Fate of Pyrrolizidine Alkaloids in Soil: Insights from Myosotis arvensis L. and Senecio vulgaris L.
Abstract
1. Introduction
2. Results
2.1. PA Content in Weed Extracts
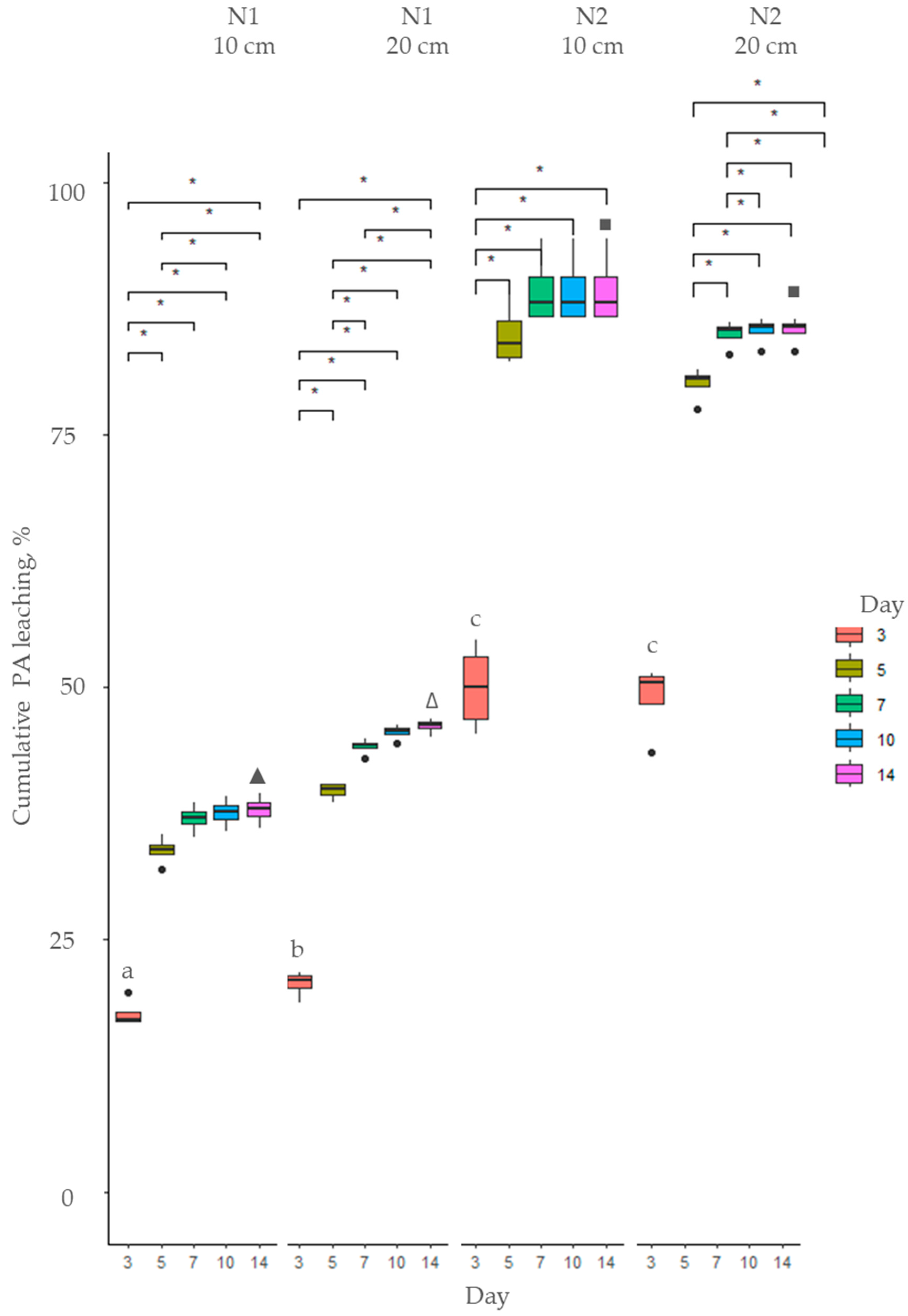
2.2. Fate of Pyrrolizidine Alkaloids in Soil
2.2.1. Myosotis arvensis Cold and Hot Extracts
2.2.2. Senecio vulgaris Cold and Hot Extracts
3. Discussion
3.1. Pyrrolizidine Alkaloid Profiles in Myosotis arvensis and Senecio vulgaris
3.2. Soil Type Effects on Pyrrolizidine Alkaloid Leaching Dynamics
3.3. Influence of Soil Physicochemical Properties
3.3.1. pH, Organic Matter, and Soil Texture Effects
3.3.2. Sorption, Desorption, and Mobility Mechanisms
3.4. Reduction of PANOs and Toxicological Impact of PA Chemical Structures
3.5. Environmental Risk and Future Directions
4. Conclusions
5. Materials and Methods
5.1. Reference Standards and Reagents
5.2. Preparation of Pyrrolizidine Alkaloid-Containing Plant Extracts
5.2.1. Weed Samples
5.2.2. Cold (CE) and Hot (HE) Extracts
5.2.3. Quantitative Analysis Using LC-HRMS
5.3. Fate of Pyrrolizidine Alkaloids in Soil
5.3.1. Soil Samples
5.3.2. Design and Settings
5.3.3. Quantitative Analysis Using LC-HRMS
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tábuas, B.; Cruz Barros, S.; Diogo, C.; Cavaleiro, C.; Sanches Silva, A. Pyrrolizidine Alkaloids in Foods, Herbal Drugs, and Food Supplements: Chemistry, Metabolism, Toxicological Significance, Analytical Methods, Occurrence, and Challenges for Future. Toxins 2024, 16, 79. [Google Scholar] [CrossRef]
- Lu, Y.-S.; Qiu, J.; Mu, X.-Y.; Qian, Y.-Z.; Chen, L. Levels, Toxic Effects, and Risk Assessment of Pyrrolizidine Alkaloids in Foods: A Review. Foods 2024, 13, 536. [Google Scholar] [CrossRef]
- Schrenk, D.; Gao, L.; Lin, G. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment-A review. Food Chem. Toxicol. 2020, 136, 111107. [Google Scholar] [CrossRef] [PubMed]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, G.A.; Hol, W.H.G.; Van Veen, J.A. Rhizosphere fungal communities are influenced by Senecio jacobaea pyrrolizidine alkaloid content and composition. Soil Biol. Biochem. 2006, 38, 2852–2859. [Google Scholar] [CrossRef]
- Selmar, D.; Abouzeid, S.; Radwan, A. Horizontal Natural Product Transfer: A Novel Attribution in Allelopathy. In Co-Evolution of Secondary Metabolites; Merillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–11. [Google Scholar] [CrossRef]
- Lewerenz, L.; Abouzeid, S.; Yahyazadeh, M.; Hijazin, T.; Selmar, D. Novel Cognitions in Allelopathy: Implications from the “Horizontal Natural Product Transfer”. Plants 2022, 11, 3264. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, R.J.; Gardner, D.L.; Colegate, S.M.; Edgar, J.A. Pyrrolizidine alkaloid toxicity in livestock: A paradigm for human poisoning? Food Addit. Contam. Part A. 2011, 3, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Wang, Z.; Han, H.; Wang, C.; Zheng, Q.; Chen, H.; Zhang, X.; Hou, R. Hepatotoxicity of Pyrrolizidine Alkaloid Compound Intermedine: Comparison with Other Pyrrolizidine Alkaloids and Its Toxicological Mechanism. Toxins 2021, 13, 849. [Google Scholar] [CrossRef]
- Schrenk, D.; Fahrer, J.; Allemang, A. Novel Insights into Pyrrolizidine Alkaloid Toxicity and Implications for Risk Assessment: Occurrence, Genotoxicity, Toxicokinetics, Risk Assessment–A Workshop Report. Planta Med. 2022, 88, 98–117. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union. 2023, 119, 103–157. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017, 15, 4908. [Google Scholar] [CrossRef]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids: Toxicity and problems. Food Addit. Contam. Part A 2011, 28, 282–292. [Google Scholar] [CrossRef]
- Peloso, M.; Minkoumba Sonfack, G.; Paduano, S.; De Martino, M.; De Santis, B.; Caprai, E. Pyrrolizidine Alkaloids in Food on the Italian Market. Molecules 2023, 28, 5346. [Google Scholar] [CrossRef] [PubMed]
- Kisielius, V.; Hama, J.R.; Skrbic, N. The invasive butterbur contaminates stream and seepage water in groundwater wells with toxic pyrrolizidine alkaloids. Sci. Rep. 2020, 10, 19784. [Google Scholar] [CrossRef]
- Merz, K.H.; Schrenk, D. Interim relative potency factors for the toxicological risk assessment of pyrrolizidine alkaloids in food and herbal medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef]
- Gao, L. Structure-dependent relative toxic potencies of selected pyrrolizidine alkaloids. Dissertation. Ph.D. Thesis, Technical University of Kaiserslautern, Kaiserslautern, Germany, 2022. [Google Scholar]
- Schrenk, D.; Allemang, A.; Fahrer, J. Toxins in Botanical Drugs and Plant-derived Food and Feed–from Science to Regulation: A Workshop Review. Planta Med. 2024, 90, 219–242. [Google Scholar] [CrossRef]
- Hama, J.R.; Strobel, B.W. Pyrrolizidine alkaloids quantified in soil and water using UPLC-MS/MS. RSC Adv. 2019, 9, 30350–30357. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Adams, Z.; Dzikunoo, J.; Asante-Donyinah, D. Uptake and accumulation of pyrrolizidine alkaloids in the tissues of maize (Zea mays L.) plants from the soil of a 4-year-old Chromolaena odorata dominated fallow farmland. Chemosphere 2020, 17, 128669. [Google Scholar] [CrossRef]
- Lu, Y.; Han, H.; Jiang, C.; Liu, H.; Wang, Z.; Chai, Y.; Zhang, X.; Qiu, J.; Chen, H. Uptake, accumulation, translocation and transformation of seneciphylline (Sp) and seneciphylline-N-oxide (SpNO) by Camellia sinensis L. Environ. Int. 2024, 188, 108765. [Google Scholar] [CrossRef]
- Lu, Y.; Han, H.; Yi, Y.; Chai, Y.; Wang, C.; Zhang, X.; Yang, X.; Chen, H. Insight into the sorption and desorption pattern of pyrrolizidine alkaloids and their N-oxides in acidic tea (Camellia sinensis) plantation soils. J. Environ. Sci. 2025, 148, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Chmit, M.S.; Müller, J.; Wiedow, D.; Horn, G.; Beuerle, T. Biodegradation and utilization of crop residues contaminated with poisonous pyrrolizidine alkaloids. J. Environ. Manag. 2021, 290, 112629. [Google Scholar] [CrossRef]
- Günthardt, F.B.; Wettstein, E.F.; Hollender, J.; Singer, H.; Härri, J.; Scheringer, M.; Hungerbühler, K.; Bucheli, D.T. Retrospective HRMS screening and dedicated target analysis reveal a wide exposure to pyrrolizidine alkaloids in small streams. Environ. Sci. Technol. 2021, 55, 1036–1044. [Google Scholar] [CrossRef]
- Hama, J.R.; Strobel, B.W. Occurrence of pyrrolizidine alkaloids in ragwort plants, soils and surface waters at the field scale in grassland. Sci. Total Environ. 2021, 755, 142822. [Google Scholar] [CrossRef] [PubMed]
- Günthardt, B.F.; Schönsee, C.D.; Hollender, J.; Hungerbühler, K.; Scheringer, M.; Bucheli, T.D. Is there anybody else out there?-First Insights from a Suspect Screening for Phytotoxins in Surface Water. Chimia 2020, 74, 129–135. [Google Scholar] [CrossRef]
- Mrkajic, N.S.; Hama, J.R.; Strobel, B.W.; Hansen, H.C.B.; Rasmussen, L.H.; Hedegaard, M.J. Removal of phytotoxins in filter sand used for drinking water treatment. Water Res. 2021, 205, 117610. [Google Scholar] [CrossRef]
- Cramer, L.; Schiebel, H.-M.; Ernst, L.; Beuerle, T. Pyrrolizidine alkaloids in the food chain: Development, validation, and application of a new HPLC-ESI-MS/MS sum parameter method. J. Agric. Food Chem. 2013, 61, 11382–11391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mulder, P.P.J.; Schaap, O.; Memelink, J.; Klinkhamer, P.G.L.; Vrieling, K. The evolution of pyrrolizidine alkaloid diversity among and within jacobaea species. J. Syst. Evol. 2020, 60, 361–376. [Google Scholar] [CrossRef]
- Schönsee, C.D.; Wettstein, F.E.; Bucheli, T.D. Phytotoxin sorption to clay minerals. Environ. Sci. Eur. 2021, 33, 36. [Google Scholar] [CrossRef]
- Ober, D.; Hartmann, T. Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 14777–14782. [Google Scholar] [CrossRef]
- Millaku, F.; Rysha, A.; Berisha, N. The growth of plants containing pyrrolizidine alkaloids (PAs) in plots cultivated with medicinal aromatic plants (MAPs) and in their natural wild habitats in Kosovo. Acta Agric. Slov. 2024, 120, 1–10. [Google Scholar] [CrossRef]
- Leiss, K.A.; Müller-Schärer, H. Adaptation of Senecio vulgaris (Asteraceae) to ruderal and agricultural habitats. Am. J. Botany 2001, 88, 1593–1599. [Google Scholar] [CrossRef]
- Priedītis, N. Latvijas Augi [Plants in Latvia]; Gandrs: Riga, Latvia, 2014; p. 888. [Google Scholar]
- Hoffmann, M.H. The distribution of Senecio vulgaris: Capacity of climatic range models for predicting adventitious ranges. Flora 2001, 196, 395–403. [Google Scholar] [CrossRef]
- Robinson, D.E.; O’Donovan, J.T.; Sharma, M.P.; Doohan, D.J.; Figueroa, R. The biology of Canadian weeds. 123. Senecio vulgaris L. Can. J. Plant Sci. 2003, 83, 629–644. [Google Scholar] [CrossRef]
- Cheng, D.; Nguyven, V.T.; Ndihokubwayo, N.; Ge, J.; Mulder, P.P.J. Pyrrolizidine alkaloid variation in Senecio vulgaris populations from native and invasive ranges. PeerJ 2017, 5, e3686. [Google Scholar] [CrossRef]
- Fernández-Pintor, B.; Casado, N.; Morante-Zarcero, S.; Sierra, I. Evaluation of the thermal stability and transfer rate of pyrrolizidine alkaloids during the brewing of herbal infusions contaminated with Echium vulgare and Senecio vulgaris weeds. Food Control 2023, 153, 109926. [Google Scholar] [CrossRef]
- Flade, J.; Beschow, H.; Wensch-Dorendorf, M.; Plescher, A.; Wätjen, W. Occurrence of Nine Pyrrolizidine Alkaloids in Senecio vulgaris L. Depending on Developmental Stage and Season. Plants 2019, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Kaltner, F. Pyrrolizidine alkaloids of European Senecio/Jacobaea species in forage and their carry-over to milk: A review. Anim. Feed Sci. Technol. 2021, 280, 115062. [Google Scholar] [CrossRef]
- Pelser, P.; de Vos, H.; Theuring, C.; Beuerle, T.; Vrieling, K.; Hatmann, T. Frequent gain and loss of pyrrolizidine alkaloids in the evolution of Senecio section Jacobaea (Asteraceae). Phytochemistry 2005, 66, 1285–1295. [Google Scholar] [CrossRef]
- Varga, F.; Lovković, J.; Mareković, M.; Carović-Stanko, K. Pyrrolizidine alkaloid plant species in the area of large-scale chamomile cultivation in Croatia. Maced. Pharm. Bulletin. 2022, 68 (Suppl. 2), 25–26. [Google Scholar] [CrossRef]
- Hsu, T.-W.; Chiang, T.-Y.; Wang, J.-C. Myosotis arvensis (L.) Hill (Boraginaceae), a Naturalized Species. Taiwania 2002, 47, 159–163. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Pyrrolizidine alkaloids in food and feed. EFSA J. 2011, 9, 2406. [Google Scholar] [CrossRef]
- Jayawickreme, K.; Świstak, D.; Ozimek, E. Pyrrolizidine Alkaloids—Pros and Cons for Pharmaceutical and Medical Applications. Int. J. Mol. Sci. 2023, 24, 16972. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Smith, L.W.; Culvenor, C.C.J. Plant Sources of Hepatotoxic Pyrrolizidine Alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef]
- Resch, J.F.; Rosberger, D.F.; Meinwald, J.; Appling, J.W. Biologically Active Pyrrolizidine Alkaloids From the True Forget-Me-Not, Myosotis scorpioides. J. Nat. Prod. 1982, 45, 358–362. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- LVGMC. Available online: https://klimats.meteo.lv/klimats_latvija/latvijas_klimatiskais_raksturojums/ (accessed on 10 May 2025). (In Latvian).
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength, and limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Nabet, N.; Gilbert-López, B.; Madani, K.; Llorca-Póras, J. Influence of extraction temperature on the bioactive compounds of plant extracts: A comparative study. Food Chem. 2019, 278, 111–120. [Google Scholar] [CrossRef]
- Nowak, M.; Selmar, D. Cellular distribution of alkaloids and their translocation via phloem and xylem: The importance of compartment pH. Plant Biol. 2016, 18, 879–882. [Google Scholar] [CrossRef]
- Prakash, J.; Agrawal, S.B.; Agrawal, M. Global Trends of Acidity in Rainfall and Its Impact on Plants and Soil. J. Soil Sci. Plant Nutr. 2023, 23, 398–419. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Hou, Q.; Chen, J.; Xu, N. Understanding of pH value and its effect on autohydrolysis pretreatment prior to popular chemi-thermomechanical pulping. Bioresour Technol. 2015, 196, 662–667. [Google Scholar] [CrossRef]
- Hijazin, T.; Radwan, A.; Lewerenz, L.; Abouzeid, S.; Selmar, D. The uptake of alkaloids by plants from the soil is determined by rhizosphere pH. Rhizosphere 2020, 15, 100234. [Google Scholar] [CrossRef]
- Schönsee, C.D.; Wettstein, F.E.; Bucheli, T.D. Disentangling Mechanisms in Natural Toxin Sorption to Soil Organic Carbon. Environ. Sci. Technol. 2021, 55, 4762–4771. [Google Scholar] [CrossRef]
- Hardie, A.G.; Olifant, K.; Smith, J.F.N.; Hoffman, J.E. Scientific evidence of soil transfer of pyrrolizidine alkaloids originating from weed species to rooibos tea. S. Afr. J. Bot. 2023, 157, 159–166. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of pyrrolizidine alkaloids in animal-and plant-derived food: Results of a survey across Europe. Food Addit. Contam. Part A 2018, 35, 118–133. [Google Scholar] [CrossRef]
- Lis-Cieplak, A.; Trześniowska, K.; Stolarczyk, K.; Stolarczyk, E.U. Pyrrolizidine Alkaloids as Hazardous Toxins in Natural Products: Current Analytical Methods and Latest Legal Regulations. Molecules 2024, 29, 3269. [Google Scholar] [CrossRef]
- Klevenhusen, F.; Pieper, R.; Winter, J.; Ronczka, S.; Speer, K. Stability of pyrrolizidine alkaloids from Senecio vernalis in grass silage under different ensilage conditions. J. Sci. Food Agric. 2019, 99, 6649–6654. [Google Scholar] [CrossRef]
- Hough, R.L.; Crews, C.; White, D.; Driffield, M.; Campbell, C.D.; Maltin, C. Degradation of yew, ragwort and rhododendron toxins during composting. Sci. Total Environ. 2010, 408, 4128–4137. [Google Scholar] [CrossRef]
- Yang, M.; Ma, J.; Ruan, J.; Ye, Y.; Fu, P.P.-C.; Lin, G. Intestinal and hepatic biotransformation of pyrrolizidine alkaloid N-oxides to toxic pyrrolizidine alkaloids. Toxicokinet. Metab. 2019, 93, 2197–2209. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Witte, L. Chemistry, Biology and Chemoecology of the Pyrrolizidine Alkaloids. In Alkaloids: Chemical and Biological Perspectives; Cordell, G.A., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 155–233. [Google Scholar]
- Agilent Technologies. ESI-TOF Reference Mass Solution Kit G1969-85001—Product Information. Available online: https://www.agilent.com/cs/library/msds/G1969-85001_NAEnglish.pdf (accessed on 20 June 2025).
- ISO 10390; Soil, Treated Biowaste and Sludge–Determination of pH. ISO: Geneva, Switzerland, 2021.
- ISO 17586; Soil Quality—Extraction of Trace Elements Using Dilute Nitric Acid. ISO: Geneva, Switzerland, 2016.
- ISO/TS 22171; Soil Quality: Determination of Potential Cation Exchange Capacity (CEC) and Exchangeable Cations. ISO: Geneva, Switzerland, 2023.
- ISO 17892-4; Geotechnical Investigation and Testing-Laboratory Testing of Soil-Part 4: Determination of Particle Size Distribution. ISO: Geneva, Switzerland, 2016.
- de Mendiburu, F.; Yaseen, M. Statistical Procedures for Agricultural Research, R Package Version 1.4.0. 2020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 12 May 2025).
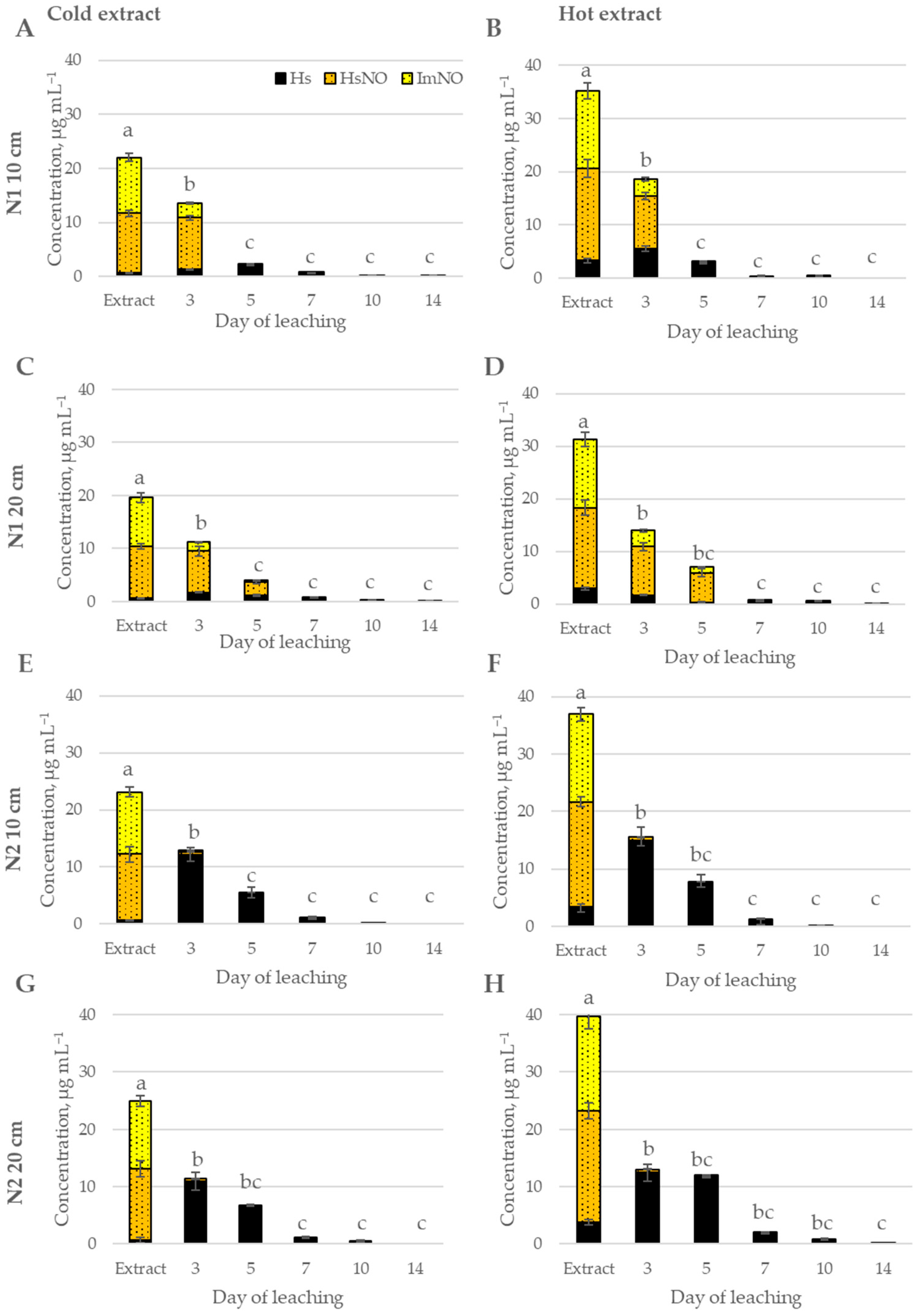

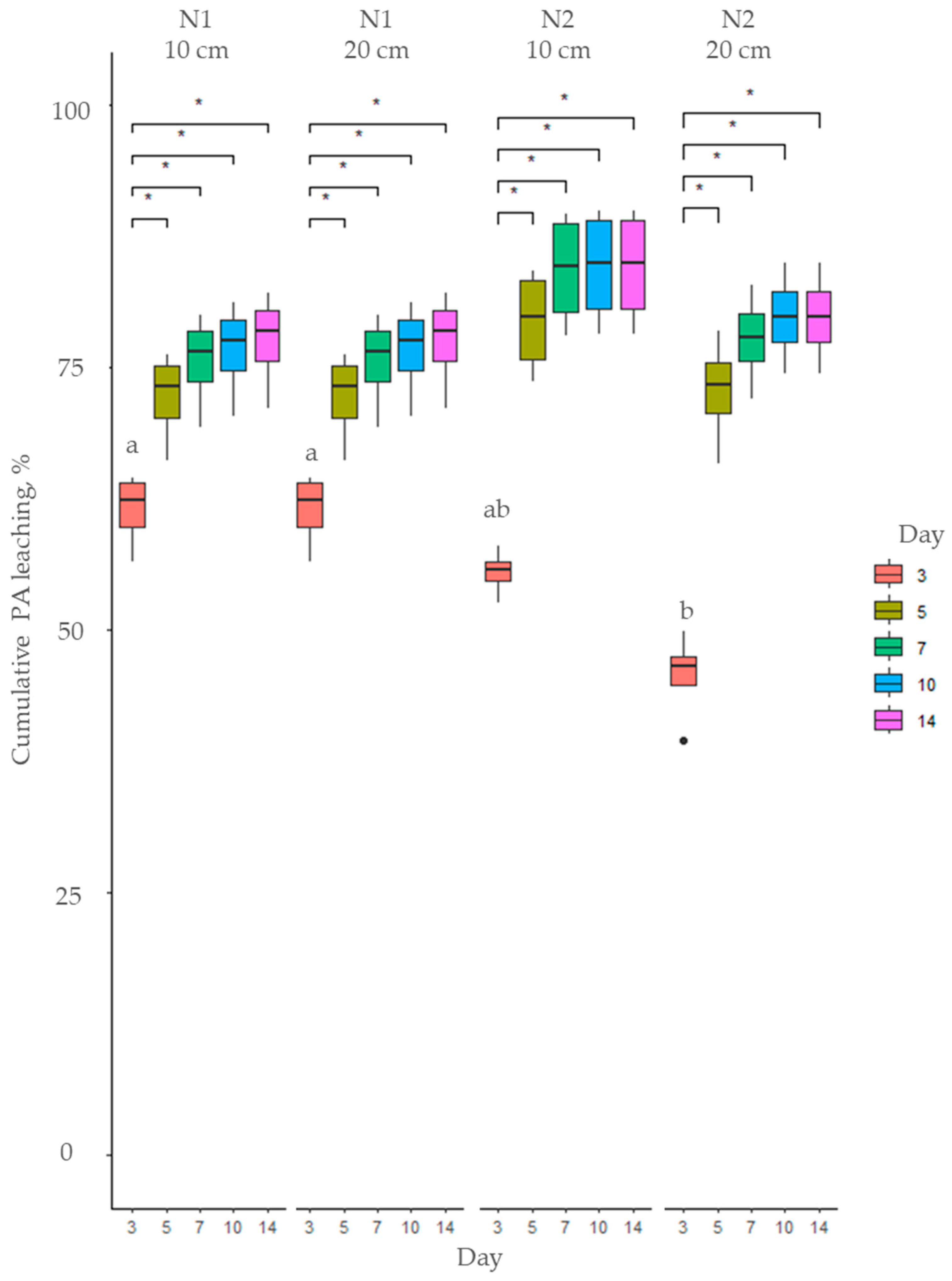
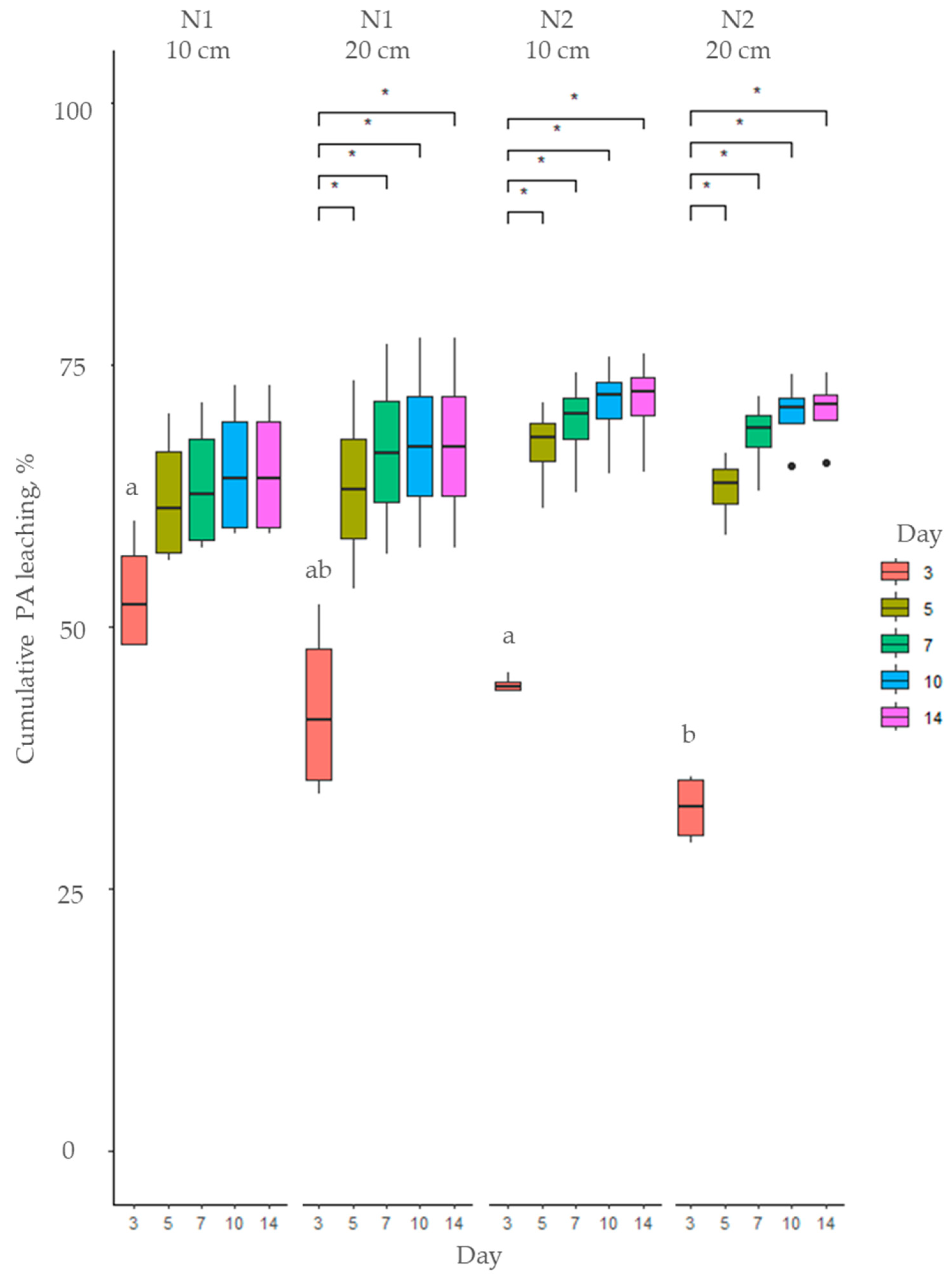
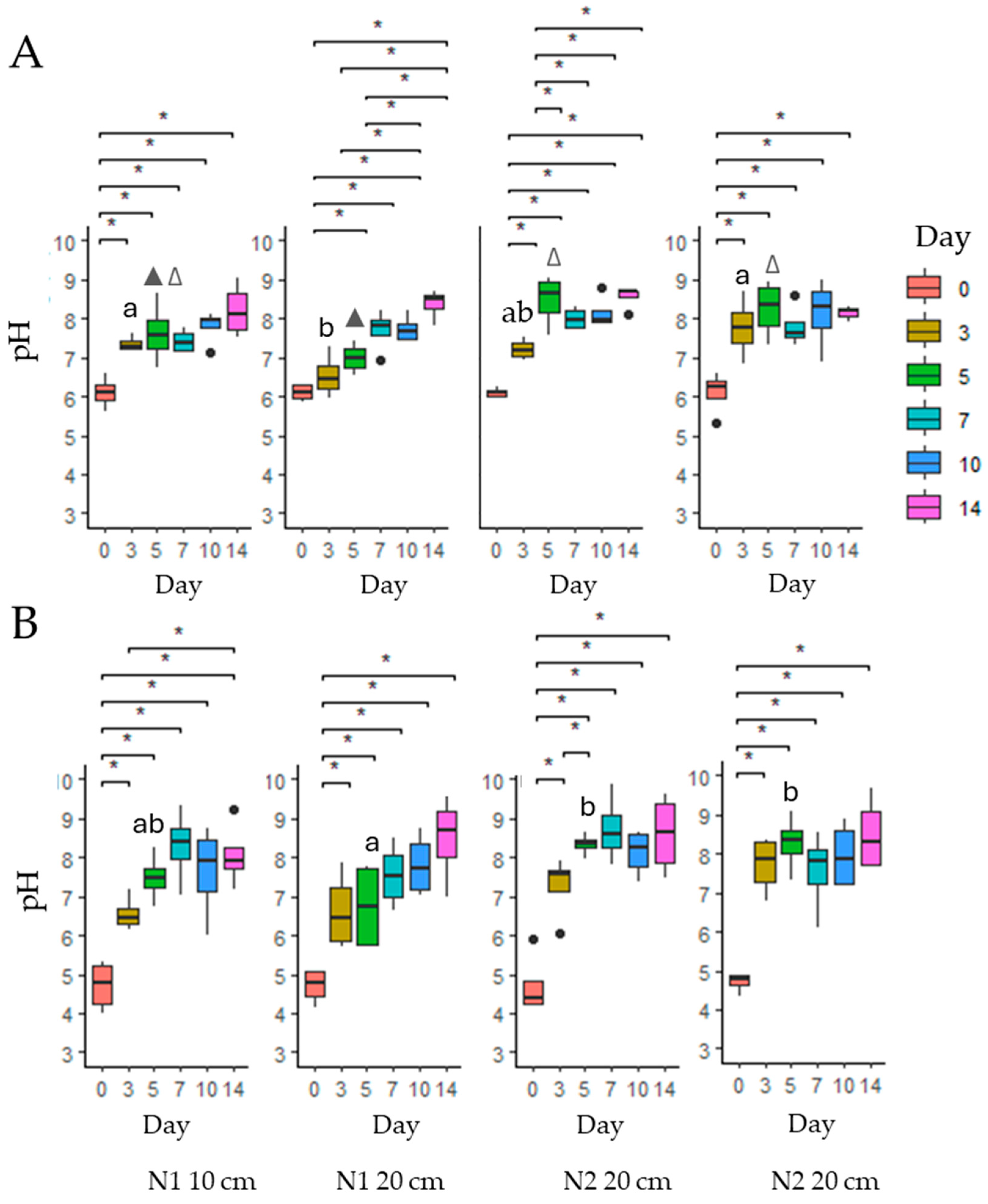


| Extract Type | Soil | Soil Layer | Hs | HsNO | ImNO |
|---|---|---|---|---|---|
| Cold extract | N1 | 10 cm | 3.54 ± 3.54 a | 0.00 ± 0.00 | |
| N1 | 20 cm | 1.12 ± 1.12 b | |||
| N2 | 10 cm | 1.84 ± 1.84 c | |||
| N2 | 20 cm | 3.62 ± 3.62 a | |||
| Hot extract | N1 | 10 cm | 6.48 ± 6.48 de | ||
| N1 | 20 cm | 7.14 ± 7.14 d | |||
| N2 | 10 cm | 5.91 ± 5.91 e | |||
| N2 | 20 cm | 8.79 ± 8.79 f | |||
| Extract Type | Soil | Soil Layer | Ir | Sp | Us | IrNO | SpNO | UsNO |
|---|---|---|---|---|---|---|---|---|
| Cold extract | N1 | 10 cm | 16.38 ± 1.61 a | 4.42 ± 0.54 a | 4.16 ± 0.35 a | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| N1 | 20 cm | 26.01 ± 2.45 b | 9.37 ± 0.32 b | 6.83 ± 0.80 b | 0.00 ± 0.00 a | |||
| N2 | 10 cm | 2.37 ± 0.31 c | 6.50 ± 0.69 c | 1.39 ± 0.11 c | ||||
| N2 | 20 cm | 6.17 ± 0.62 d | 1.43 ± 0.19 d | 2.76 ± 0.14 d | ||||
| Hot extract | N1 | 10 cm | 27.43 ± 2.19 b | 5.92 ± 0.61 c | 4.69 ± 0.36 a | 0.08 ± 0.01 b | ||
| N1 | 20 cm | 40.16 ± 2.85 e | 7.35 ± 0.51 e | 6.92 ± 1.17 b | 0.13 ± 0.01 c | |||
| N2 | 10 cm | 4.34 ± 0.09 cd | 0.76 ± 0.04 f | 1.17 ± 0.17 c | 0.08 ± 0.01 b | |||
| N2 | 20 cm | 12.42 ± 0.90 f | 2.21 ± 0.26 g | 2.92 ± 0.25 d | 0.13 ± 0.01 c |
| PA | tR, min | [M+H]t+ | [M+H]p+ | Δ, ppm | a | b | r2 | LOQ, ng mL−1 | Esterification |
|---|---|---|---|---|---|---|---|---|---|
| Intermedine-N-oxide | 4.70 | 316.1755 | 316.1753 | −0.64 | 7086 | −59125 | 0.9992 | 7.09 | ME |
| Seneciphylline | 5.13 | 334.1649 | 334.1653 | 1.20 | 8238 | −24026 | 0.9987 | 5.00 | CDE |
| Integerrimine | 5.60 | 336.1805 | 336.1814 | 2.68 | 7671 | −84340 | 0.9962 | 8.96 | CDE |
| Seneciphylline-N-oxide | 5.30 | 350.1598 | 350.1599 | 0.29 | 6297 | −25866 | 0.9990 | 2.14 | CDE |
| Usuramine | 4.91 | 352.1755 | 352.1756 | 0.28 | 3421 | −25668 | 0.9966 | 5.11 | CDE |
| Integerrimine-N-oxide | 5.79 | 352.1755 | 352.1760 | 1.42 | 5540 | −34947 | 0.9988 | 4.39 | CDE |
| Usuramine-N-oxide | 5.06 | 368.1704 | 368.1714 | 2.72 | 2055 | −14862 | 0.9928 | 9.71 | CDE |
| Heliosupine | 6.26 | 398.2173 | 398.2163 | −2.51 | 7691 | −35718 | 0.9930 | 3.59 | DE |
| Heliosupine-N-oxide | 6.77 | 414.2122 | 414.2123 | 0.24 | 6403 | −12066 | 0.9957 | 5.50 | DE |
| Soil Properties | Soil N1 | Soil N2 |
|---|---|---|
| * pH (KCl) | 4.9 | 7.5 |
| * pH (H2O) | 6.0 | 8.3 |
| Organic matter, % | 2.5 | 1.9 |
| ** P2O2, mg kg−1 | 124 | 159 |
| ** K2O, mg kg−1 | 119 | 139 |
| ** Mg, mg kg−1 | 82 | 1003 |
| ** Ca, mg kg−1 | 783 | 2259 |
| B, mg kg−1 | 0.8 | 0.8 |
| ** Cu, mg kg−1 | 2.1 | 2.1 |
| ** Mn, mg kg−1 | 65.5 | 102.0 |
| ** Zn, mg kg−1 | 1.2 | 3.5 |
| ** S-SO4, mg kg−1 | <1.0 | 2.0 |
| ** Fe, mg kg−1 | 633 | 571 |
| ** Na, mg kg−1 | 2.0 | 2.2 |
| *** CEC, cmol (+)/kg | 4.7 | 19.7 |
| **** Sand, % | 73 | 48 |
| **** Silt, % | 18 | 43 |
| **** Clay, % | 9 | 9 |
| **** Soil Type | sandy loam | loam |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakurte, I.; Skudriņš, G.; Mežaka, I. Fate of Pyrrolizidine Alkaloids in Soil: Insights from Myosotis arvensis L. and Senecio vulgaris L. Toxins 2025, 17, 335. https://doi.org/10.3390/toxins17070335
Nakurte I, Skudriņš G, Mežaka I. Fate of Pyrrolizidine Alkaloids in Soil: Insights from Myosotis arvensis L. and Senecio vulgaris L. Toxins. 2025; 17(7):335. https://doi.org/10.3390/toxins17070335
Chicago/Turabian StyleNakurte, Ilva, Gundars Skudriņš, and Ieva Mežaka. 2025. "Fate of Pyrrolizidine Alkaloids in Soil: Insights from Myosotis arvensis L. and Senecio vulgaris L." Toxins 17, no. 7: 335. https://doi.org/10.3390/toxins17070335
APA StyleNakurte, I., Skudriņš, G., & Mežaka, I. (2025). Fate of Pyrrolizidine Alkaloids in Soil: Insights from Myosotis arvensis L. and Senecio vulgaris L. Toxins, 17(7), 335. https://doi.org/10.3390/toxins17070335





