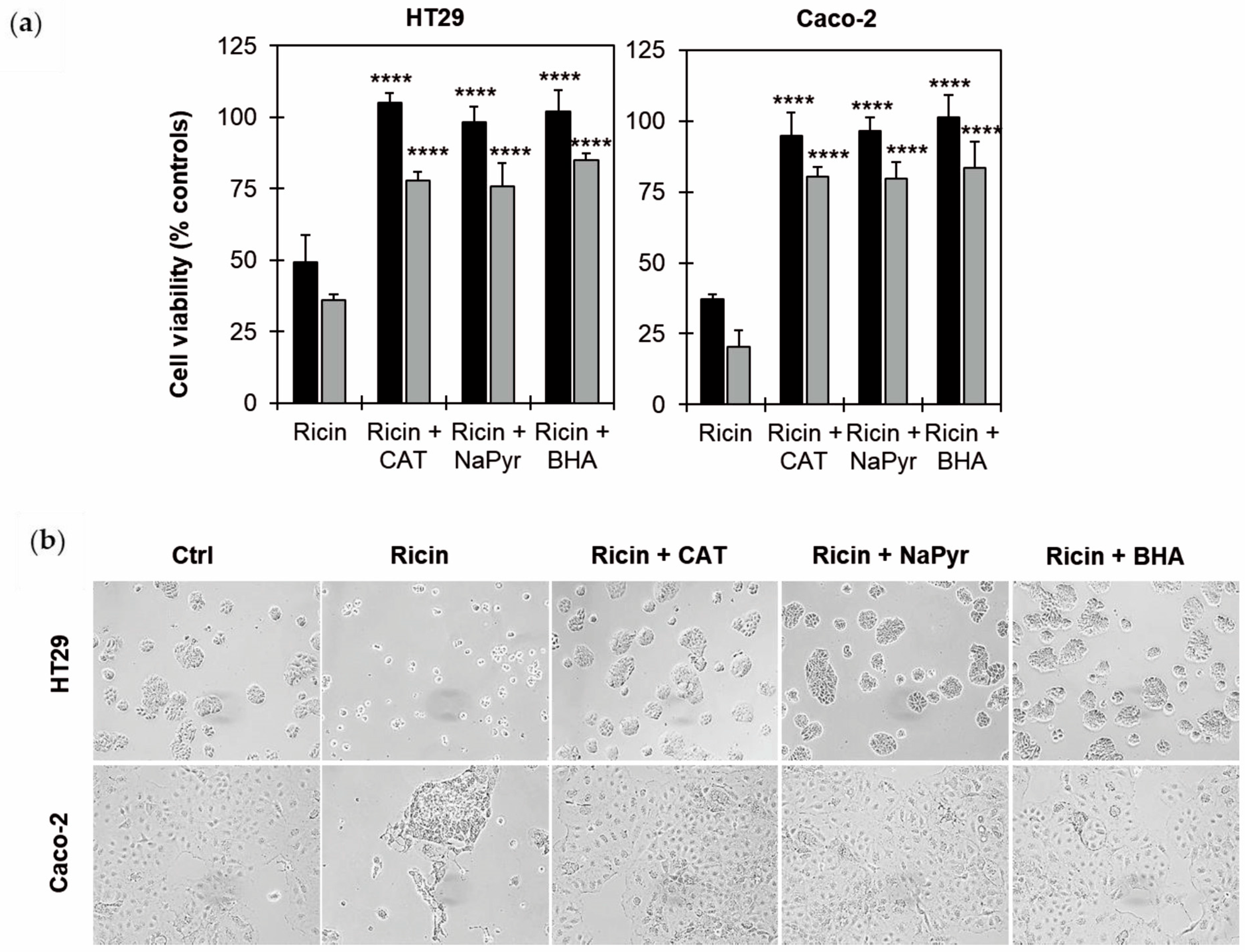
2.5. Effects of ROS Scavengers on Ricin Cytotoxicity in HT29 and Caco-2 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ricin Purification
5.2. Cell Maintenance
5.3. Cell Viability
5.4. Measurement of Trans-Epithelial Electrical Resistance (TEER)
5.5. Evaluation of Cell Death Mechanisms Involved Through Flow Cytometry Analysis
5.6. Evaluation of the Involvement of Apoptosis/Necroptosis and Oxidative Stress Using Cell Death Inhibitors and ROS Scavengers
5.7. Morphological Analysis of Ricin-Treated Intestinal Cells
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHA | Butylated hydroxyanisole |
| C.T. | Continuous treatment |
| CAT | Catalase |
| EC50 | Effective Concentration reducing cell viability by 50% |
| EGFP | Enhanced Green Fluorescent Protein |
| NaPyr | Sodium Pyruvate |
| NEC | Necrostatin-1 |
| PBS | Sodium Phosphate Buffer |
| PI | Propidium Iodide |
| RIP | Ribosome-Inactivating Protein |
| ROS | Reactive Oxygen Species |
| TEER | Trans-Epithelial Electrical Resistance |
References
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from plants: A historical overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef]
- Stirpe, F.; Bolognesi, A.; Bortolotti, M.; Farini, V.; Lubelli, C.; Pelosi, E.; Polito, L.; Dozza, B.; Strocchi, P.; Chambery, A.; et al. Characterization of highly toxic type 2 ribosome-inactivating proteins from Adenia lanceolata and Adenia stenodactyla (Passifloraceae). Toxicon 2007, 50, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.W.; Wong, K.B.; Shaw, P.C. Structural and functional investigation and pharmacological mechanism of trichosanthin, a type 1 Ribosome-Inactivating Protein. Toxins 2018, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Farini, V.; Battelli, M.G.; Barbieri, L.; Bolognesi, A. Saporin induces multiple death pathways in lymphoma cells with different intensity and timing as compared to ricin. Int. J. Biochem. Cell Biol. 2009, 41, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Rogozińska, N.; Sominka, H.; Nowakowska-Gołacka, J.; Sandvig, K.; Słomińska-Wojewódzka, M. Intracellular transport and cytotoxicity of the protein toxin ricin. Toxins 2019, 11, 350. [Google Scholar] [CrossRef]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–23451. [Google Scholar] [CrossRef]
- Roy, C.J.; Song, K.; Sivasubramani, S.K.; Gardner, D.J.; Pincus, S.H. Animal models of ricin toxicosis. Curr. Top. Microbiol. Immunol. 2012, 357, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S. Ricin and abrin. Medicine 2016, 44, 109–110. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Avril, A. Medical countermeasures against ricin intoxication. Toxins 2023, 15, 100. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Dickers, K.J.; Rice, P.; Griffiths, G.D.; Vale, J.A. Ricin poisoning. Toxicol. Rev. 2003, 22, 65–70. [Google Scholar] [CrossRef]
- EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2002/32/oj/eng (accessed on 1 July 2025).
- Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on ricin (from Ricinus communis) as undesirable substances in animal feed. EFSA J. 2008, 726, 1–38. [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Calafato, G.; Bolognesi, A. Ricin: An ancient story for a timeless plant toxin. Toxins 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Guerci, A. Various uses of the castor oil plant (Ricinus communis L.). A review. J. Ethnopharmacol. 1982, 5, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Ladda, P.L.; Kamthane, R.B. Ricinus communis (castor): An overview. Int. J. Res. Pharmacol. Pharmacother. 2014, 3, 136–144. [Google Scholar]
- Ziaei, A.; Sahranavard, S.; Gharagozlou, M.J.; Faizi, M. Preliminary investigation of the effects of topical Mix. of Lawsonia inermis L. and Ricinus communis L. leaves extract in treatment of osteoarthritis using MIA model in rats. Daru 2016, 24, 12. [Google Scholar] [CrossRef]
- Wong, W. Some folk medicinal plants from Trinidad. Econ. Bot. 1976, 30, 103–142. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Bolognesi, A. Plants producing Ribosome-Inactivating Proteins in traditional medicine. Molecules 2016, 21, 1560. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Cui, M.; Li, X.; Zhai, H.; Wu, D.; Chu, X. Therapeutic effects of medicinal and food-based traditional herbal couples on type 2 diabetes mellitus based on pharmacodynamics and pharmacokinetics. Front. Pharmacol. 2025, 16, 1560271. [Google Scholar] [CrossRef]
- Pietkiewicz, S.; Schmidt, J.H.; Lavrik, I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods 2015, 423, 99–103. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; Chapter 11; pp. 113–124. [Google Scholar] [CrossRef]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 cell line standardization with pharmaceutical requirements and in vitro model suitability for permeability assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef]
- Zhang, J.; Penny, J.; Lu, J.R. Development of a novel in vitro 3D intestinal model for permeability evaluations. Int. J. Food Sci. Nutr. 2020, 71, 549–562. [Google Scholar] [CrossRef]
- Yoder, J.M.; Aslam, R.U.; Mantis, N.J. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect. Immun. 2007, 75, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Smallshaw, J.E.; Richardson, J.A.; Vitetta, E.S. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 2007, 25, 7459–7469. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; McGuinness, C.R.; Sonuyi, O.; Edwards, G.; Farrant, S.A. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect. Immun. 2006, 74, 3455–3462. [Google Scholar] [CrossRef]
- Flora, A.D.; Teel, L.D.; Smith, M.A.; Sinclair, J.F.; Melton-Celsa, A.R.; O’Brien, A.D. Ricin crosses polarized human intestinal cells and intestines of ricin-gavaged mice without evident damage and then disseminates to mouse kidneys. PLoS ONE 2013, 8, e69706. [Google Scholar] [CrossRef]
- Franke, H.; Scholl, R.; Aigner, A. Ricin and Ricinus communis in pharmacology and toxicology-from ancient use and “Papyrus Ebers” to modern perspectives and “poisonous plant of the year 2018”. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1181–1208. [Google Scholar] [CrossRef]
- Korcheva, V.; Wong, J.; Corless, C.; Iordanov, M.; Magun, B. Administration of ricin induces a severe inflammatory response via nonredundant stimulation of ERK, JNK, and P38 MAPK and provides a mouse model of hemolytic uremic syndrome. Am. J. Pathol. 2005, 166, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Jetzt, A.E.; Cheng, J.S.; Tumer, N.E.; Cohick, W.S. Ricin A-chain requires c-Jun N-terminal kinase to induce apoptosis in nontransformed epithelial cells. Int. J. Biochem. Cell Biol. 2009, 41, 2503–2510. [Google Scholar] [CrossRef]
- Wong, J.; Korcheva, V.; Jacoby, D.B.; Magun, B.E. Proinflammatory responses of human airway cells to ricin involve stress-activated protein kinases and NF-kappaB. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L1385–L1394. [Google Scholar] [CrossRef]
- Jandhyala, D.M.; Thorpe, C.M.; Magun, B. Ricin and Shiga toxins: Effects on host cell signal transduction. Curr. Top. Microbiol. Immunol. 2012, 357, 41–65. [Google Scholar] [CrossRef]
- Bortolotti, M.; Biscotti, F.; Zanello, A.; Polito, L.; Bolognesi, A. Heterophyllin: A new Adenia toxic lectin with peculiar biological properties. Toxins 2023, 16, 1. [Google Scholar] [CrossRef]
- Mercatelli, D.; Bortolotti, M.; Andresen, V.; Sulen, A.; Polito, L.; Gjertsen, B.T.; Bolognesi, A. Early response to the plant toxin stenodactylin in acute myeloid leukemia cells involves inflammatory and apoptotic signaling. Front Pharmacol. 2020, 11, 630. [Google Scholar] [CrossRef]
- Xie, Y.; Zhao, G.; Lei, X.; Cui, N.; Wang, H. Advances in the regulatory mechanisms of mTOR in necroptosis. Front. Immunol. 2023, 14, 1297408. [Google Scholar] [CrossRef]
- Patankar, J.V.; Bubeck, M.; Acera, M.G.; Becker, C. Breaking bad: Necroptosis in the pathogenesis of gastrointestinal diseases. Front. Immunol. 2023, 14, 1203903. [Google Scholar] [CrossRef]
- Akanyibah, F.A.; Zhu, Y.; Jin, T.; Ocansey, D.K.W.; Mao, F.; Qiu, W. The function of necroptosis and its treatment target in IBD. Mediat. Inflamm. 2024, 2024, 7275309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, O.; Sugendran, K.; Vijayaraghavan, R. Oxidative stress associated hepatic and renal toxicity induced by ricin in mice. Toxicon 2003, 41, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.M.S.; Narrowe, A.B.; Firrman, J.; Mahalak, K.K.; Liu, L.; Higgins, S.; Moustafa, A.M.; Baudot, A.; Deyaert, S.; Van den Abbeele, P. The food additive butylated hydroxyanisole minimally affects the human gut microbiome ex vivo. Food Chem. 2025, 473, 143037. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Y.; Wu, D.; Shou, J.; Wang, S.; Ye, J.; Tang, X.; Wang, X.J. Butylated hydroxyanisole induces distinct expression patterns of Nrf2 and detoxification enzymes in the liver and small intestine of C57BL/6 mice. Toxicol. Appl. Pharmacol. 2015, 288, 339–348. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Y.; Wang, H.; Wang, S.; Liu, K.; Li, X.; Wang, X.J.; Tang, X. Mkp-1 protects mice against toxin-induced liver damage by promoting the Nrf2 cytoprotective response. Free Radic. Biol. Med. 2018, 115, 361–370. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Blaustein, J.; Etzler, M.E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry 1974, 13, 196–204. [Google Scholar] [CrossRef] [PubMed]

| EC50 (M) | ||||
|---|---|---|---|---|
| Cell Line | Treatment | 24 h | 48 h | 72 h |
| HT29 | C.T. | 6.96 × 10−8 | 8.82 × 10−10 | 4.31 × 10−10 |
| 2 h | >10−7 | 1.52 × 10−9 | 7.22 × 10−10 | |
| Caco-2 | C.T. | 3.30 × 10−9 | 6.14 × 10−11 | 1.80 × 10−11 |
| 2 h | >10−7 | 4.99 × 10−10 | 9.99 × 10−11 | |
| Cell Line | Treatment | Live | Early Apoptotic | Late Apoptotic/ Necroptotic | Necrotic |
|---|---|---|---|---|---|
| Relative Percentage of Ricin-Treated Cells | |||||
| HT29 | C.T. | 41.69 | 35.90 | 20.41 | 2.00 |
| 2 h | 54.45 | 42.74 | 2.25 | 0.56 | |
| Caco-2 | C.T. | 32.10 | 30.88 | 35.78 | 1.25 |
| 2 h | 40.97 | 44.80 | 14.09 | 0.14 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscotti, F.; Bortolotti, M.; Falà, F.; Di Maro, A.; Bolognesi, A.; Polito, L. Ricin Toxicity to Intestinal Cells Leads to Multiple Cell Death Pathways Mediated by Oxidative Stress. Toxins 2025, 17, 400. https://doi.org/10.3390/toxins17080400
Biscotti F, Bortolotti M, Falà F, Di Maro A, Bolognesi A, Polito L. Ricin Toxicity to Intestinal Cells Leads to Multiple Cell Death Pathways Mediated by Oxidative Stress. Toxins. 2025; 17(8):400. https://doi.org/10.3390/toxins17080400
Chicago/Turabian StyleBiscotti, Francesco, Massimo Bortolotti, Federica Falà, Antimo Di Maro, Andrea Bolognesi, and Letizia Polito. 2025. "Ricin Toxicity to Intestinal Cells Leads to Multiple Cell Death Pathways Mediated by Oxidative Stress" Toxins 17, no. 8: 400. https://doi.org/10.3390/toxins17080400
APA StyleBiscotti, F., Bortolotti, M., Falà, F., Di Maro, A., Bolognesi, A., & Polito, L. (2025). Ricin Toxicity to Intestinal Cells Leads to Multiple Cell Death Pathways Mediated by Oxidative Stress. Toxins, 17(8), 400. https://doi.org/10.3390/toxins17080400










