The Complex Role of Botulinum Toxin in Enhancing Goal Achievement for Post-Stroke Patients
Abstract
1. Introduction
2. Results
- -
- The number of prior injections at the wrist, with an additional injection being associated with an increase in the improved GAS-T score of 0.93 points.
- -
- Improved proximal motor control, with an increase of 1 point being associated with an increase in the improved GAS-T score of 3.3 points.
- -
- Improved intermediate motor control, with an increase of 1 point being associated with an increase in the improved GAS-T score of 3.3 points.
- -
- Spasticity decrease across all muscle groups (overall upper limb spasticity reduction) of the affected upper limb;
- -
- Overall pain decrease at the affected upper limb (shoulder/elbow/wrist);
- -
- Number of prior BoNT-A injections of each patient;
- -
- Improvement in motor control of the affected upper limb (proximal, intermediate, distal); composite variables (baseline and follow-up) were employed, quantifying the mentioned parameters at all levels of the affected upper limb.
3. Discussion
3.1. Role of BoNT-A Treatment in GAS
3.2. Influence of Motor Control over Goal Setting and Achieving
3.3. Pain Assessment, Management and Impact on GAS
3.4. Splints and Orthoses as Adjunctive Therapy
3.5. Oral Antispasticity Medication
3.6. Study Limitations
3.7. Future Perspectives
4. Conclusions
5. Materials and Methods
5.1. Study Design
5.2. Patient Selection
5.2.1. Inclusion Criteria
- Patients who have fulfilled the written informed consent;
- Age ≥ 18 and ≤80 years;
- Hemiparesis due to a single stroke occurred ≥2 months before the assessment;
- Presence of muscle hypertonia of shoulder, elbow, wrist, and/or finger level;
- Clinical assessment performed just before (T0 = baseline, at the moment of inpatient hospital admission) and after BoNT-A treatment (T1 = follow-up evaluation at 20 days ± 5 days after hospital discharge), which included: (a) motor control at shoulder, elbow, wrist, and fingers levels; (b) pain perceived in shoulder, elbow, wrist, and fingers during passive mobilization; (c) muscle tone of pectoralis major, elbow, wrist and finger flexors; and (d) goal setting and GAS assessment.
5.2.2. Exclusion Criteria
- Use of intrathecal baclofen [60];
- Patients who experienced adverse effects from previous BoNT-A injections (e.g., myalgia, muscle weakness, asthenia, flu-like syndrome, local reactions at the injection site, etc.);
- Severe cognitive impairment;
- Severe aphasia interfering with patient’s assessment;
- Degree of spasticity <= 1 or 4 on MAS (Modified Ashworth Scale);
- Patients who have refused the written informed consent.
5.3. Intramuscular Diffusion of BoNT-A
5.4. GAS Assessment
- 1.a.
- Motor control;
- 1.b.
- Joint pain;
- 1.c.
- Joint mobility.
5.5. Muscle Tone Assessment
5.6. Pain Assessment
5.7. Motor Control Assessment
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wade, D.T. Goal setting in rehabilitation: An overview of what, why and how. Clin. Rehabil. 2009, 23, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.D.; Siegert, R.; Levack, W.; Freeman, J. Areas of consensus and controversy about goal setting in rehabilitation: A conference report. Clin. Rehabil. 2009, 23, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Plant, S.E.; Tyson, S.F.; Kirk, S.; Parsons, J. What are the barriers and facilitators to goal-setting during rehabilitation for stroke and other acquired brain injuries? A systematic review and meta-synthesis. Clin. Rehabil. 2016, 30, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Scobbie, L.; Brady, M.C.; Duncan, E.A.S.; Wyke, S. Goal attainment, adjustment and disengagement in the first year after stroke: A qualitative study. Neuropsychol. Rehabil. 2021, 31, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Kiresuk, T.; Sherman, R. Goal attainment scaling: A general method 14. of evaluating comprehensive mental health programmes. Com. Mental Health J. 1968, 4, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Fheodoroff, K. Goal Setting with ICF (International Classification of Functioning, Disability and Health) and Multidisciplinary Team Approach in Stroke Rehabilitation. In Clinical Pathways in Stroke Rehabilitation: Evidence-Based Clinical Practice Recommendations [Internet]; Platz, T., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Lance, J. 1980 symposium synopsis. In Spasticity Disordered Motor Control; Feldman, R.G., Feldman, G., Young, R.R., Koella, W.P., Eds.; Year Book Medical Publishers: Chicago, IL, USA, 1980; pp. 485–494. [Google Scholar]
- Gracies, J. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 2005, 31, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.; Gregoric, M.; Barnes, M.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G. Spasticity: Clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef]
- Sheean, G. The pathophysiology of spasticity. Eur. J. Neurol. 2002, 9, 3–9. [Google Scholar] [CrossRef]
- Potcovaru, C.G.; Salmen, T.; Chitu, M.C.; Dima, V.; Mihai, M.B.; Bohiltea, R.E.; Cinteza, D.; Berteanu, M. Assessment tools of disability status after stroke. Rom. J. Neurol. 2022, 21, 208–212. [Google Scholar] [CrossRef]
- Bhakta, B.B.; Cozens, J.A.; Chamberlain, M.A.; Bamford, J.M. Impact of 2. botulinum toxin type A on disability and carer burden due to arm spas-ticity after stroke: A randomised double blind placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2000, 69, 217–221, Erratum in J. Neurol. Neurosurg. Psychiatry 2001, 70, 821. [Google Scholar] [CrossRef]
- Bhakta, B.B.; Cozens, J.A.; Bamford, J.M.; Chamberlain, M.A. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J. Neurol. Neurosurg. Psychiatry 1996, 61, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bakheit, A.M.; Sawyer, J. The effects of botulinum toxin treatment 1. on associated reactions of the upper limb on hemiplegic gait—A pilot study. Disabil. Rehabil. 2002, 24, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Do, M.; Lee, C.-H.; Jenkins, S.; Turkel, C. Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke [comment]. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Jahnke, M.; Luecke, D.; Mauritz, K. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci. Lett. 1995, 201, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Edwards, S.; Sheean, G.L.; Greenwood, R.J.; Thompson, A.J. The effect of botulinum toxin on hand function after incomplete spinal cord injury at the level of C5/6: A case report. Clin. Rehabil. 1997, 11, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, A.A.; McGinn, M.; Chappell, R. Botulinum toxin injection of spastic finger flexors in hemiplegic patients. Am. J. Phys. Med. Rehabil. 2000, 79, 44–47. [Google Scholar] [CrossRef]
- Simpson, D.M.; Alexander, D.N.; O’Brien, C.F.; Tagliati, M.; Aswad, A.S.; Leon, J.M.; Gibson, J.; Mordaunt, J.M.; Monaghan, E.P. Botulinum toxin type A in the treatment of upper extremity spasticity: A randomized, double-blind, placebo-controlled trial. Neurology 1996, 46, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Ellis, E.; White, S.; Moore, A.P. A double-blind placebo-controlled study of botulinum toxin in upper limb spasticity after stroke or head injury. Clin. Rehabil. 2000, 14, 5–13. [Google Scholar] [CrossRef]
- Escaldi, S.; Bianchi, F.; Bavikatte, G.; Molteni, F.; Moraleda, S.; Deltombe, T.; Francisco, G.E. Module 1: Pathophysiology and assessment of spasticity; Goal setting. J. Int. Soc. Phys. Rehabil. Med. 2022, 5, S3–S22. [Google Scholar] [CrossRef]
- Jacinto, J.; Camões-Barbosa, A.; Carda, S.; Hoad, D.; Wissel, J. A Practical Guide to Botulinum Neurotoxin Treatment of Shoulder Spasticity 1: Anatomy, Physiology, And Goal Setting. Front. Neurol. 2022, 13, 1004629. [Google Scholar] [CrossRef]
- Gracies, J.; O’Dell, M.; Vecchio, M.; Hedera, P.; Kocer, S.; Rudzinska-Bar, M.; Rubin, B.; Timerbaeva, S.L.; Lusakowska, A.; Boyer, F.C.; et al. Effects of repeated abobotulinumtoxinA injections in upper limb spasticity. Muscle Nerve 2018, 57, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Fheodoroff, K.; Hoonhorst, M.; Müngersdorf, M.; Gallien, P.; Meier, N.; Hamacher, J.; Hefter, H.; Maisonobe, P.; Koch, M. Effectiveness of AbobotulinumtoxinA in Post-Stroke Upper Limb Spasticity in Relation to Timing of Treatment. Front. Neurol. 2020, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; Novak, I.; Cusick, A. Repeat injection of botulinum toxin 20. A is safe and effective for upper limb movement and function in children with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Marinelli, L.; Mori, L.; Bragazzi, N.; Maggi, G.; Cotellessa, F.; Puce, L.; Vestito, L.; Molteni, F.; Gasperini, G.; et al. Increasing the Passive Range of Joint Motion in Stroke Patients Using Botulinum Toxin: The Role of Pain Relief. Toxins 2023, 15, 335. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.C.; Price, C.I.M.; van Wijck, F.M.J.; Shackley, P.; Steen, N.; Barnes, M.P.; Ford, G.A.; Graham, L.A.; Rodgers, H. Botulinum Toxin for the Upper Limb After Stroke (BoTULS) Trial: Effect on Impairment, Activity Limitation, and Pain. Stroke 2011, 42, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Kong, K.H.; Goh, K.J.; Kumthornthip, W.; Mok, V.C.T.; Delgado-De Los Santos, M.M.; Chua, K.S.G.; Abdullah, S.J.B.F.; Zakine, B.; Maisonobe, P.; et al. Botulinum Toxin Injection for Hypertonicity of the Upper Extremity within 12 Weeks after Stroke: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2012, 26, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Camões-Barbosa, A.; Comes, G.; Althaus, M.; Scheschonka, A.; Simpson, D.M. Pain Reduction in Adults with Limb Spasticity Following Treatment with IncobotulinumtoxinA: A Pooled Analysis. Toxins 2021, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Marinelli, L.; Mori, L.; Puce, L.; Avanti, C.; Saretti, E.; Biasotti, G.; Amella, R.; Cotellessa, F.; Restivo, D.A.; et al. Effectiveness of Botulinum Toxin on Pain in Stroke Patients Suffering from Upper Limb Spastic Dystonia. Toxins 2022, 14, 39. [Google Scholar] [CrossRef]
- Pavone, F.; Luvisetto, S. Botulinum Neurotoxin for Pain Management: Insights from Animal Models. Toxins 2010, 2, 2890–2913. [Google Scholar] [CrossRef]
- Wang, J.; Ding, Z.; Xu, W.; He, L.; Huang, J.; Zhang, C.; Guo, Q.; Zou, W. Botulinum Toxin Type A Counteracts Neuropathic Pain by Countering the Increase of GlyT2 Expression in the Spinal Cord of CCI Rats. Brain Res. 2022, 1796, 148095. [Google Scholar] [CrossRef]
- Poenaru, D.; Sandulescu, M.I.; Cinteza, D. Pain Modulation in Chronic Musculoskeletal Disorders: Botulinum Toxin, a Descriptive Analysis. Biomedicines 2023, 11, 1888. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians, British Society of Rehabilitation Medicine, Chartered Society of Physiotherapy, Association of Chartered Physiotherapists Interested in Neurology. Spasticity in Adults: Management Using Botulinum Toxin. National Guide-lines; RCP: London, UK, 2009. [Google Scholar]
- Hawthorne, G.; Richardson, J.; Osborne, R. The Assessment of Quality 29. of Life (AQoL) instrument: A psychometric measure of health-related quality of life. Qual. Life Res. 1999, 8, 209–224. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Func-33. Tioning, Disability and Health; World Health Organisation: Geneva, Switzerland, 2002. [Google Scholar]
- Potcovaru, C.-G.; Salmen, T.; Bîgu, D.; Săndulescu, M.I.; Filip, P.V.; Diaconu, L.S.; Pop, C.; Ciobanu, I.; Cinteză, D.; Berteanu, M. Assessing the Effectiveness of Rehabilitation Interventions through the World Health Organization Disability Assessment Schedule 2.0 on Disability—A Systematic Review. J. Clin. Med. 2024, 13, 1252. [Google Scholar] [CrossRef]
- Becker, H.; Stuifbergen, A.; Rogers, S.; Timmerman, G. Goal attainment scaling to measure individual change in intervention studies. Nurs. Res. 2000, 49, 176–180. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Turner-Stokes, L.; Baguley, I.J.; De Graaff, S.; Katrak, P.; Sandanam, J.; Davies, L.; Munns, M.; Hughes, A. Botulinum toxin A for treatment of upper limb spasticity following stroke: A multi-centre randomised placebo-controlled study of the effects on quality of life and other person-centred outcomes. J. Rehabil. Med. 2009, 41, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes; Baguley, I.J.; De Graaff, S.; Katrak, P.; Davies, L.; Mccrory, P.; Hughes, A. Goal Attainment Scaling in The Evaluation of Treatment of Upper Limb Spasticity With Botulinum Toxin: A Secondary Analysis from a Double-Blind Placebo-Controlled Randomized Clinical Trial. J. Rehabil. Med. 2010, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.-M.; Lugassy, M.; Weisz, D.J.; Vecchio, M.; Flanagan, S.; Simpson, D.M. Botulinum toxin dilution and endplate targeting in spasticity: A double-blind controlled study. Arch. Phys. Med. Rehabil. 2009, 90, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Turner-Stokes, L.; Allison, R.; Duke, L.; Moore, P.; Bavikatte, G.; Kirker, S.; Ward, T.; Bilton, D. Spasticity in Adults: Management Using Botulinum Toxin. National Guidelines, 2nd ed.; RCP: London, UK, 2018. [Google Scholar]
- DYSPORT® (Abobotulinumtoxina) for Injection, for Intramuscular Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125274s107lbl.pdf (accessed on 7 January 2019).
- Excel Spread Sheet GAS Calculator. Available online: https://www.kcl.ac.uk/cicelysaunders/resources/tools/gas (accessed on 17 October 2022).
- Andringa, A.; van de Port, I.; Meijer, J.-W. Long-term use of a static hand-wrist orthosis in chronic stroke patients: A pilot study. Stroke Res. Treat. 2013, 2013, 546093. [Google Scholar] [CrossRef]
- Alshahrani, A. Oral Antispasticity Drugs and Non-Progressive Neurological Diseases: A Meta-Analysis on Safety and Efficacy. J. Pharm. Bioallied Sci. 2023, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montané, E.; Vallano, A.; Laporte, J.R. Oral antispastic drugs in nonprogressive neurologic diseases. Neurology 2004, 63, 1357–1363. [Google Scholar] [CrossRef]
- Dario, A.; Tomei, G. A Benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf. 2004, 27, 799–818. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraten, M.C.; Ploeg, R.J.O.; Burg, W.V.D.; Vreeling, A.; Marle, S.; Minderhoud, J.M. Tizanidine versus baclofen in the treatment of spasticity in multiple sclerosis patients. Acta Neurol. Scand. 1988, 77, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Chen, L.L. Gabapentin in Pain Management. Obstet. Anesth. Dig. 2000, 91, 680–687. [Google Scholar] [CrossRef]
- Gruenthal, M.; Mueller, M.; Priebe, M.M.; Sherwood, A.M.; Olson, W.H. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord 1997, 35, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Young, R.R.; Delwaide, P.J. Drug therapy: Spasticity (first of two parts). N. Engl. J. Med. 1981, 304, 28–33. [Google Scholar] [PubMed]
- Jang, S.H.; Cho, I.T.; Lim, J.W. Recovery of aphasia and change of injured arcuate fasciculus in the dominant hemisphere in stroke patients. NeuroRehabilitation 2017, 41, 759–764. [Google Scholar] [CrossRef]
- Cinteza, D. Update in physical medicine and rehabilitation: New technologies and robots versus classical training in gait rehabilitation after stroke. Maedica 2011, 6, 160–161. [Google Scholar] [PubMed]
- Poenaru, D.; Cinteza, D.; Petrusca, I.; Cioc, L.; Dumitrascu, D. Local Application of Vibration in Motor Rehabilitation—Scientific and Practical Considerations. Maedica 2016, 11, 227–231. [Google Scholar] [PubMed]
- Struyf, P.; Triccas, L.T.; Schillebeeckx, F.; Struyf, F. The Place of Botulinum Toxin in Spastic Hemiplegic Shoulder Pain after Stroke: A Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 2797. [Google Scholar] [CrossRef]
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef]
- Holmes, R.J.; Connell, L.A. A survey of the current practice of intramuscular Botulinum toxin injections for hemiplegic shoulder pain in the UK. Disabil. Rehabil. 2019, 41, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Nalysnyk, L.; Papapetropoulos, S.; Rotella, P.; Simeone, J.C.; Alter, K.E.; Esquenazi, A. OnabotulinumtoxinA muscle injection patterns in adult spasticity: A systematic literature review. BMC Neurol. 2013, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-R. Intrathecal Baclofen Therapy: Pros and Cons. Ann. Rehabil. Med. 2023, 47, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kistner, K.; Zimmermann, K.; Ehnert, C.; Reeh, P.W.; Leffler, A. The tetrodotoxin-resistant Na+ channel Nav1.8 reduces the potency of local anesthetics in blocking C-fiber nociceptors. Pflügers Arch. Eur. J. Physiol. 2010, 459, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Scholz, A.; Vogel, W. Tetrodotoxin-resistant action potentials in dorsal root ganglion neurons are blocked by local anesthetics. Pain 2000, 89, 47–52. [Google Scholar] [CrossRef]
- Cuvillon, P.; Nouvellon, E.; Ripart, J.; Boyer, J.-C.; Dehour, L.; Mahamat, A.; L’hermite, J.; Boisson, C.; Vialles, N.; Lefrant, J.Y.; et al. A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: A double-blind randomized study. Anesth. Analg. 2009, 108, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Lee, J.; Hur, H.; Lee, H.; Choi, Y.; Kim, H. Distribution of the intramuscular innervation of the triceps brachii: Clinical importance in the treatment of spasticity with botulinum neurotoxin. Clin. Anat. 2023, 36, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Kishner, S. Modified Ashworth Scale. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15, S17–S24. [Google Scholar] [CrossRef]
- Jensen, M.P.; Karoly, P.; Braver, S. The measurement of clinical pain intensity: A comparison of six methods. Pain 1986, 27, 117–126. [Google Scholar] [CrossRef]
- Kremer, E.; Atkinson, H.J.; Ignelzi, R.J. Measurement of pain: Patient preference does not confound pain measurement. Pain 1981, 10, 241–248. [Google Scholar] [CrossRef] [PubMed]
- MRC Scale Link. Available online: https://www.criteria.blood.gov.au/NeurologicalScales/GeneratePDF?section=2 (accessed on 10 January 2019).
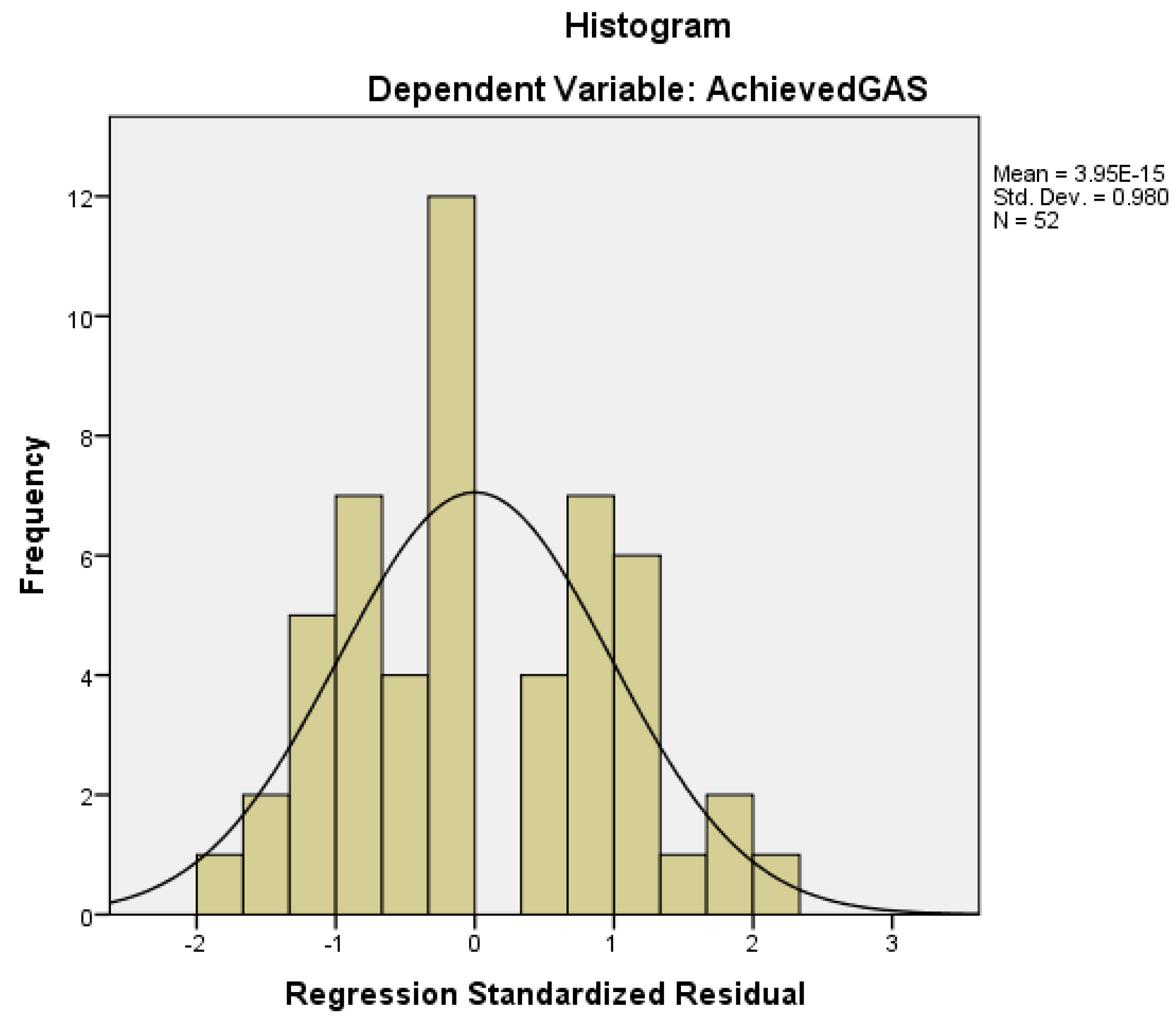
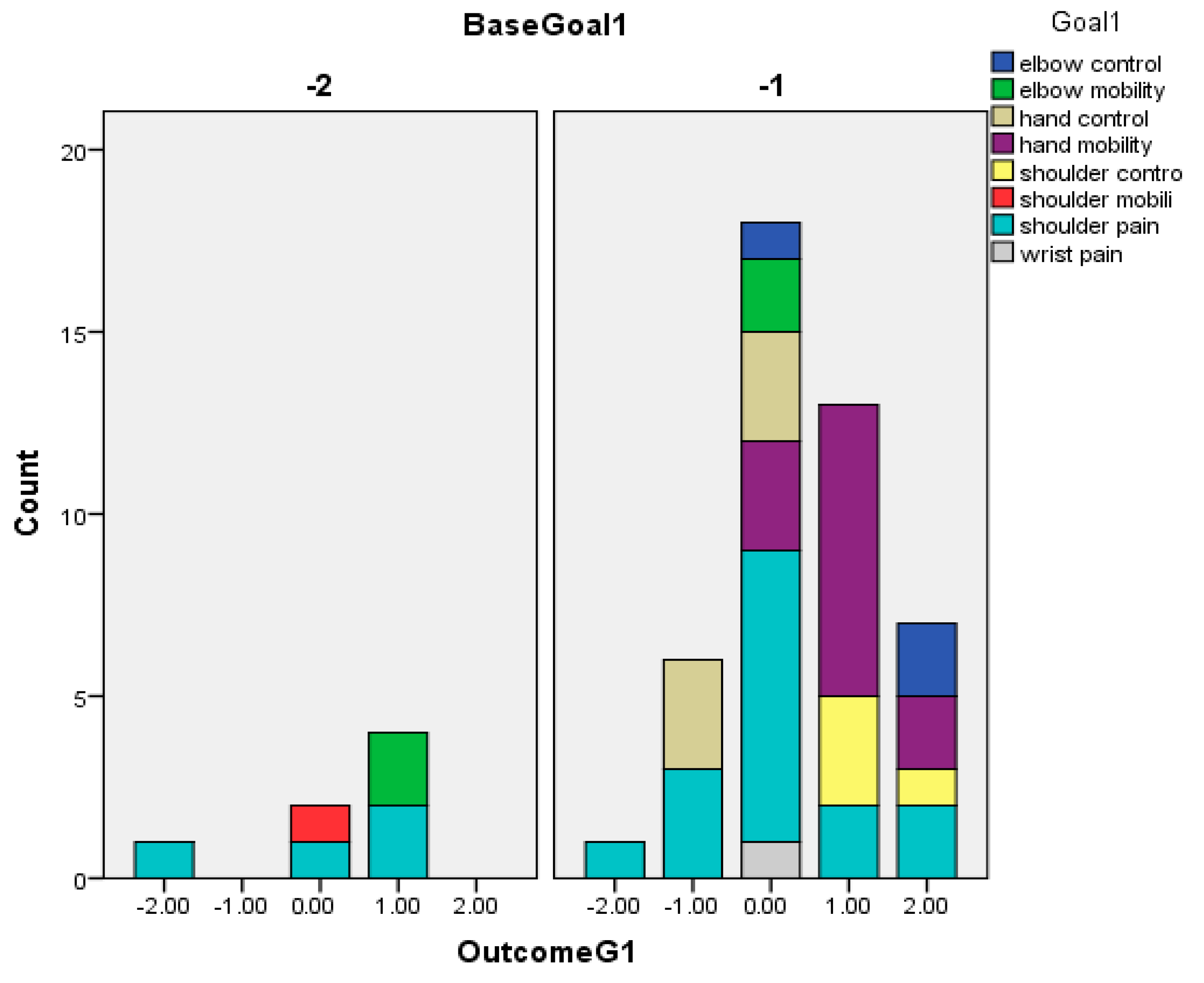

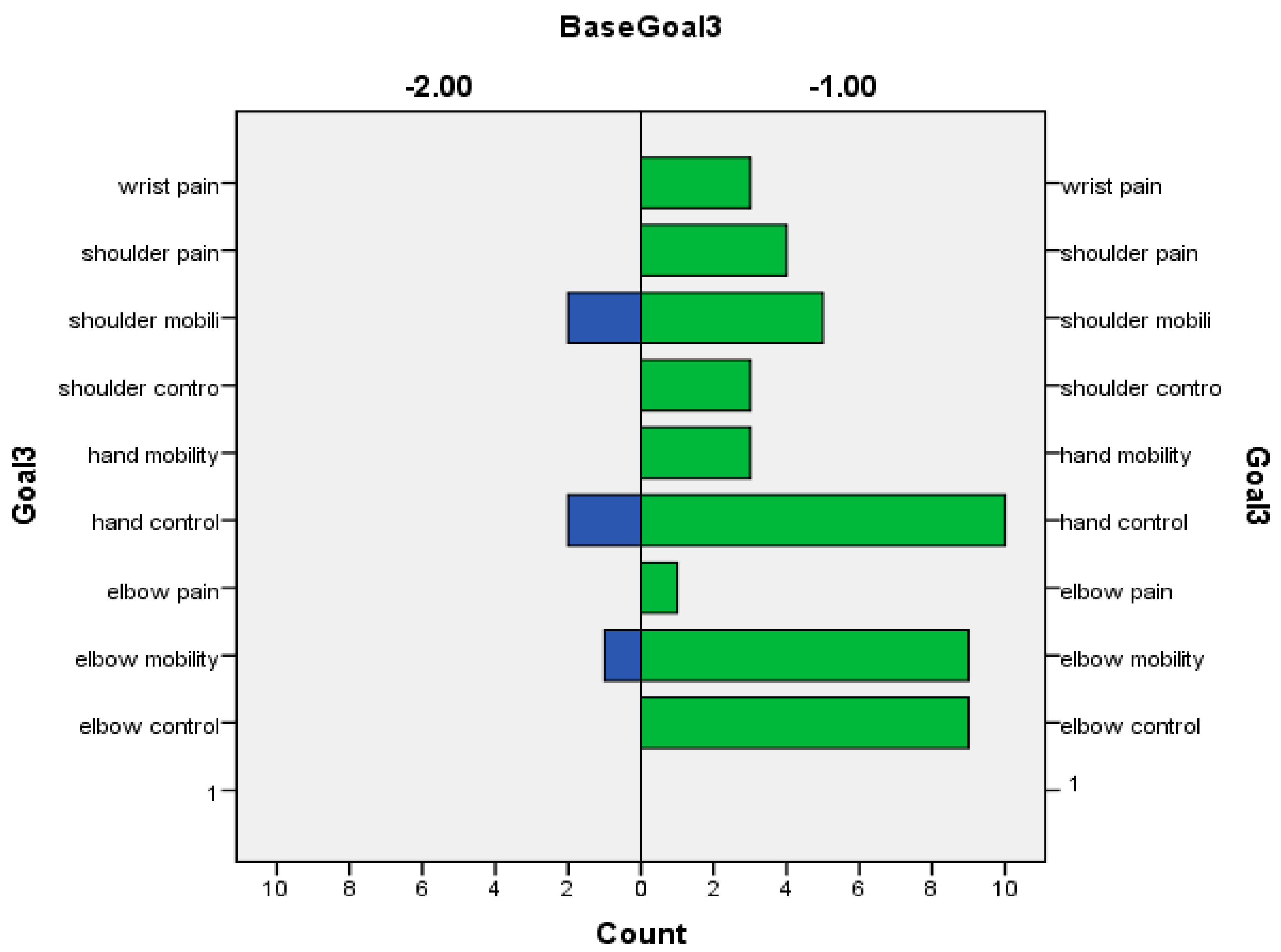

| Variable | N = 52 |
|---|---|
| Sex, n (%) | |
| F | 20 (38) |
| M | 32 (62) |
| Age, Mean (SD) | 56.40 (12.66) |
| Stroke etiology, n (%) | |
| Hemorrhage | 15 (29) |
| Ischemia | 37 (71) |
| Time since stroke onset, Mean (SD) | 16.08 (14.00) months |
| Damaged hemisphere, n (%) | |
| Right | 19 (37) |
| Left | 33 (63) |
| Oral antispastic treatment (baclofenum), n (%) | |
| Yes | 28 (54) |
| No | 24 (46) |
| Prior Nr. of BoNT-A injections (i.e.), Mean (SD) | 1.46 (1.97) |
| Splints/orthoses post-injection, n (%) | |
| Yes | 26 (51) |
| No | 26 (51) |
| FIM score admission, Mean (SD) | 5.04 (1.49) |
| FIM score follow-up, Mean (SD) | 5.23 (1.57) |
| Barthel score admission, Mean (SD) | 75.48 (21.13) |
| Barthel score follow-up, Mean (SD) | 76.92 (21.42) |
| Pectoralis major BoNT-A dose (N = 39), Mean (SD) | 130.51 (48.77) |
| MAS Pectoralis major T0, Mean (SD) | 1.72 (0.85) |
| MAS Pectoralis major T1, Mean (SD) | 0.94 (0.52) |
| Prior Nr. BoNT-A injections shoulder, Mean (SD) | 0.96 (1.56) |
| NRS score shoulder T0, Mean (SD) | 3.04 (1.90) |
| NRS score shoulder T1, Mean (SD) | 1.50 (1.31) |
| Biceps brachii BoNT-A dose, Mean (SD) | 139.02 (59.90) |
| MAS Biceps brachii T0, Mean (SD) | 2.02 (0.54) |
| MAS Biceps brachii T1, Mean (SD) | 1.08 (0.48) |
| Brachialis BoNT-A dose (N = 47), Mean (SD) | 111.70 (45.70) |
| MAS Brachialis T0, Mean (SD) | 1.82 (0.70) |
| MAS Brachialis T1, Mean (SD) | 0.97 (0.51) |
| Brachioradialis BoNT-A dose (N = 40), Mean (SD) | 105.00 (38.89) |
| MAS Brachioradialis T0, Mean (SD) | 1.65 (0.79) |
| MAS Brachioradialis T1, Mean (SD) | 0.91 (0.56) |
| Pronator teres BoNT-A dose (N = 48), Mean (SD) | 119.58 (38.41) |
| MAS Pronator teres T0, Mean (SD) | 1.92 (0.62) |
| MAS Pronator teres T1, Mean (SD) | 1.02 (0.53) |
| Prior Nr. BoNT-A injections elbow, Mean (SD) | 1.42 (1.92) |
| NRS score elbow T0, Mean (SD) | 1.15 (1.38) |
| NRS score elbow T1, Mean (SD) | 0.60 (0.82) |
| Flexor digitorum superficialis BoNT-A dose (N = 51), Mean (SD) | 128.53 (45.36) |
| MAS Flexor digitorum superficialis T0, Mean (SD) | 2.16 (0.56) |
| MAS Flexor digitorum superficialis T1, Mean (SD) | 1.18 (0.46) |
| Flexor digitorum profundus BoNT-A dose (N = 46), Mean (SD) | 103.48 (36.30) |
| MAS Flexor digitorum profundus T0, Mean (SD) | 1.86 (0.59) |
| MAS Flexor digitorum profundus T1, Mean (SD) | 1.07 (0.46) |
| Flexor carpi radialis BoNT-A dose (N = 26), Mean (SD) | 117.88 (27.50) |
| MAS Flexor carpi radialis T0, Mean (SD) | 1.45 (1.00) |
| MAS Flexor carpi radialis T1, Mean (SD) | 0.99 (0.53) |
| Flexor carpi ulnaris BoNT-A dose (N = 14), Mean (SD) | 99.29 (25.86) |
| MAS Flexor carpi ulnaris T0, Mean (SD) | 0.85 (0.90) |
| MAS Flexor carpi ulnaris T1, Mean (SD) | 0.56 (0.60) |
| Prior Nr. BoNT-A injections wrist, Mean (SD) | 1.37 (1.85) |
| NRS score wrist T0, Mean (SD) | 0.92 (1.37) |
| NRS score wrist T1, Mean (SD) | 0.52 (0.78) |
| Flexor pollicis longus BoNT-A dose (N = 16), Mean (SD) | 79.69 (24.53) |
| MAS Flexor pollicis longus T0, Mean (SD) | 0.88 (1.06) |
| MAS Flexor pollicis longus T1, Mean (SD) | 0.48 (0.54) |
| Improved proximal motor control, Mean (SD) | 0.69 (0.64) |
| Improved intermediate motor control, Mean (SD) | 1.00 (0.66) |
| Improved distal motor control, Mean (SD) | 0.62 (0.60) |
| Outcome of Changed GAS-T score, Mean (SD) | 19.33 (3.73) |
| FIM score dif., Mean (SD) | 0.19 (0.40) |
| Barthel score dif., Mean (SD) | 1.44 (3.62) |
| NRS shoulder score dif., Mean (SD) | 1.54 (1.26) |
| NRS elbow score dif., Mean (SD) | 0.56 (0.83) |
| NRS wrist score dif., Mean (SD) | 0.40 (0.75) |
| MAS Pectoralis major score dif., Mean (SD) | 0.78 (0.61) |
| MAS Biceps brachii score dif., Mean (SD) | 0.94 (0.43) |
| MAS Brachialis score dif., Mean (SD) | 0.85 (0.48) |
| MAS Pronator teres score dif., Mean (SD) | 0.90 (0.42) |
| MAS Flexor digitorum superficialis score dif., Mean (SD) | 1.10 (0.51) |
| MAS Flexor carpi radialis score dif., Mean (SD) | 0.46 (0.73) |
| MAS Flexor carpi ulnaris score dif., Mean (SD) | 0.29 (0.55) |
| MAS Flexor pollicis longus score dif., Mean (SD) | 0.39 (0.66) |
| Muscle | Number of Injected Patients (Percentage) | Dose of BoNT-A (UI): Mean ± SD |
|---|---|---|
| Shoulder muscles | ||
| Pectoralis major | 39/52 (75%) | 130.51 ± 48.77 |
| Elbow flexor muscles | ||
| Biceps brachii | 51/52 (98%) | 139.02 ± 59.90 |
| Brachialis | 47/52 (90%) | 111.70 ± 45.70 |
| Brachioradialis | 40/52 (77%) | 105.00 ± 38.89 |
| Pronator teres | 48/52 (92%) | 119.58 ± 38.41 |
| Wrist flexor muscles | ||
| Flexor carpi radialis | 26/52 (50%) | 117.88 ± 27.50 |
| Flexor carpi ulnaris | 14/52 (27%) | 99.29 ± 25.86 |
| Finger flexor muscles | ||
| Flexor digitorum superficialis | 51/52 (98%) | 128.53 ± 45.36 |
| Flexor digitorum profundus | 46/52 (88%) | 103.48 ± 36.30 |
| Flexor pollicis longus | 16/52 (31%) | 79.69 ± 24.53 |
| Predictors | N | Beta (95% CI) 1 | p-Value |
|---|---|---|---|
| Sex | |||
| F | 20 | — | — |
| M | 22 | −0.81 (−2.9 to 1.3) | 0.45 |
| Age | — | −0.04 (−0.13 to 0.04) | 0.29 |
| Stroke etiology | |||
| Hemorrhagic | 15 | — | — |
| Ischemic | 37 | −1.3 (−3.6 to 0.89) | 0.24 |
| Time since stroke onset | — | 0.06 (−0.01 to 0.13) | 0.12 |
| Damaged hemisphere | |||
| Left | 19 | — | — |
| Right | 33 | 2.5 (0.51 to 4.5) | 0.017 |
| Oral myorelaxant treatment | |||
| Yes | 28 | — | — |
| No | 24 | −1.9 (−3.9 to 0.10) | 0.068 |
| Non-BoNT-naïve patients | 27 | 0.92 (0.46 to 1.4) | <0.001 |
| Predictors | N | Beta (95% CI) 1 | p-Value |
|---|---|---|---|
| Splints/orthoses | |||
| Yes | 26 | — | — |
| No | 26 | −2.5 (−4.4 to −0.55) | 0.015 |
| FIM score dif. 2 | — | 2.9 (0.46 to 5.4) | 0.024 |
| Barthel score dif. 3 | — | 0.18 (−0.10 to 0.46) | 0.21 |
| Nr. of prior BoNT-A injections (i.e.,) | 25 | 0.92 (0.46 to 1.4) | <0.001 |
| Predictors | N | Beta (95% CI) 1 | p-Value |
|---|---|---|---|
| NRS shoulder score dif. 2 | — | 0.72 (−0.08 to 1.5) | 0.082 |
| Nr. of prior BoNT-A injections Pectoralis major | — | 0.61 (−0.03 to 1.3) | 0.068 |
| Improvement in proximal motor control 3 | — | 3.3 (2.0 to 4.6) | <0.001 |
| MAS Pectoralis major score dif. 4 | — | 1.1 (−0.54 to 2.8) | 0.19 |
| Pectoralis major BoNT-A dose | — | 0.01 (−0.01 to 0.02) | 0.46 |
| Predictors | N | Beta (95% CI) 1 | p-Value |
|---|---|---|---|
| Nr. of prior BoNT-A injections for elbow flexor muscles (i.e.,) | — | 0.91 (0.44 to 1.4) | <0.001 |
| MAS Biceps brachii score dif. 2 | — | 1.4 (−1.05 to 3.83) | 0.26 |
| MAS Brachialis score dif. 2 | — | 1.36 (−0.8 to 3.53) | 0.21 |
| MAS Brachioradialis score dif. 2 | — | 0.01 (−0.01 to 0.03) | 0.17 |
| MAS Pronator teres score dif. 2 | — | 1.8 (−0.61 to 4.2) | 0.15 |
| NRS elbow score dif. 3 | — | 0.9 (−0.08 to 1.8) | 0.09 |
| Biceps brachii BoNT-A dose | — | 0.00 (−0.02 to 0.02) | 0.99 |
| Brachialis BoNT-A dose | — | 0.00 (−0.02 to 0.02) | 0.74 |
| Brachioradialis BoNT-A dose | — | 0.01 (−0.01 to 0.03) | 0.17 |
| Pronator teres BoNT-A dose | — | 0.01 (−0.01 to 0.03) | 0.31 |
| Improvement in intermediate motor control 4 | — | 3.1 (1.8 to 4.4) | <0.001 |
| Predictors | N | Beta (95% CI) 1 | p-Value |
|---|---|---|---|
| Nr. of prior BoNT-A injections for wrist/fingers flexor muscles (i.e.,) | — | 0.93 (0.43 to 1.4) | <0.001 |
| NRS wrist score dif. 2 | — | −0.51 (−1.9 to 0.87) | 0.47 |
| Flexor digitorum superficialis BoNT-A dose | — | 0.00 (−0.03 to 0.02) | 0.65 |
| Flexor digitorum profundus BoNT-A dose | — | 0.01 (−0.01 to 0.03) | 0.54 |
| Flexor carpi radialis BoNT-A dose | — | 0.00 (−0.02 to 0.01) | 0.83 |
| Flexor carpi ulnaris BoNT-A dose | — | 0.00 (−0.02 to 0.02) | 0.93 |
| Flexor pollicis longus BoNT-A dose | — | 0.02 (−0.01 to 0.04) | 0.22 |
| MAS score dif. Flexor digitorum superficialis 3 | — | 0.52 (−1.5 to 2.6) | 0.62 |
| MAS score dif. Flexor digitorum Profundus 3 | — | 0.3 (−0.6 to 0.3) | 0.09 |
| MAS score dif. Flexor carpi radialis 3 | — | 0.00 (−1.4 to 1.4) | 0.99 |
| MAS score dif. Flexor carpi ulnaris 3 | — | 0.21 (−1.7 to 2.1) | 0.83 |
| MAS score dif. Flexor pollicis longus 3 | — | 1.3 (−0.25 to 2.8) | 0.11 |
| Improvement in distal motor control 4 | — | 2.4 (0.85 to 4.0) | 0.004 |
| Predictors | N | Beta (95% CI) 1 | p-Value |
|---|---|---|---|
| A. Nr. of prior BoNT-A injections (i.e.,) | 0.72 | 0.73 (0.31 to 1.1) | 0.001 |
| B. Improvement in proximal motor control 2 | 2.8 | 2.8 (1.6 to 4.0) | <0.001 |
| MAS2 | AchievedGAS | |||
|---|---|---|---|---|
| Spearman’s rho | MAS2 | Correlation Coefficient | 1.000 | −0.362 ** |
| Sig. (1-tailed) | . | 0.004 | ||
| N | 52 | 52 | ||
| AchievedGAS | Correlation Coefficient | −0.362 ** | 1.000 | |
| Sig. (1-tailed) | 0.004 | . | ||
| N | 52 | 52 | ||
| MAS2 | AchievedGAS | BaselineGAS | |||
|---|---|---|---|---|---|
| Spearman’s rho | MAS2 | Correlation Coefficient | 1.000 | −0.362 ** | −0.320 * |
| Sig. (1-tailed) | . | 0.004 | 0.010 | ||
| N | 52 | 52 | 52 | ||
| AchievedGAS | Correlation Coefficient | −0.362 ** | 1.000 | 0.228 | |
| Sig. (1-tailed) | 0.004 | . | 0.052 | ||
| N | 52 | 52 | 52 | ||
| BaselineGAS | Correlation Coefficient | −0.320 * | 0.228 | 1.000 | |
| Sig. (1-tailed) | 0.010 | 0.052 | . | ||
| N | 52 | 52 | 52 | ||
| MAS1 | MAS2 | ||
|---|---|---|---|
| MAS1 | Pearson Correlation | 1 | 0.745 ** |
| Sig. (1-tailed) | 0.000 | ||
| N | 52 | 52 | |
| MAS2 | Pearson Correlation | 0.745 ** | 1 |
| Sig. (1-tailed) | 0.000 | ||
| N | 52 | 52 | |
| AchievedGAS | Pain2 | |||
|---|---|---|---|---|
| Spearman’s rho | AchievedGAS | Correlation Coefficient | 1.000 | −0.333 ** |
| Sig. (1-tailed) | . | 0.008 | ||
| N | 52 | 52 | ||
| Pain2 | Correlation Coefficient | −0.333 ** | 1.000 | |
| Sig. (1-tailed) | 0.008 | . | ||
| N | 52 | 52 | ||
| Pain1 | Pain2 | ||
|---|---|---|---|
| Pain1 | Pearson Correlation | 1 | 0.843 ** |
| Sig. (1-tailed) | 0.000 | ||
| N | 52 | 52 | |
| Pain2 | Pearson Correlation | 0.843 ** | 1 |
| Sig. (1-tailed) | 0.000 | ||
| N | 52 | 52 | |
| Motor Control Global Upper Limb Increase | Achieved GAS-T | |||
|---|---|---|---|---|
| Spearman’s rho | Motor Control Global Upper Limb Increase | Correlation Coefficient | 1.000 | 0.591 ** |
| Sig. (1-tailed) | . | 0.000 | ||
| N | 52 | 52 | ||
| Achieved GAS-T | Correlation Coefficient | 0.591 ** | 1.000 | |
| Sig. (1-tailed) | 0.000 | . | ||
| N | 52 | 52 | ||
| PriorBTinj | BaselineGAS | AchievedGAS | |||
|---|---|---|---|---|---|
| Spearman’s rho | PriorBTinj | Correlation Coefficient | 1.000 | −0.002 | 0.453 ** |
| Sig. (2-tailed) | . | 0.988 | 0.001 | ||
| N | 52 | 52 | 52 | ||
| BaselineGAS | Correlation Coefficient | −0.002 | 1.000 | 0.228 | |
| Sig. (2-tailed) | 0.988 | . | 0.104 | ||
| N | 52 | 52 | 52 | ||
| AchievedGAS | Correlation Coefficient | 0.453 ** | 0.228 | 1.000 | |
| Sig. (2-tailed) | 0.001 | 0.104 | . | ||
| N | 52 | 52 | 52 | ||
| Orthos | Barthel1 | |||
|---|---|---|---|---|
| Spearman’s rho | orthos | Correlation Coefficient | 1.000 | 0.220 * |
| Sig. (1-tailed) | . | 0.049 | ||
| N | 52 | 52 | ||
| barthel1 | Correlation Coefficient | 0.220 * | 1.000 | |
| Sig. (1-tailed) | 0.049 | . | ||
| N | 52 | 52 | ||
| Baclofen | AchievedGAS | ||
|---|---|---|---|
| baclofen | Pearson Correlation | 1 | −0.111 |
| Sig. (2-tailed) | 0.434 | ||
| N | 52 | 52 | |
| AchievedGAS | Pearson Correlation | −0.111 | 1 |
| Sig. (2-tailed) | 0.434 | ||
| N | 52 | 52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Săndulescu, M.I.; Cinteză, D.; Poenaru, D.; Potcovaru, C.-G.; Păunescu, H.; Coman, O.A. The Complex Role of Botulinum Toxin in Enhancing Goal Achievement for Post-Stroke Patients. Toxins 2024, 16, 172. https://doi.org/10.3390/toxins16040172
Săndulescu MI, Cinteză D, Poenaru D, Potcovaru C-G, Păunescu H, Coman OA. The Complex Role of Botulinum Toxin in Enhancing Goal Achievement for Post-Stroke Patients. Toxins. 2024; 16(4):172. https://doi.org/10.3390/toxins16040172
Chicago/Turabian StyleSăndulescu, Miruna Ioana, Delia Cinteză, Daniela Poenaru, Claudia-Gabriela Potcovaru, Horia Păunescu, and Oana Andreia Coman. 2024. "The Complex Role of Botulinum Toxin in Enhancing Goal Achievement for Post-Stroke Patients" Toxins 16, no. 4: 172. https://doi.org/10.3390/toxins16040172
APA StyleSăndulescu, M. I., Cinteză, D., Poenaru, D., Potcovaru, C.-G., Păunescu, H., & Coman, O. A. (2024). The Complex Role of Botulinum Toxin in Enhancing Goal Achievement for Post-Stroke Patients. Toxins, 16(4), 172. https://doi.org/10.3390/toxins16040172







