The Emerging Fusarium graminearum NA3 Population Produces High Levels of Mycotoxins in Wheat and Barley
Abstract
1. Introduction
2. Results
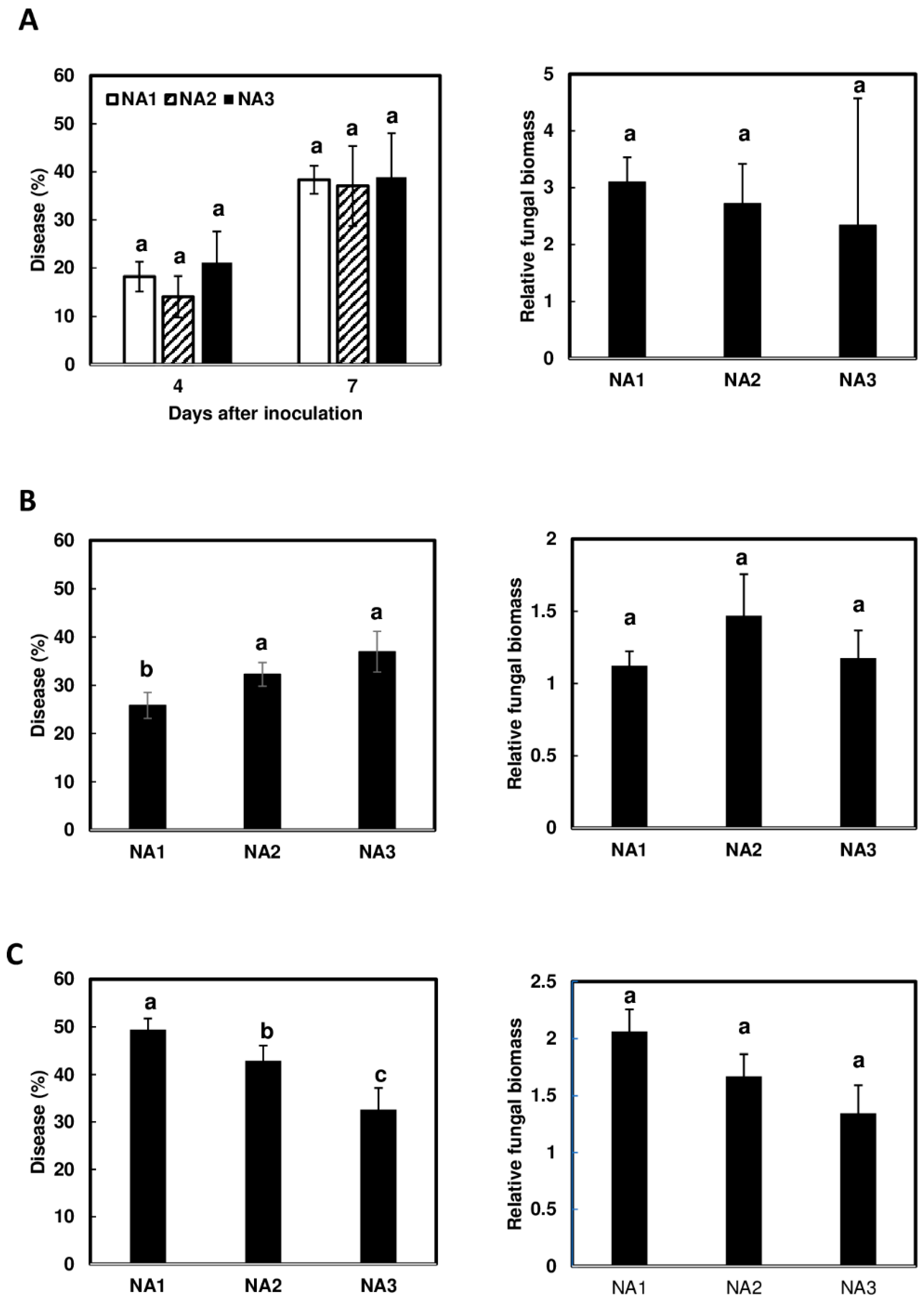
2.1. No Effect of Fg Populations on Initial Infection in Wheat cv. Alsen
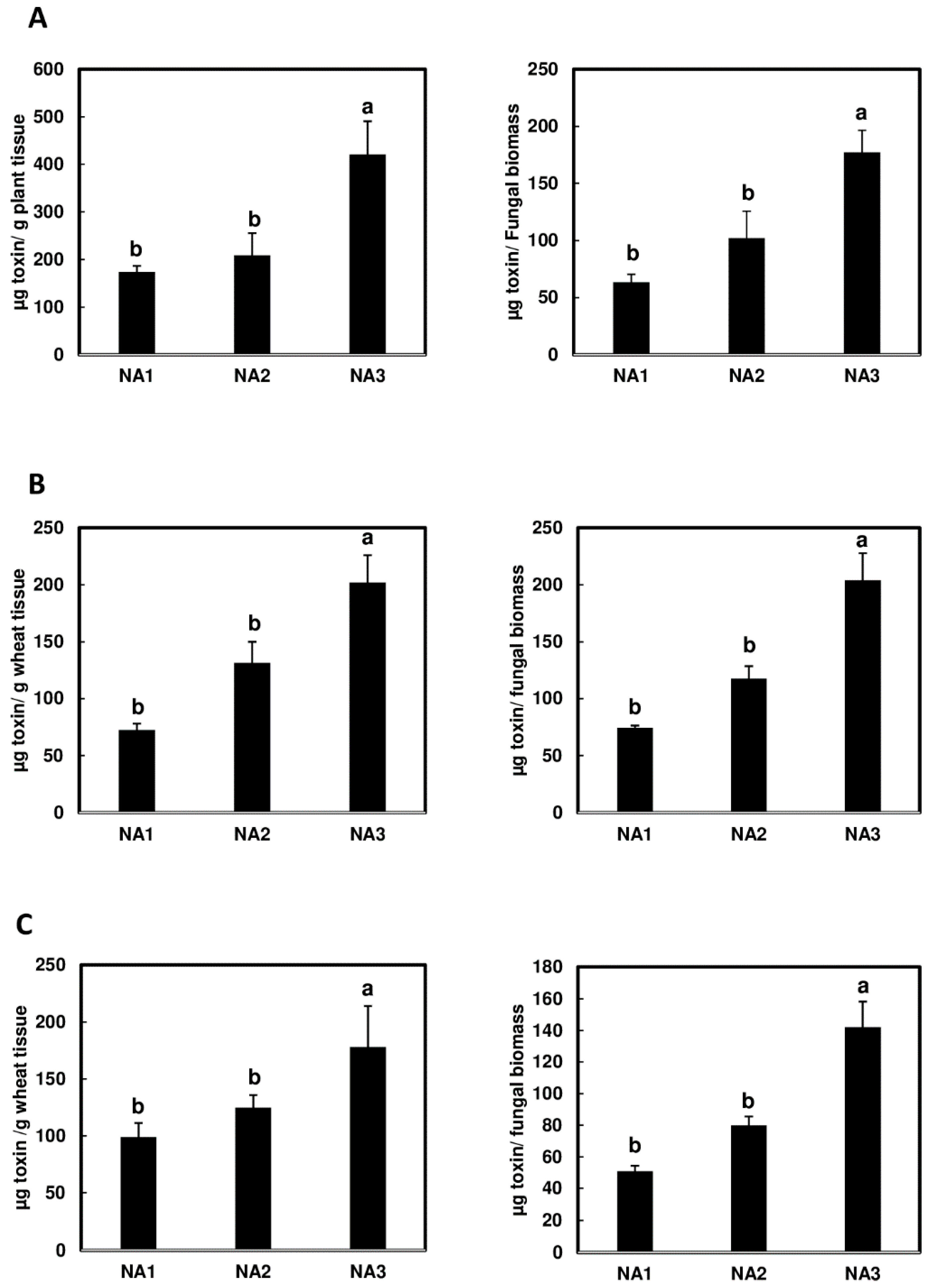
2.2. Significantly Higher Levels of NX-3 Toxin Detected in Wheat Spikes during Initial Infection
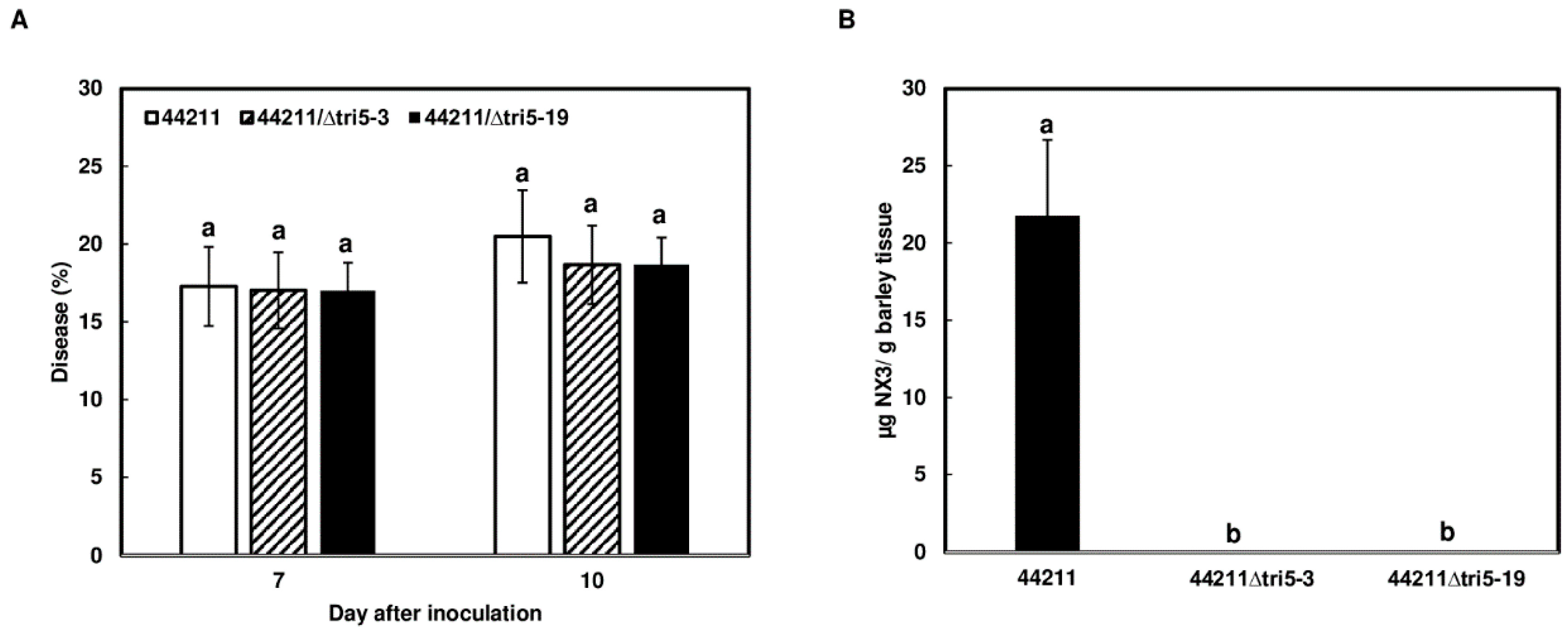
2.3. NX Toxin Is Not Involved in Barley Infection
2.4. NA1 Population Displays High Virulence during Infection of the Barley cv. Voyager
2.5. NA3 Population Produces Higher Levels of Toxin per Unit of Fungal Biomass in Barley Compared to NA1 and NA2 Populations
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fusarium Strains and Cultivation
5.2. Cultivation of Wheat and Barley
5.3. FHB Virulence Assays
5.4. Fungal Biomass Quantification
5.5. Trichothecene Detection in Planta
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium head blight in wheat and barley. Rev. Agric. Econ. 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Dey, D.K.; Kang, J.I.; Bajpai, V.K.; Kim, K.; Lee, H.; Sonwal, S.; Simal-Gandara, J.; Xiao, J.B.; Ali, S.; Huh, Y.S.; et al. Mycotoxins in food and feed: Toxicity, preventive challenges, and advanced detection techniques for associated diseases. Crit. Rev. Food Sci. 2023, 63, 8489–8510. [Google Scholar] [CrossRef]
- Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium head blight populations and trichothecene toxin types reveals regional differences in pathogen composition and temporal dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [Google Scholar] [CrossRef]
- Liang, J.; Lofgren, L.; Ma, Z.; Ward, T.J.; Kistler, H.C. Population subdivision of Fusarium graminearum from barley and wheat in the upper midwestern United States at the turn of the century. Phytopathology 2015, 105, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Clear, R.M.; Rooney, A.P.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Tiley, H.; Gutiérrez, S.; Yulfo-Soto, G.; Vaughan, M.M.; Ward, T.J. NX trichothecenes are required for Fusarium graminearum infection of wheat. MPMI 2023, 36, 294–304. [Google Scholar] [CrossRef]
- Pestka, J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Pierron, A.; Neves, M.; Puel, S.; Lippi, Y.; Soler, L.; Miller, J.D.; Oswald, I.P. Intestinal toxicity of the new type A trichothecenes, NX and 3ANX. Chemosphere 2022, 288, 132415. [Google Scholar] [CrossRef]
- Chen, L.Q.; Yang, J.H.; Wang, H.Y.; Yang, X.L.; Zhang, C.K.; Zhao, Z.H.; Wang, J.H. NX toxins: New threat posed by Fusarium graminearum species complex. Trends Food Sci. Technol. 2022, 119, 179–191. [Google Scholar] [CrossRef]
- Woelflingseder, L.; Del Favero, G.; Blazevic, T.; Heiss, E.H.; Haider, M.; Warth, B.; Adam, G.; Marko, D. Impact of glutathione modulation on the toxicity of the Fusarium mycotoxins deoxynivalenol (DON), NX-3 and butenolide in human liver cells. Toxicol. Lett. 2018, 299, 104–117. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Mueller, B.; Groves, C.L.; Smith, D.L. Chemotype and aggressiveness evaluation of Fusarium graminearum and Fusarium culmorum isolates from wheat fields in Wisconsin. Plant Dis. 2021, 105, 3686–3693. [Google Scholar] [CrossRef]
- Oghenekaro, A.O.; Oviedo-Ludena, M.A.; Serajazari, M.; Wang, X.; Henriquez, M.A.; Wenner, N.G.; Kuldau, G.A.; Navabi, A.; Kutcher, H.R.; Fernando, W.G.D. Population genetic structure and chemotype diversity of Fusarium graminearum populations from wheat in Canada and north eastern United States. Toxins 2021, 13, 180. [Google Scholar] [CrossRef]
- Puri, K.D.; Yan, C.; Leng, Y.; Zhong, S. RNA-Seq revealed differences in transcriptomes between 3ADON and 15ADON populations of Fusarium graminearum in vitro and in planta. PLoS ONE 2016, 11, e0163803. [Google Scholar] [CrossRef]
- Gale, L.R.; Harrison, S.A.; Ward, T.J.; O’Donnell, K.; Milus, E.A.; Gale, S.W.; Kistler, H.C. Nivalenol-type populations of Fusarium graminearum and F. asiaticum are prevalent on wheat in southern Louisiana. Phytopathology 2011, 101, 124–134. [Google Scholar] [CrossRef]
- Kelly, A.; Proctor, R.H.; Belzile, F.; Chulze, S.N.; Clear, R.M.; Cowger, C.; Elmer, W.; Lee, T.; Obanor, F.; Waalwijk, C.; et al. The geographic distribution and complex evolutionary history of the NX-2 trichothecene chemotype from Fusarium graminearum. Fungal Genet. Biol. 2016, 95, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.; Riddle, J.; Dong, Y.; Kuhnem, P.R.; Cummings, J.A.; Del Ponte, E.M.; Bergstrom, G.C.; Kistler, H.C. A high proportion of NX-2 genotype strains are found among Fusarium graminearum isolates from northeastern New York State. Eur. J. Plant Pathol. 2018, 150, 791–796. [Google Scholar] [CrossRef]
- Varga, E.; Wiesenberger, G.; Hametner, C.; Ward, T.J.; Dong, Y.; Schöfbeck, D.; McCormick, S.; Broz, K.; Stückler, R.; Schuhmacher, R.; et al. New tricks of an old enemy: Isolates of Fusarium graminearum produce a type A trichothecene mycotoxin. Environ. Microbiol. 2015, 17, 2588–2600. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hohn, T.M.; Mccormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe 1995, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, M.; Zhang, J.; Yang, X.; Abdallah, M.F.; Wang, J. Phylogenetic variation of Tri1 gene and development of PCR-RFLP analysis for the identification of NX genotypes in Fusarium graminearum species complex. Toxins 2023, 15, 692. [Google Scholar] [CrossRef] [PubMed]
- Zeller, K.A.; Bowden, R.L.; Leslie, J.F. Diversity of epidemic populations of Gibberella zeae from small quadrats in Kansas and North Dakota. Phytopathology 2003, 93, 874–880. [Google Scholar] [CrossRef]
- Fulcher, M.R.; Winans, J.B.; Quan, M.; Oladipo, E.D.; Bergstrom, G.C. Population genetics of Fusarium graminearum at the interface of wheat and wild grass communities in New York. Phytopathology 2019, 109, 2124–2131. [Google Scholar] [CrossRef]
- Kelly, A.C.; Ward, T.J. Population genomics of Fusarium graminearum reveals signatures of divergent evolution within a major cereal pathogen. PLoS ONE 2018, 13, e0194616. [Google Scholar] [CrossRef]
- Soler, L.; Miller, I.; Terciolo, C.; Hummel, K.; Nöbauer, K.; Neves, M.; Oswald, I.P. Exposure of intestinal explants to NX, but not to DON, enriches the secretome in mitochondrial proteins. Arch. Toxicol. 2022, 96, 2609–2619. [Google Scholar] [CrossRef]
- Woelflingseder, L.; Gruber, N.; Adam, G.; Marko, D. Pro-Inflammatory Effects of NX-3 Toxin Are Comparable to Deoxynivalenol and not Modulated by the Co-Occurring Pro-Oxidant Aurofusarin. Microorganisms 2020, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Bokore, F.E.; Knox, R.E.; DePauw, R.M.; Clarke, F.; Cuthbert, R.D.; Campbell, H.L.; Brûlé-Babel, A.L.; Gilbert, J.; Ruan, Y. Validation of Molecular Markers for Use with Adapted Sources of Fusarium Head Blight Resistance in Wheat. Plant Dis. 2017, 101, 1292–1299. [Google Scholar] [CrossRef]
- Laraba, I.; Ward, T.J.; Cuperlovic-Culf, M.; Azimi, H.; Xi, P.; McCormick, S.P.; Hay, W.T.; Hao, G.; Vaughan, M.M. Insights into the aggressiveness of the emerging North American population 3 (NA3) of Fusarium graminearum. Plant Dis. 2023, 107, 2687–2700. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.K.; Xie, W.; Dill-Macky, R.; Mirocha, C.J. Biosynthesis of deoxynivalenol in spikelets of barley inoculated with macroconidia of Fusarium graminearum. Plant Dis. 2000, 84, 654–660. [Google Scholar] [CrossRef]
- Jansen, C.; von Wettstein, D.; Schäfer, W.; Kogel, K.-H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef]
- Tucker, J.R.; Legge, W.G.; Maiti, S.; Hiebert, C.W.; Simsek, S.; Yao, Z.; Xu, W.; Badea, A.; Fernando, W.G.D. Transcriptome alterations of an in vitro-selected, moderately resistant, two-Row malting barley in response to 3ADON, 15ADON, and NIV chemotypes of Fusarium graminearum. Front. Plant Sci. 2021, 12, 701969. [Google Scholar] [CrossRef]
- Foroud, N.A.; McCormick, S.P.; MacMillan, T.; Badea, A.; Kendra, D.F.; Ellis, B.E.; Eudes, F. Greenhouse studies reveal increased aggressiveness of emergent Canadian Fusarium graminearum chemotypes in wheat. Plant Dis. 2012, 96, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Clear, R.M.; Ward, T.J.; Gaba, D.; Tekauz, A.; Turkington, T.K.; Woods, S.M.; Nowicki, T.; O’Donnell, K. Relative aggressiveness and production of 3- or 15-acetyl deoxynivalenol and deoxynivalenol by Fusarium graminearumin spring wheat. Can. J. Plant Pathol. 2010, 32, 146–152. [Google Scholar] [CrossRef]
- Bowden, R.L.; Leslie, J.F. Nitrate-Nonutilizing Mutants of Gibberella zeae (Fusarium graminearum) and Their Use in Determining Vegetative Compatibility. Exp. Mycol. 1992, 16, 308–315. [Google Scholar] [CrossRef]
- Trail, F.; Common, R. Perithecial development by Gibberella zeae: A light microscopy study. Mycologia 2000, 92, 130–138. [Google Scholar] [CrossRef]
- Gale, L.R.; Ward, T.J.; Balmas, V.; Kistler, H.C. Population subdivision of Fusarium graminearum sensu stricto in the upper midwestern United States. Phytopathology 2007, 97, 1434–1439. [Google Scholar] [CrossRef]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef]
- Alouane, T.; Rimbert, H.; Bormann, J.; González-Montiel, G.A.; Loesgen, S.; Schäfer, W.; Freitag, M.; Langin, T.; Bonhomme, L. Comparative genomics of eight Fusarium graminearum strains with contrasting aggressiveness reveals an expanded open pangenome and extended effector content signatures. Int. J. Mol. Sci. 2021, 22, 6257. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Ward, T.J.; Van Coller, G.J.; Flett, B.; Lamprecht, S.C.; O’Donnell, K.; Viljoen, A. Analysis of the Fusarium graminearum species complex from wheat, barley and maize in South Africa provides evidence of species-specific differences in host preference. Fungal Genet. Biol. 2011, 48, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Wiesenberger, G.; Woelflingseder, L.; Twaruschek, K.; Hametner, C.; Vaclaviková, M.; Malachová, A.; Marko, D.; Berthiller, F.; Adam, G. Less-toxic rearrangement products of NX-toxins are formed during storage and food processing. Toxicol. Lett. 2018, 284, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.X.; Naumann, T.A.; Chen, H.; Bai, G.H.; Mccormick, S.; Kim, H.S.; Tian, B.; Trick, H.N.; Naldrett, M.J.; Proctor, R. Fusarium graminearum Effector FgNls1 targets plant nuclei to induce wheat head blight. Mol. Plant Microbe 2023, 36, 478–488. [Google Scholar] [CrossRef]
- Hao, G.X.; McCormick, S.; Usgaard, T.; Tiley, H.; Vaughan, M.M. Characterization of three effectors and their roles during Fusarium head blight. Front. Plant Sci. 2020, 11, 579553. [Google Scholar] [CrossRef]

| NRRL # | Alternate Name | Population | Chemotype | Isolate Location | Host | Reference |
|---|---|---|---|---|---|---|
| GZ3639 | NA1 | 15ADON | Kansas (KS), USA | Wheat | [37] | |
| PH1 | NA1 | 15ADON | Michigan (MI), USA | Wheat | [38] | |
| 64387 | 13MN1-6 | NA1 | 15ADON | Minnesota (MN), USA | Wheat | [4] |
| 64388 | F333 | NA1 | 15ADON | North Dakota (ND), USA | Wheat | [4] |
| 38746 | ON-05-85 | NA1 | 15ADON | Ontario (ON), Canada | Wheat | [3] |
| 38581 | Q-05-105 | NA2 | 3ADON | Quebec (QC), Canada | Wheat | [3] |
| 37525 | S8A-04-2 | NA2 | 3ADON | Saskatchewan (SK), Canada | Wheat | [26] |
| 38964 | ON-05-92 | NA2 | 3ADON | Ontario (ON), Canada | Wheat | [3] |
| 38763 | Q-05-17 | NA2 | 3ADON | Quebec (QC), Canada | Wheat | [3] |
| 46422 | 00-566 | NA2 | 3ADON | Minnesota (MN), USA | Wheat | [39] |
| 44211 | 5B-06-4 | NA3 | NX2 | Saskatchewan (SK), Canada | Wheat | [3] |
| 43884 | ON-06-4 | NA3 | NX2 | Ontario (ON), Canada | Wheat | [3] |
| 43161 | M11-05-oat4 | NA3 | NX2 | Manitoba (MB), Canada | Oat | [3] |
| 66044 | 03-348 | NA3 | NX2 | North Dakota (ND), USA | Wheat | [4] |
| 64394 | F322 | NA3 | NX2 | Minnesota (MN), USA | Wheat | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhoades, N.A.; McCormick, S.P.; Vaughan, M.M.; Hao, G. The Emerging Fusarium graminearum NA3 Population Produces High Levels of Mycotoxins in Wheat and Barley. Toxins 2024, 16, 408. https://doi.org/10.3390/toxins16090408
Rhoades NA, McCormick SP, Vaughan MM, Hao G. The Emerging Fusarium graminearum NA3 Population Produces High Levels of Mycotoxins in Wheat and Barley. Toxins. 2024; 16(9):408. https://doi.org/10.3390/toxins16090408
Chicago/Turabian StyleRhoades, Nicholas A., Susan P. McCormick, Martha M. Vaughan, and Guixia Hao. 2024. "The Emerging Fusarium graminearum NA3 Population Produces High Levels of Mycotoxins in Wheat and Barley" Toxins 16, no. 9: 408. https://doi.org/10.3390/toxins16090408
APA StyleRhoades, N. A., McCormick, S. P., Vaughan, M. M., & Hao, G. (2024). The Emerging Fusarium graminearum NA3 Population Produces High Levels of Mycotoxins in Wheat and Barley. Toxins, 16(9), 408. https://doi.org/10.3390/toxins16090408





