Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review
Abstract
1. Introduction
2. Results
2.1. Description of Included Studies
2.2. Study Characteristics
2.3. Included Interventions
2.3.1. Stretching Exercise
2.3.2. Static Stretching with Positioning Orthoses
2.3.3. Transcutaneous Electrical Nerve Simulation (TENS) as an Adjuvant Therapy
2.3.4. Extracorporeal Shock Wave Therapy (ESWT)
2.3.5. Repetitive Peripheral Magnetic Stimulation (rPMS)
2.3.6. Non-Invasive Brain Stimulation (NIBS)
2.3.7. Botulinum Toxin A (BoNT-A) Injection
- The injection dose may be modified based on the patient’s age, body weight, muscle mass, and effectiveness.
- We agreed to lower the dose for patients in hot-climate countries or with small muscle mass [39].
- The injection intervals should be 12 weeks or more.
- The maximum injection doses accepted are as follows:
- Botox® (OnaBoNT-A) injections range from 5 to 100 units per muscle, with a maximum dose of 400 units per visit [40].
- Dysport® (AboBoNT-A) injections range from 100 to 250 units per muscle, with a maximum dose of 1500–2000 units per visit. In small- and medium-volume muscles, the mean/median dose often varied between 100 U and 200 U when only values for 50 or more treated patients were taken into account. The same pattern was noted for the muscle group with a significant volume; however, the average/middle AboBoNT-A dosage was more inclined to range from 200 U to 500 U, especially in larger muscles [19].
- Xeomin® (IncoBoNT-A) injections range from 5 to 100 units per muscle, with a maximum dose of 400 units per visit.
- In available settings, US -guided BoNT-A injections can be useful, especially in distal upper extremities’ muscles, such as wrist and finger flexors.
2.3.8. Dry Needling (DN)
2.3.9. Intrathecal Baclofen (ITB)
2.3.10. Whole-Body Vibration Therapy (WBV)
2.3.11. Localized Muscle Vibration (LMV)
3. Discussion
Strengths and Limitations
4. Conclusions
5. Materials and Methods
5.1. Search Strategy and Selection Criteria
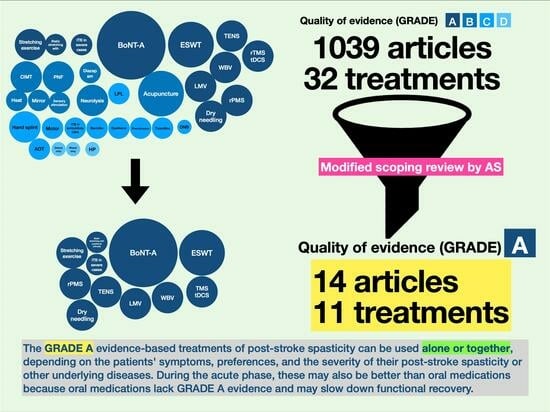
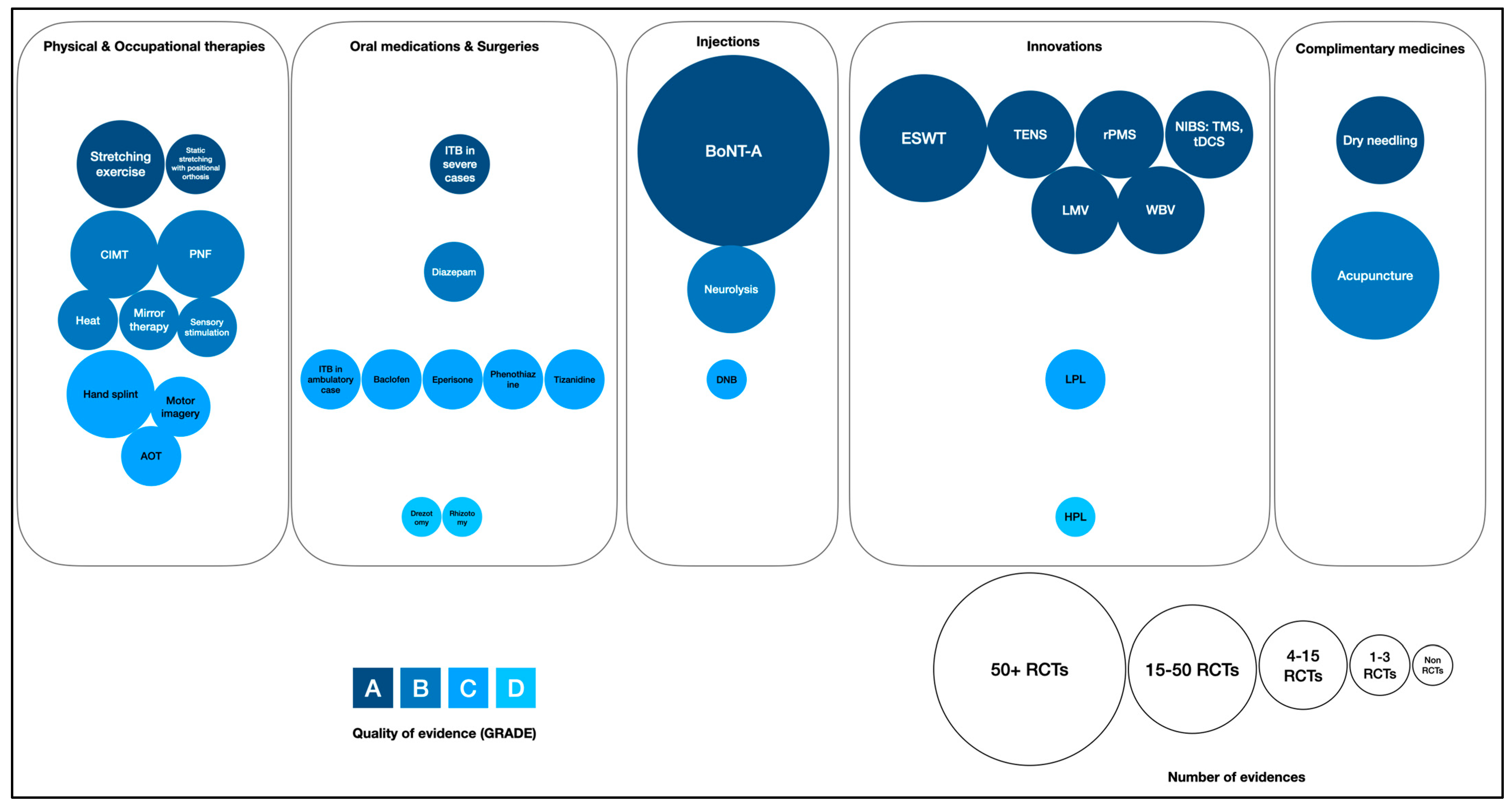
5.2. Modified Scoping Review by Areerat Suputtitada (AS)
- Color coding: In this chart, various shades of blue were used to signify the quality of evidence for each type of intervention, with a spectrum ranging from dark navy to light blue. This gradation represented the quality of the evidence, categorized from GRADE A (highest quality, indicated by dark navy) to GRADE D (lower quality, denoted by light blue).
- Circle size (circumference): The circumference of each circle within the chart also reflected the number of randomized controlled trials associated with each intervention. Larger circles indicated a higher number of trials. Smaller circles, on the other hand, represented a lower amount of evidence.
5.3. Eligibility Criteria
5.4. Information Sources
5.5. Search and Selection of Sources of Evidence
5.6. Risk of Bias
5.7. Data Charging Process and Data Items
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef]
- Watkins, C.L.; Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K. Prevalence of spasticity post stroke. Clin. Rehabil. 2002, 16, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ashford, S.; Turner-Stokes, L.; Allison, R.; Duke, L.; Moore, P.; Bavikatte, G.; Kirker, S.; Moore, P.; Ward, A.B.; Bilton, D.; et al. Spasticity in Adults: Management Using Botulinum Toxin, 2nd ed.; National Guidelines; The Royal College of Physicians: London, UK, 2018. [Google Scholar]
- Francisco, G.E.; McGuire, J.R. Poststroke spasticity management. Stroke 2012, 43, 3132–3136. [Google Scholar] [CrossRef]
- Bavikatte, G.; Subramanian, G.; Ashford, S.; Allison, R.; Hicklin, D. ELarly Identification, Intervention and Management of Post-stroke Spasticity: Expert Consensus Recommendations. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211036576. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. What is “quality of evidence” and why is it important to clinicians? Bmj 2008, 336, 995–998. [Google Scholar] [CrossRef]
- Brozek, J.L.; Akl, E.A.; Alonso-Coello, P.; Lang, D.; Jaeschke, R.; Williams, J.W.; Phillips, B.; Lelgemann, M.; Lethaby, A.; Bousquet, J.; et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 2009, 64, 669–677. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Mc Namara, M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Gomez-Cuaresma, L.; Lucena-Anton, D.; Gonzalez-Medina, G.; Martin-Vega, F.J.; Galan-Mercant, A.; Luque-Moreno, C. Effectiveness of Stretching in Post-Stroke Spasticity and Range of Motion: Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1074. [Google Scholar] [CrossRef]
- Salazar, A.P.; Pinto, C.; Mossi, J.V.R.; Figueiro, B.; Lukrafka, J.L.; Pagnussat, A.S. Effectiveness of static stretching positioning on post-stroke upper-limb spasticity and mobility: Systematic review with meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 274–282. [Google Scholar] [CrossRef]
- Mahmood, A.; Veluswamy, S.K.; Hombali, A.; Mullick, A.; Manikandan, N.; Solomon, J.M. Effect of Transcutaneous Electrical Nerve Stimulation on Spasticity in Adults with Stroke: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 751–768. [Google Scholar] [CrossRef]
- Zhang, H.L.; Jin, R.J.; Guan, L.; Zhong, D.L.; Li, Y.X.; Liu, X.B.; Xiao, Q.W.; Xiao, X.L.; Li, J. Extracorporeal Shock Wave Therapy on Spasticity After Upper Motor Neuron Injury: A Systematic Review and Meta-analysis. Am. J. Phys. Med. Rehabil. 2022, 101, 615–623. [Google Scholar] [CrossRef]
- Pan, J.-X.; Diao, Y.-X.; Peng, H.-Y.; Wang, X.-Z.; Liao, L.-R.; Wang, M.-Y.; Wen, Y.-L.; Jia, Y.-B.; Liu, H. Effects of repetitive peripheral magnetic stimulation on spasticity evaluated with modified Ashworth scale/Ashworth scale in patients with spastic paralysis: A systematic review and meta-analysis. Front. Neurol. 2022, 13, 997913. [Google Scholar] [CrossRef]
- Wang, X.; Ge, L.; Hu, H.; Yan, L.; Li, L. Effects of Non-Invasive Brain Stimulation on Post-Stroke Spasticity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Brain Sci. 2022, 12, 836. [Google Scholar] [CrossRef]
- Schnitzler, A.; Dince, C.; Freitag, A.; Iheanacho, I.; Fahrbach, K.; Lavoie, L.; Loze, J.-Y.; Forestier, A.; Gasq, D. AbobotulinumtoxinA Doses in Upper and Lower Limb Spasticity: A Systematic Literature Review. Toxins 2022, 14, 734. [Google Scholar] [CrossRef]
- Sun, L.-C.; Chen, R.; Fu, C.; Chen, Y.; Wu, Q.; Chen, R.; Lin, X.; Luo, S. Efficacy and Safety of Botulinum Toxin Type A for Limb Spasticity after Stroke: A Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2019, 2019, 8329306. [Google Scholar] [CrossRef]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]
- Doan, T.-N.; Kuo, M.-Y.; Chou, L.-W. Efficacy and Optimal Dose of Botulinum Toxin A in Post-Stroke Lower Extremity Spasticity: A Systematic Review and Meta-Analysis. Toxins 2021, 13, 428. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Pérez-Bellmunt, A.; Llurda-Almuzara, L.; Plaza-Manzano, G.; I De-La-Llave-Rincón, A.; Navarro-Santana, M.J. Is Dry Needling Effective for the Management of Spasticity, Pain, and Motor Function in Post-Stroke Patients? A Systematic Review and Meta-Analysis. Pain. Med. 2021, 22, 131–141. [Google Scholar] [CrossRef]
- Creamer, M.; Cloud, G.; Kossmehl, P.; Yochelson, M.; Francisco, G.E.; Ward, A.B.; Wissel, J.; Zampolini, M.; Abouihia, A.; Calabrese, A.; et al. Effect of Intrathecal Baclofen on Pain and Quality of Life in Poststroke Spasticity. Stroke 2018, 49, 2129–2137. [Google Scholar] [CrossRef]
- Creamer, M.; Cloud, G.; Kossmehl, P.; Yochelson, M.; E Francisco, G.; Ward, A.B.; Wissel, J.; Zampolini, M.; Abouihia, A.; Berthuy, N.; et al. Intrathecal baclofen therapy versus conventional medical management for severe poststroke spasticity: Results from a multicentre, randomised, controlled, open-label trial (SISTERS). J. Neurol. Neurosurg. Psychiatry 2018, 89, 642–650. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, S.; Li, S.; Zeng, Y.; Chen, L.; Li, G.; Li, S.; He, L.; Chen, S.; Zheng, X.; et al. Efficacy and safety of whole-body vibration therapy for post-stroke spasticity: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1074922. [Google Scholar] [CrossRef]
- Avvantaggiato, C.; Casale, R.; Cinone, N.; Facciorusso, S.; Turitto, A.; Stuppiello, L.; Picelli, A.; Ranieri, M.; Intiso, D.; Fiore, P.; et al. Localized muscle vibration in the treatment of motor impairment and spasticity in post-stroke patients: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 57, 44–60. [Google Scholar] [CrossRef]
- Pradhan, S.; Bansal, R. Role of corrected-assisted-synchronized-periodic therapy in post-stroke rehabilitation. Neurol. India 2018, 66, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Lannin, N.A.; Cusick, A.; McCluskey, A.; Herbert, R.D. Effects of splinting on wrist contracture after stroke: A randomized controlled trial. Stroke 2007, 38, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Marcolino, M.A.Z.; Hauck, M.; Stein, C.; Schardong, J.; Pagnussat, A.d.S.; Plentz, R.D.M. Effects of transcutaneous electrical nerve stimulation alone or as additional therapy on chronic post-stroke spasticity: Systematic review and meta-analysis of randomized controlled trials. Disabil. Rehabil. 2020, 42, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Lian, Y.; Jing, Y.; Xing, J.; Li, Z. Research progress in extracorporeal shock wave therapy for upper limb spasticity after stroke. Front. Neurol. 2023, 14, 1121026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, S.-N.; Lee, I.-S.; Jung, H.; Lee, K.-S.; Koh, S.-E. Effects of Extracorporeal Shock Wave Therapy on Spasticity in Patients after Brain Injury: A Meta-analysis. J. Phys. Ther. Sci. 2014, 26, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Dymarek, R.; Ptaszkowski, K.; Ptaszkowska, L.; Kowal, M.; Sopel, M.; Taradaj, J.; Rosińczuk, J. Shock Waves as a Treatment Modality for Spasticity Reduction and Recovery Improvement in Post-Stroke Adults-Current Evidence and Qualitative Systematic Review. Clin. Interv. Aging 2020, 15, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Gao, F.; Zhao, T.; Sun, W.; Wang, B.; Li, Z. Positive Effects of Extracorporeal Shock Wave Therapy on Spasticity in Poststroke Patients: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, W.; Jiang, W.; Qian, Q. Effects of extracorporeal shock wave therapy on spasticity in post-stroke patients: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2018, 50, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Park, H.D.; Han, S.H.; Shim, G.Y.; Choi, K.Y. Duration of Treatment Effect of Extracorporeal Shock Wave on Spasticity and Subgroup-Analysis According to Number of Shocks and Application Site: A Meta-Analysis. Ann. Rehabil. Med. 2019, 43, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdés, R.; Calvo-Sanz, J.; Urrùtia, G.; Serra-Llobet, P.; Pérez-Bellmunt, A.; Germán-Romero, A. The effectiveness of extracorporeal shock wave therapy to reduce lower limb spasticity in stroke patients: A systematic review and meta-analysis. Top. Stroke Rehabil. 2020, 27, 137–157. [Google Scholar] [CrossRef]
- Jia, G.; Ma, J.; Wang, S.; Wu, D.; Tan, B.; Yin, Y.; Jia, L.; Cheng, L. Long-term Effects of Extracorporeal Shock Wave Therapy on Poststroke Spasticity: A Meta-analysis of Randomized Controlled Trials. J. Stroke Cerebrovasc. Dis. 2020, 29, 104591. [Google Scholar] [CrossRef]
- Suputtitada, A.; Suwanwela, N. The lowest effective dose of botulinum A toxin in adult patients with upper limb spasticity. Disabil. Rehabil. 2005, 27, 176–184. [Google Scholar] [CrossRef]
- Abo, M.; Shigematsu, T.; Hara, H.; Matsuda, Y.; Nimura, A.; Yamashita, Y.; Takahashi, K. Efficacy and Safety of OnabotulinumtoxinA 400 Units in Patients with Post-Stroke Upper Limb Spasticity: Final Report of a Randomized, Double-Blind, Placebo-Controlled Trial with an Open-Label Extension Phase. Toxins 2020, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E. The Use of Botulinum Toxin for Treatment of Spasticity. Handb. Exp. Pharmacol. 2021, 263, 127–146. [Google Scholar]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Hesse, S.; Mach, H.; Fröhlich, S.; Behrend, S.; Werner, C.; Melzer, I. An early botulinum toxin A treatment in subacute stroke patients may prevent a disabling finger flexor stiffness six months later: A randomized controlled trial. Clin. Rehabil. 2012, 26, 237–245. [Google Scholar] [CrossRef]
- Huynh, W.; Krishnan, A.V.; Lin, C.S.; Vucic, S.; Katrak, P.; Hornberger, M.; Kiernan, M.C. Botulinum toxin modulates cortical maladaptation in post-stroke spasticity. Muscle Nerve 2013, 48, 93–99. [Google Scholar] [CrossRef]
- Toliopoulos, A. In-Plane Ultrasound-Guided Botulinum Toxin Injection to Lumbrical and Interosseus Upper Limb Muscles: Technical Report. Cureus 2023, 15, e45073. [Google Scholar] [CrossRef]
- Lagnau, P.; Lo, A.; Sandarage, R.; Alter, K.; Picelli, A.; Wissel, J.; Verduzco-Gutierrez, M.; Suputtitada, A.; Munin, M.C.; Carda, S.; et al. Ergonomic Recommendations in Ultrasound-Guided Botulinum Neurotoxin Chemodenervation for Spasticity: An International Expert Group Opinion. Toxins 2021, 13, 249. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Ashford, S.; Esquenazi, A.; Wissel, J.; Ward, A.B.; Francisco, G.; Lains, J.; Suputtitada, A.; Serrano, S.; Baguley, I.J.; et al. A comprehensive person-centered approach to adult spastic paresis: A consensus-based framework. Eur. J. Phys. Rehabil. Med. 2018, 54, 605–617. [Google Scholar] [CrossRef]
- Schillebeeckx, F.; Mills, P.B.; Ip, A.; Schinwelski, M.; Teixeira, J.E.M.; Ashford, S.; Bayle, N.; Chemello, E.; Jacinto, J.; Nayar, M.; et al. Worldwide Survey of Clinician Practice on use of Adjunctive Therapies following Botulinum Toxin Injection for Spasticity. J. Rehabil. Med. 2022, 54, jrm00320. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D.; Centra, A.M.; Gravina, M.; Chiaramonte, A.; Bartolo, M.; Di Rienzo, F. Botulinum Toxin—A High-Dosage Effect on Functional Outcome and Spasticity-Related Pain in Subjects with Stroke. Toxins 2023, 15, 509. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, T.; Hu, X.; Wang, T. Efficacy and safety of botulinum toxin type A for upper limb spasticity after stroke or traumatic brain injury: A systematic review with meta-analysis and trial sequential analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Grigoriu, A.I.; Dinomais, M.; Rémy-Néris, O.; Brochard, S. Impact of Injection-Guiding Techniques on the Effectiveness of Botulinum Toxin for the Treatment of Focal Spasticity and Dystonia: A Systematic Review. Arch. Phys. Med. Rehabil. 2015, 96, 2067–2078.e1. [Google Scholar] [CrossRef]
- Picelli, A.; Tamburin, S.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Santilli, V.; Smania, N. Botulinum toxin type A injection into the gastrocnemius muscle for spastic equinus in adults with stroke: A randomized controlled trial comparing manual needle placement, electrical stimulation and ultrasonography-guided injection techniques. Am. J. Phys. Med. Rehabil. 2012, 91, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Asimakidou, E.; Sidiropoulos, C. A Bayesian Network Meta-Analysis and Systematic Review of Guidance Techniques in Botulinum Toxin Injections and Their Hierarchy in the Treatment of Limb Spasticity. Toxins 2023, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; DeLisa, J.A. DeLisa’s Physical Medicine & Rehabilitation: Principles and Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- E Francisco, G.; Boake, C. Improvement in walking speed in poststroke spastic hemiplegia after intrathecal baclofen therapy: A preliminary study. Arch. Phys. Med. Rehabil. 2003, 84, 1194–1199. [Google Scholar]
- Li, S.; Francisco, G.E.; Rymer, W.Z. A New Definition of Poststroke Spasticity and the Interference of Spasticity with Motor Recovery from Acute to Chronic Stages. Neurorehabilit. Neural Repair. 2021, 35, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Suputtitada, A. Emerging theory of sensitization in post-stroke muscle spasticity. Front. Rehabil. Sci. 2023, 4, 1169087. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.; Kouzouna, A.; Simcox, C.; Pandyan, A.D. Pharmacological interventions other than botulinum toxin for spasticity after stroke. Cochrane Database Syst. Rev. 2016, 10, CD010362. [Google Scholar] [CrossRef]
- Simon, O.; Yelnik, A.P. Managing spasticity with drugs. Eur. J. Phys. Rehabil. Med. 2010, 46, 401–410. [Google Scholar]
- Thibaut, A.; Beaudart, C.; Martens, G.; Bornheim, S.; Kaux, J.F. Common Bias and Challenges in Physical and Rehabilitation Medicine Research: How to Tackle Them. Front. Rehabil. Sci. 2022, 3, 873241. [Google Scholar] [CrossRef]
- Negrini, S.; Selb, M.; Kiekens, C.; Todhunter-Brown, A.; Arienti, C.; Stucki, G.; Meyer, T. 3rd Cochrane Rehabilitation Methodology Meeting participants. Rehabilitation definition for research purposes. A global stakeholders’ initiative by Cochrane Rehabilitation. Eur. J. Phys. Rehabil. Med. 2022, 58, 333–341. [Google Scholar] [CrossRef]
- Suputtitada, A. Editorial: Highlights in medical and surgical rehabilitation 2021/22. Front. Rehabil. Sci. 2023, 4, 1219924. [Google Scholar] [CrossRef]

| Authors | Types of Studies | RCTs/ Population | Population | Interventions | Parameters | Control | Outcome Measures | Future Studies/ Limitation |
|---|---|---|---|---|---|---|---|---|
| 1. Stretching exercise | ||||||||
| Gomez et al., 2021 [13] | SR and meta- analysis | 8 RCTs included in the SR; 6 RCTs included in the meta- analysis (332 patients) | Chronic stroke (3–6 months) | Passive static/ dynamic stretching by PT, self-stretching | Varied greatly, but none of them exceed 60 min/ session | No treatment | MAS p = 0.45, I2 = 92% | Larger sample size and optimal protocol |
| 2. Static stretching with positioning orthoses | ||||||||
| Salazar et al., 2018 [14] | SR and meta- analysis | 3 RCTs for spasticity outcome (57 patients) | Chronic stroke | Static stretching with wrist devices | 6–7 days/week, 20–45 min/day (separated by 2–3 time/day), 3–4 weeks | No treatment | MAS p < 0.01, I2 = 82% | Larger sample size |
| 3. Transcutaneous electrical nerve stimulation (TENS) | ||||||||
| Mahmood et al., 2018 [15] | SR and meta- analysis | 15 RCTs included in the SR; 7 RCTs Included in the meta- analysis (427 patients) | Chronic stroke | TENS | High frequency (100 Hz), duration > 30 min/session, electrodes placement along the nerve/ muscle belly, intensity twice of the sensory threshold, and treatment duration > 2 weeks | No treatment, or placebo- controlled interventions, or active controls, or PT | MAS p = 0.001, I2 = 17% | Focusing on effect in UE, duration of efficacy, and effect of low TENS |
| 4. Extracorporeal shock wave therapy (ESWT) | ||||||||
| Zhang et al., 2022 [16] | SR and meta- analysis | 42 RCTs Included in the SR; 34 RCTs Included in the meta-analysis (1973 patients) | Upper motor neuron injury (29 studies focused on stroke) | ESWT | Radial ESWT >2 sessions | Sham ESWT/ conventional RT | MAS p < 0.0001, I2 = 78% | Focusing on the duration of efficacy, dose response relationship, mechanism |
| 5. Repetitive peripheral magnetic stimulation (rPMS) | ||||||||
| Pan et al., 2022 [17] | SR and meta- analysis | 8 RCTs included in the SR; 6 RCTs Included in the meta-analysis (297 patients) | Spastic paralysis patients (170 chronic stroke) | rPMS | 5/25 Hz of frequency, 3–30 min/ session, and round/ figure of eight coil types | Sham rPMS and/or PT, and PT | AS, MAS, MTS, and FMA | Focusing on the duration of efficacy, with larger sample size, and optimal protocol |
| 6. Non-invasive brain stimulation (NIBS) | ||||||||
| Wang et al., 2022 [18] | SR and meta- analysis | 14 RCTs (232 patients on TMS, and 345 patients on tDCS) | Stroke patients | 11 RCTs on TMS | Low frequency at unaffected hemisphere | Sham TMS, Sham TMS plus PT, Sham TMS plus RT | MAS in UE p < 0.00001, I2 = 3% | Further study in LE spasticity, mechanism, and the duration of efficacy |
| 7 RCTs on tDCS | Anodal stimulation at affected hemisphere, 0.7 mA or 1.2 mA | Sham tDCS, Sham tDCS plus PT, Sham tDCS plus VR | MAS in UE p = 0.003, I2 = 78% | |||||
| 7. Botulinum toxin A (BoNT-A) injection | ||||||||
| 7.1 In the upper and lower extremities | ||||||||
| Schnitzler et al., 2022 [19] | SR | 49 primary studies | Spasticity of any Etiology (38 studies in stroke/ brain injury) | BoNT-A injection | AboBoNT-A | Placebo/ control or another BoNT-A treatment | AboBoNT-A dose given per muscle in clinical practice varies considerably, with only a slight trend toward a relationship between dose and muscle volume | - |
| 7.2 In the upper extremities | ||||||||
| Sun et al., 2019 [20] | meta- analysis | 27 RCTs (2793 patients) (16 trials UE; 12 trials of muscle tone in UE) | Stroke patients | BoNT- A injection | AboBoNT-A/ OnaBoNT-A/ IncoBoNT-A | Placebo | Muscle tone p < 0.001, I2 = 52.1% | - |
| Andringa et al., 2019 [21] | SR and meta- analysis | 40 RCTs (2718 patients) | Stroke patients | UE BoNT-A injection | Placebo and/or PT | MAS and AS | - | |
| 7.3 In the lower extremities | ||||||||
| Doan et al., 2021 [22] | SR and meta- analysis | 12 RCTs included in SR, 9 RCTs included in Meta- Analysis (1601patients) | PSS in LE | BoNT-A Injection in LE | 300 units of OnaBoNT-A and 1000 units of AboBoNT-A | Placebo/ dose- ranging | MAS, AS at week 4th, 8th, and 12th | Further studies on functional improvement |
| 8. Dry needling (DN) | ||||||||
| Fernández et al., 2021 [23] | SR and meta- analysis | 7 RCTs (83 patients) | Stroke patients | DN | - | RT/ sham DN/ non-TrP DN | MAS, MMAS p = 0.0007, I2 = 66%: UE p = 0.18, LE p < 0.0001 | Larger sample size and examining the long-term effect |
| 9. Intrathecal baclofen (ITB) | ||||||||
| Creamer et al., 2018 [24,25] | Multi- center phase 4 RCT | 60 patients (ITB: 31; control: 29) | PSS in ≥2 extremities and ASS of ≥3 in ≥2 affected LE | ITB | - | CMM with oral antispastic drugs | ASS in LE, NRS, and quality of life | Larger sample sizes and longer follow-up |
| 10. Whole-body vibration (WBV) | ||||||||
| Zhang et al., 2023 [26] | SR and meta- analysis | 11 RCTs (475 patients) | Stroke patients | WBV or PT with WBV | Variable | Sham WBV or PT | MAS | Focusing on the duration of efficacy, severe spasticity |
| 11. Localized muscle vibration (LMV) | ||||||||
| Avvantaggiato et al., 2021 [27] | SR and meta- analysis | 14 RCTs (425 patients) | Stroke patients | LMV plus PT | Variable from frequency 30, 80, 90, 91, 100, 120, and 300 Hz; amplitude 0.01, 0.2–0.5, 1, and 2 mm; duration 5, 20, 30, and 60 min | PT/sham plus PT | Neurophysiological parameters (TMS, ENG, and EMG), MAS (elbow: p = 0.001, I2 = 0%; wrist: p = 0.04, I2 = 36%; shoulder: p = 0.26, I2 = 0%) Functional scales | Larger size of homogeneous patients (shared methodology), on LE |
| Rank | Explanation | Examples |
|---|---|---|
| High | Further research is very unlikely to change our confidence in the estimate of the effect |
|
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
|
| Low | Further research is very likely to have an important impact on our confidence in the estimate of the effect and is likely to change the estimate |
|
| Very low | Any estimate of the effect is very uncertain |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suputtitada, A.; Chatromyen, S.; Chen, C.P.C.; Simpson, D.M. Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review. Toxins 2024, 16, 98. https://doi.org/10.3390/toxins16020098
Suputtitada A, Chatromyen S, Chen CPC, Simpson DM. Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review. Toxins. 2024; 16(2):98. https://doi.org/10.3390/toxins16020098
Chicago/Turabian StyleSuputtitada, Areerat, Supattana Chatromyen, Carl P. C. Chen, and David M. Simpson. 2024. "Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review" Toxins 16, no. 2: 98. https://doi.org/10.3390/toxins16020098
APA StyleSuputtitada, A., Chatromyen, S., Chen, C. P. C., & Simpson, D. M. (2024). Best Practice Guidelines for the Management of Patients with Post-Stroke Spasticity: A Modified Scoping Review. Toxins, 16(2), 98. https://doi.org/10.3390/toxins16020098