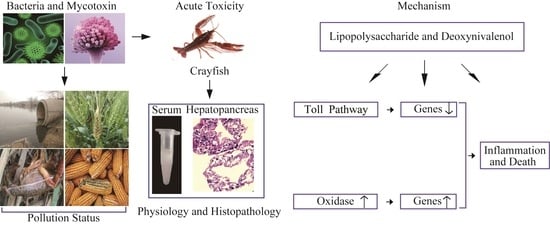
Effects of Lipopolysaccharide and Deoxynivalenol on the Survival, Antioxidant and Immune Response, and Histopathology of Crayfish (Procambarus clarkii)
Abstract
1. Introduction
2. Results
2.1. Survival Rate
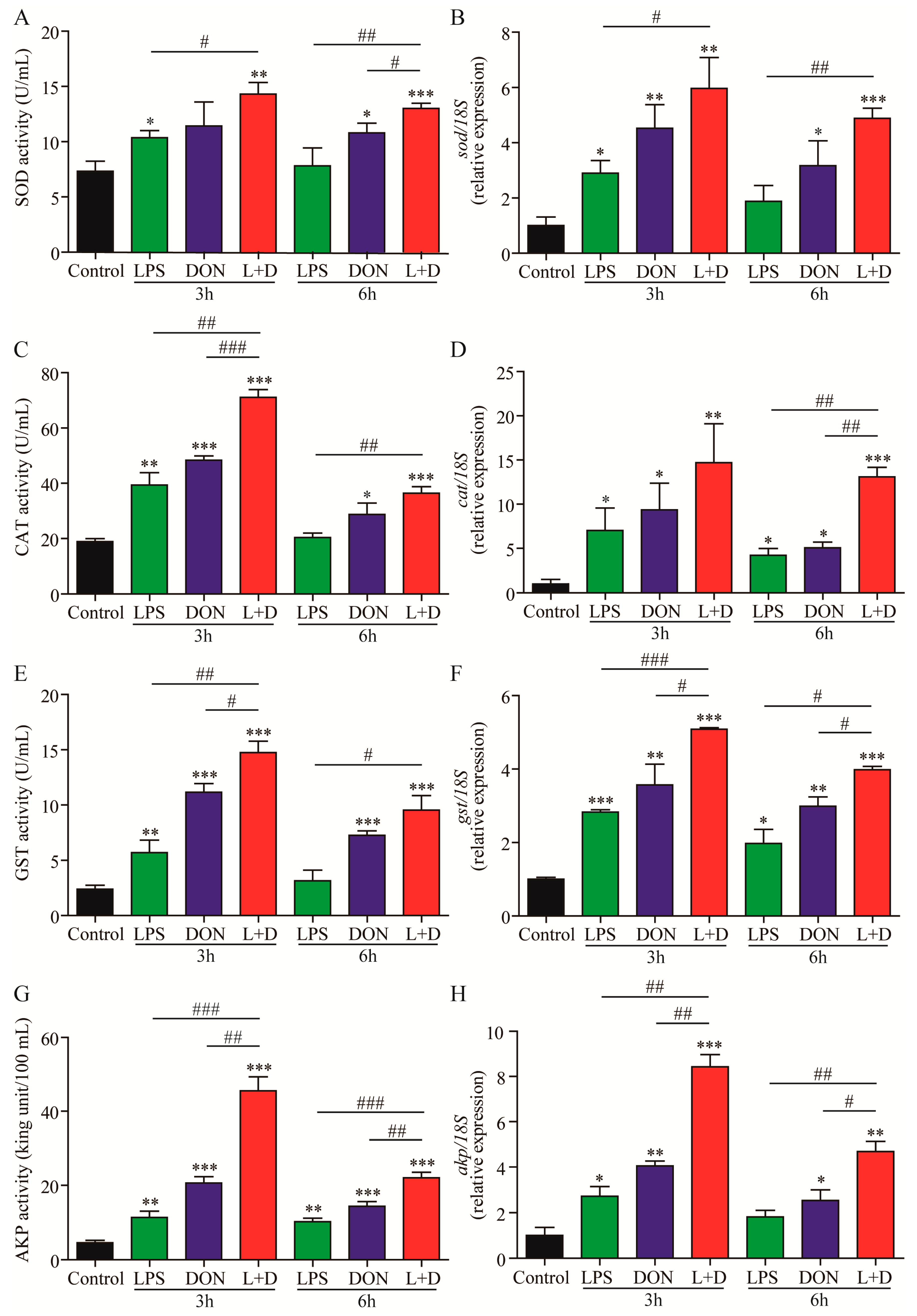
2.2. Activities of Enzyme and Expressions of Enzyme-Related Genes
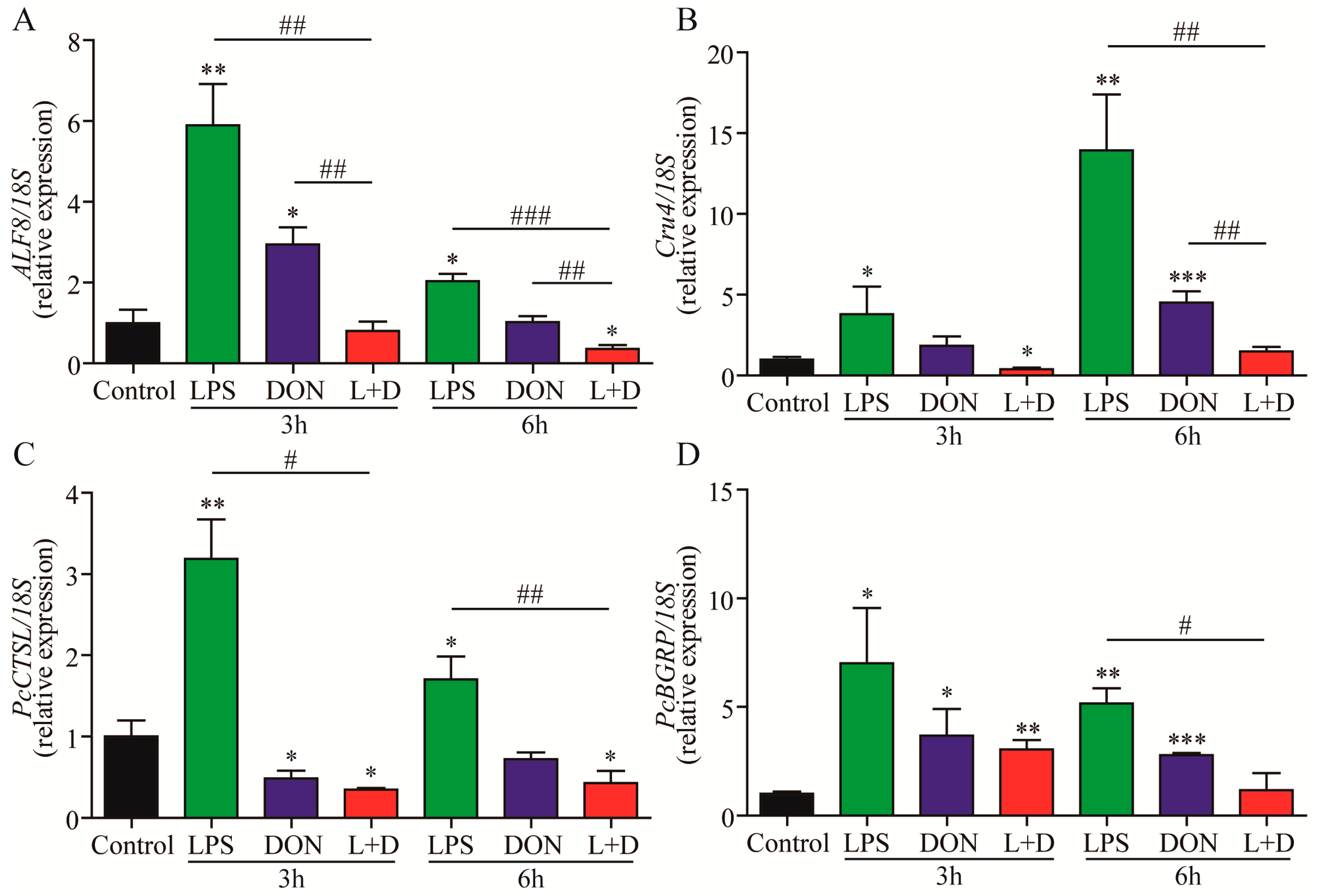
2.3. Expressions of Immune-Related Genes
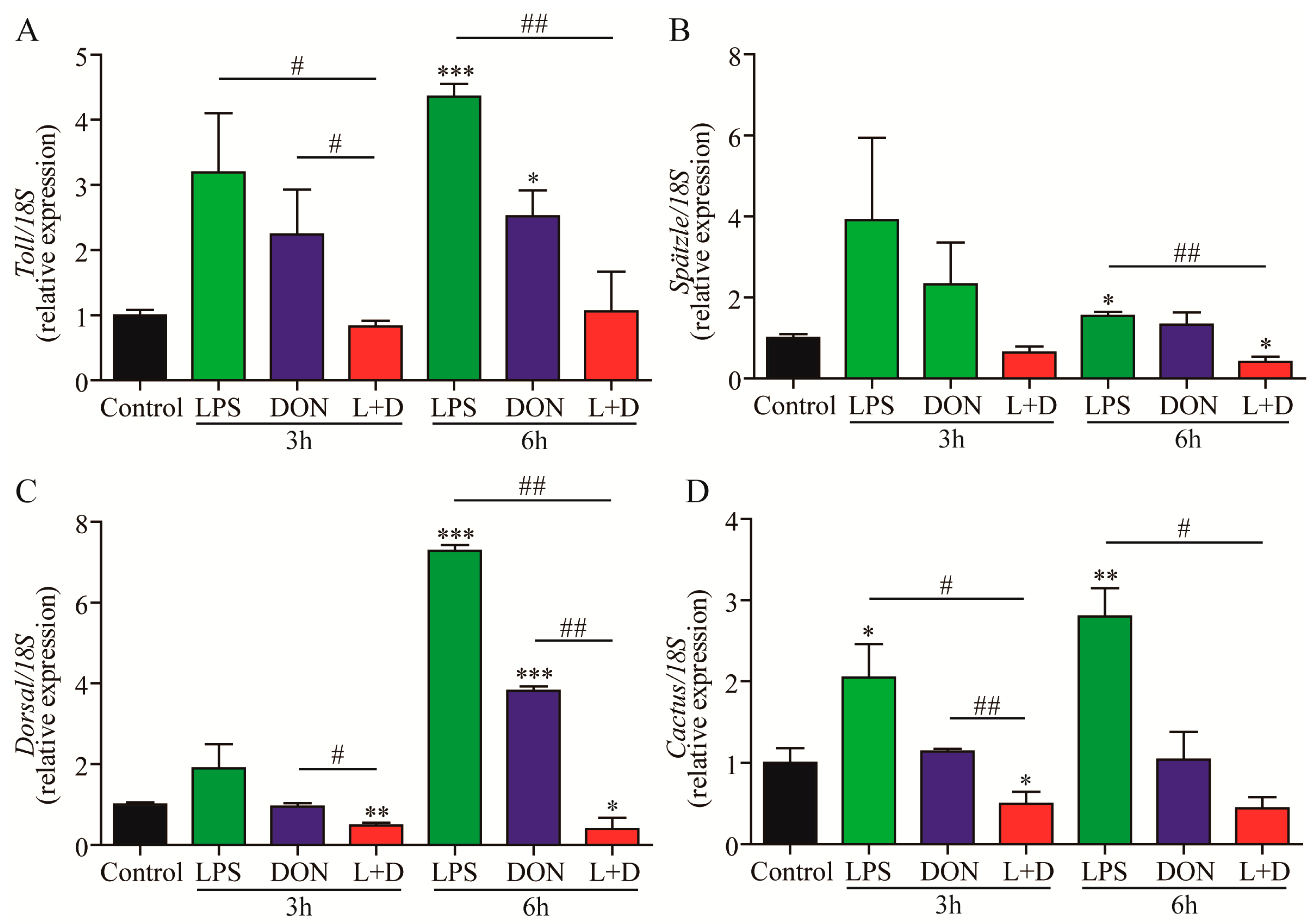
2.4. Expressions of Toll Pathway-Related Genes
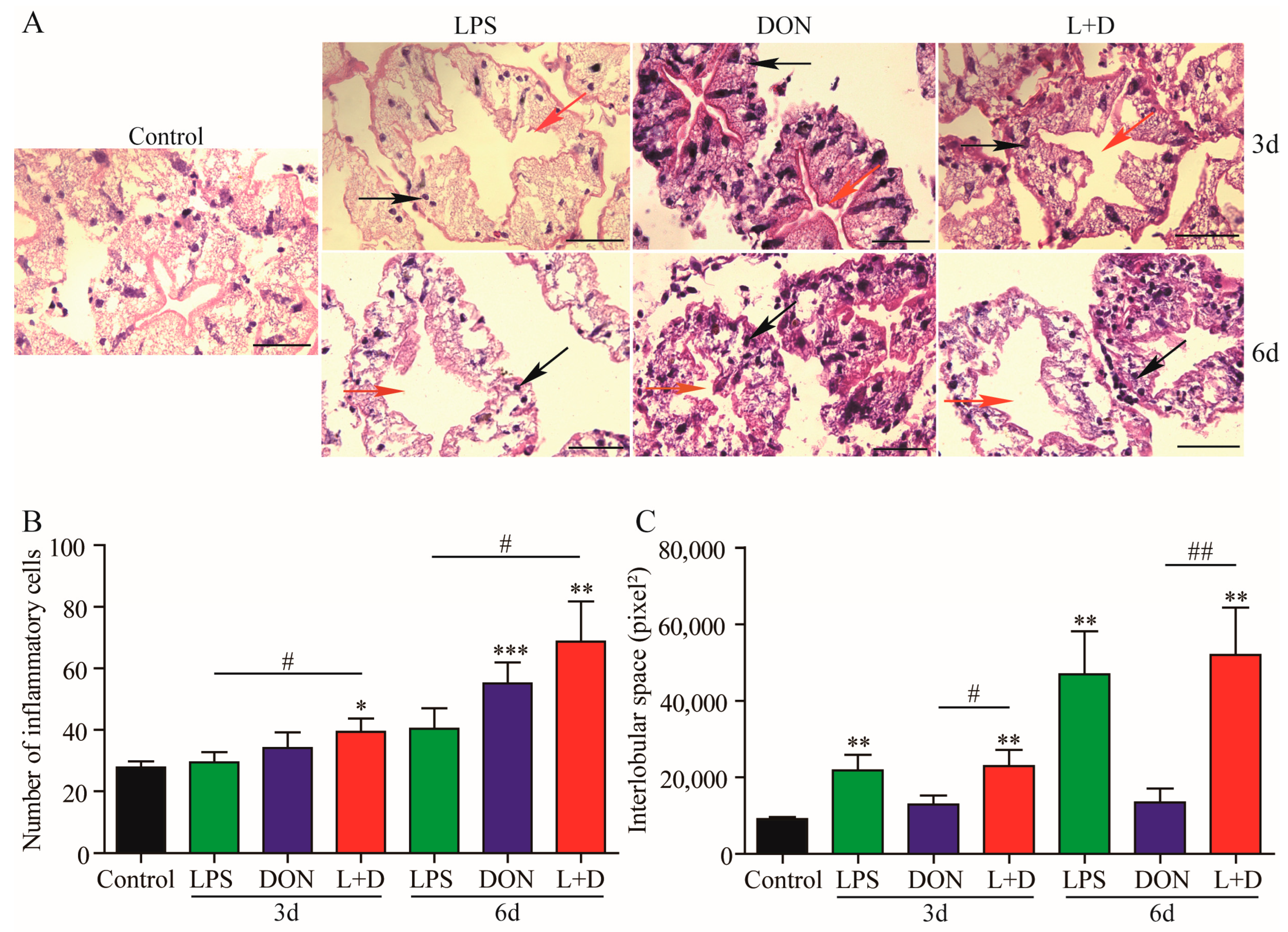
2.5. Histological Changes in Hepatopancreas
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Chemicals
5.2. Experimental Protocol
5.3. Survival Rate
5.4. Enzyme Activity Analysis
5.5. RNA Isolation and Quantitative RT-qPCR
5.6. Histopathological Analysis
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.F.; Zhou, Y.L.; Liu, M.; Li, Z.; Zhou, L.; Wang, Z.W.; Gui, J.F. A high-density genetic map and QTL fine mapping for growth- and sex-related traits in red swamp crayfish (Procambarus clarkii). Front. Genet. 2022, 13, 852280. [Google Scholar] [CrossRef]
- Yi, W.; Chen, W.; Yonglun, C.; Dongdong, Z.; Mingming, Z.; Hailan, L.; Peng, G. Microbiome analysis reveals microecological balance in the emerging rice–crayfish integrated breeding mode. Front. Microbiol. 2021, 12, 669570. [Google Scholar]
- Ma, X.; Zhu, F.; Jin, Q. Antibiotics and chemical disease-control agents reduce innate disease resistance in crayfish. Fish Shellfish Immunol. 2019, 86, 169–178. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.; Soderhall, K.; Sirikharin, R.; Jiravanichpaisal, P.; Soderhall, I. Vibrio areninigrae as a pathogenic bacterium in a crustacean. J. Invertebr.Pathol. 2021, 178, 107517. [Google Scholar] [CrossRef]
- Luo, S.W.; Xiong, N.X.; Luo, Z.Y.; Luo, K.K.; Liu, S.J.; Wu, C.; Wang, S.; Wen, M. Effect of Lipopolysaccharide (LPS) stimulation on apoptotic process and oxidative stress in fibroblast cell of hybrid crucian carp compared with those of Carassius cuvieri and Carassius auratus red var. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 248, 109085. [Google Scholar] [CrossRef]
- Noonin, C.; Jiravanichpaisal, P.; Soderhall, I.; Merino, S.; Tomas, J.M.; Soderhall, K. Melanization and pathogenicity in the insect, Tenebrio molitor, and the crustacean, Pacifastacus leniusculus, by Aeromonas hydrophila AH-3. PLoS ONE 2010, 5, e15728. [Google Scholar] [CrossRef]
- Soderhall, K.; Wingren, A.; Johansson, M.W.; Bertheussen, K. The cytotoxic reaction of hemocytes from the freshwater crayfish, Astacus astacus. Cell Immunol. 1985, 94, 326–332. [Google Scholar] [CrossRef]
- Pietsch, C.; Kersten, S.; Burkhardt-Holm, P.; Valenta, H.; Danicke, S. Occurrence of deoxynivalenol and zearalenone in commercial fish feed: An initial study. Toxins 2013, 5, 184–192. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B(1), deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Animal Feed Sci. Technol. Soc. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- European Commission. Recommendation 2006/576/ECon the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intendedfor animal feeding. Off. J. Eur. 2006, 229, 7–9. [Google Scholar]
- Hooft, J.M.; Elmor, A.E.H.I.; Encarnação, P.; Bureau, D.P. Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed-borne Fusarium mycotoxin deoxynivalenol (DON). Aquaculture 2011, 311, 224–232. [Google Scholar] [CrossRef]
- Xie, S.; Zheng, L.; Wan, M.; Niu, J.; Liu, Y.; Tian, L. Effect of deoxynivalenol on growth performance, histological morphology, anti-oxidative ability and immune response of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 82, 442–452. [Google Scholar] [CrossRef]
- Fang, Z.; Zhou, L.; Wang, Y.; Sun, L.; Gooneratne, R. Evaluation the effect of mycotoxins on shrimp (Litopenaeus vannamei) muscle and their limited exposure dose for preserving the shrimp quality. J. Food Process. Pres. 2019, 43, e13902. [Google Scholar] [CrossRef]
- Pietsch, C.; Bucheli, T.D.; Wettstein, F.E.; Burkhardt-Holm, P. Frequent biphasic cellular responses of permanent fish cell cultures to deoxynivalenol (DON). Toxicol. Appl. Pharmacol. 2011, 256, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, S.; Yalin, G.; Xiaojing, L. Deoxynivalenol induces carp neutrophil apoptosis and necroptosis via CYP450s/ROS/PI3K/AKT pathway. Aquaculture 2021, 545, 737182. [Google Scholar]
- Liu, M.; Zhang, L.; Mo, Y.; Li, J.; Yang, J.; Wang, J.; Karrow, N.A.; Wu, H.; Sun, L. Ferroptosis is involved in deoxynivalenol-induced intestinal damage in pigs. J. Anim. Sci. Biotechnol. 2023, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Pestka, J.J. LPS priming potentiates and prolongs proinflammatory cytokine response to the trichothecene deoxynivalenol in the mouse. Toxicol. Appl. Pharmacol. 2006, 211, 53–63. [Google Scholar] [CrossRef]
- Lucke, A.; Bohm, J.; Zebeli, Q.; Metzler-Zebeli, B.U. Dietary deoxynivalenol and oral lipopolysaccharide challenge differently affect intestinal innate immune response and barrier function in broiler chickens. J. Anim. Sci. 2018, 96, 5134–5143. [Google Scholar] [PubMed]
- Halawa, A.; Danicke, S.; Kersten, S.; Breves, G. Intestinal transport of deoxynivalenol across porcine small intestines. Arch. Anim. Nutr. 2013, 67, 134–146. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, T.; Espinosa, G.; Hernández-López, J.; Gollas-Galván, T.; Marrero, J.; Borrell, Y.; Alonso, M.E.; Bécquer, U.; Alonso, M. Effects of Echerichia coli lipopolysaccharides and dissolved ammonia on immune response in southern white shrimp Litopenaeus schmitti. Aquaculture 2008, 274, 118–125. [Google Scholar] [CrossRef]
- Zhou, H.R.; Harkema, J.R.; Yan, D.; Pestka, J.J. Amplified proinflammatory cytokine expression and toxicity in mice coexposed to lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol). J. Toxicol. Environ. Health A 1999, 57, 115–136. [Google Scholar] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Feng, Y.; Ren, N.; Sun, K. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci. Total. Environ. 2019, 666, 944–955. [Google Scholar] [CrossRef]
- Bodea, G.; Munteanu, M.C.; Dinu, D.; Serban, A.; Israel-Roming, F.; Costache, M.; Dinischiotu, A. Influence of deoxynivalenol on the oxidative status of HepG2 cells. Rom. Biotechnol. Lett. 2009, 14, 4349–4359. [Google Scholar]
- Liu, Z.; Qiuqian, L.; Yao, Z.; Wang, X.; Huang, L.; Zheng, J.; Wang, K.; Li, L.; Zhang, D. Effects of a commercial microbial agent on the bacterial communities in shrimp culture system. Front. Microbiol. 2018, 9, 2430. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Lucke, A.; Doupovec, B.; Zebeli, Q.; Bohm, J. A multicomponent mycotoxin deactivator modifies the response of the jejunal mucosal and cecal bacterial community to deoxynivalenol contaminated feed and oral lipopolysaccharide challenge in chickens1. J. Anim. Sci. 2020, 98, 377. [Google Scholar] [CrossRef]
- Feher, M.; Fauszt, P.; Tolnai, E.; Fidler, G.; Pesti-Asboth, G.; Stagel, A.; Szucs, I.; Biro, S.; Remenyik, J.; Paholcsek, M.; et al. Effects of phytonutrient-supplemented diets on the intestinal microbiota of Cyprinus carpio. PLoS ONE 2021, 16, e0248537. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Sun, Y.; Han, W.; Liu, J.; Liu, F.; Cheng, Y. Microbiota comparison in the intestine of juvenile Chinese mitten crab Eriocheir sinensis fed different diets. Aquaculture 2020, 515, 734518. [Google Scholar] [CrossRef]
- Chiodini, R.J.; Dowd, S.E.; Chamberlin, W.M.; Galandiuk, S.; Davis, B.; Glassing, A. Microbial population differentials between mucosal and submucosal intestinal tissues in advanced Crohn’s disease of the ileum. PLoS ONE 2015, 10, e0134382. [Google Scholar] [CrossRef]
- Otake, T.; Fujimoto, M.; Hoshino, Y.; Ishihara, T.; Haneda, T.; Okada, N.; Miki, T. Twin-arginine translocation system is involved in Citrobacter rodentium fitness in the intestinal tract. Infect. Immun. 2020, 88, e00892-19. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Akira, S. Toll-like receptors and innate immunity. Adv. Immunol. 2001, 78, 1–56. [Google Scholar] [PubMed]
- Dai, L.S.; Chu, S.H.; Yu, X.M.; Li, Y.Y. A role of cathepsin L gene in innate immune response of crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2017, 71, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Q.; Meng, J.H.; Gao, J.; Xu, Y.H.; Wang, X.W. Identification of a crustacean beta-1,3-glucanase related protein as a pattern recognition protein in antibacterial response. Fish Shellfish Immunol. 2018, 80, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol. 2015, 43, 510–519. [Google Scholar] [CrossRef]
- Yang, J.; Cougui, C. Crayfish–rice integrated system of production: An agriculture success story in China. A review. Agron. Sustain. Dev. 2021, 41, 68. [Google Scholar]
- Supamattaya, K.; Sukrakanchana, N.; Boonyaratpalin, M.; Schatzmayr, D.; Chittiwan, V. Effects of ochratoxin A and deoxynivalenol on growth performance and immuno-physiological parameters in black tiger shrimp (Penaeus monodon). Songklanakarin J. Sci. Technol. 2005, 27, 91. [Google Scholar]
- Zhu, L.; Yuan, G.; Wang, X.; Zhao, T.; Hou, L.; Li, C.; Jiang, X.; Zhang, J.; Zhao, X.; Pei, C.; et al. Molecular characterization of Rab7 and its involvement in innate immunity in red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2022, 127, 318–328. [Google Scholar] [CrossRef]
- Chen, D.; Guo, L.; Yi, C.; Wang, S.; Ru, Y.; Wang, H. Hepatopancreatic transcriptome analysis and humoral immune factor assays in red claw crayfish (Cherax quadricarinatus) provide insight into innate immunomodulation under Vibrio parahaemolyticus infection. Ecotoxicol. Environ. Saf. 2021, 217, 112266. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dai, L.S.; Wu, B.L.; Zhang, Y.; Chen, J.; He, J.X. Identification and immunoregulatory role of cathepsin A in the red swamp crayfish, Procambarus clarkii. Int. J. Biol. Macromol. 2020, 153, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Arayamethakorn, S.; Supungul, P.; Tassanakajon, A.; Krusong, K. Corrigendum to ‘Characterization of molecular properties and regulatory pathways of CrustinPm1 and CrustinPm7 from the black tiger shrimp Penaeus monodon’. Dev. Comp. Immunol. 2017, 76, 420. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Y.; Lu, K.; Xiong, H.; Zhang, Y.; Wei, W. Herbicide atrazine exposure induce oxidative stress, immune dysfunction and WSSV proliferation in red swamp crayfish Procambarus clarkii. Chemosphere 2021, 283, 131227. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Song, X.; Teng, P.; Ji, H.; Peng, L.; Qiu, Y.; Guo, D.; Jiang, S. Effect of maduramicin on crayfish (Procambius clarkii): Hematological parameters, oxidative stress, histopathological changes and stress response. Ecotoxicol. Environ. Saf. 2021, 211, 111896. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, K.; Dai, X.; Zhang, R.; Cao, X.; Zhang, C.; Wang, K.; Huang, X.; Ren, Q. Identification of two LGBPs (isoform1 and isoform2) and their function in AMP expression and PO activation in male hepatopancreas. Fish Shellfish Immunol. 2019, 95, 624–634. [Google Scholar] [CrossRef]
- Osuna-Jimenez, I.; Abril, N.; Vioque-Fernandez, A.; Gomez-Ariza, J.L.; Prieto-Alamo, M.J.; Pueyo, C. The environmental quality of Donana surrounding areas affects the immune transcriptional profile of inhabitant crayfish Procambarus clarkii. Fish Shellfish Immunol. 2014, 40, 136–145. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Chen, Y.; Si, Q.; Tian, J.; Jiang, Q.; Yang, J. Exposure time relevance of response to nitrite exposure: Insight from transcriptional responses of immune and antioxidant defense in the crayfish, Procambarus clarkii. Aquat. Toxicol. 2019, 214, 105262. [Google Scholar] [CrossRef]
- Liu, Q.N.; Tang, Y.Y.; Li, Y.T.; Zha, X.H.; Yang, T.T.; Zhang, D.Z.; Wang, J.L.; Jiang, S.H.; Zhou, C.L.; Tang, B.P.; et al. Differentially expressed genes in hemocytes of red swamp crayfish Procambarus clarkii following lipopolysaccharide challenge. Aquaculture 2021, 533, 735943. [Google Scholar] [CrossRef]
- Dai, Y.J.; Wang, Y.Q.; Zhang, Y.H.; Liu, Y.; Li, J.Q.; Wei, S.; Zhao, L.J.; Zhou, Y.C.; Lin, L.; Lan, J.F. The role of ficolin-like protein (PcFLP1) in the antibacterial immunity of red swamp crayfish (Procambarus clarkii). Mol. Immunol. 2017, 81, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, L.; Chen, W.; Zheng, X.; Zhang, Y.; Liu, Q.; Yang, D. Neutrophil plays critical role during Edwardsiella piscicida immersion infection in zebrafish larvae. Fish Shellfish Immunol. 2019, 87, 565–572. [Google Scholar] [CrossRef] [PubMed]

| Name | Sequence (5’-3’) | Reference |
|---|---|---|
| Toll sense | GCTGTTGCTGCTTAGGCTCA | [43] |
| Toll antisense | TCCTCCACAGCTCTTCATTCC | |
| Cactus sense | CTTGTGAGAGAGCCGTGTG | [43] |
| Cactus antisense | CAGTACAAGCAGCAGCAGCA | |
| Spätzle sense | GTCGGCAGCAACGACATACA | [43] |
| Spätzle antisense | GGTGTCATGGTTGGCTGTGA | |
| PcBGRPRT sense | CCCACGCTGACTATTCGG | [36] |
| PcBGRPRT antisense | GGTTGTCCAGGGAGTTGTCG | |
| Dorsal sense | TCACTGTTGACCCACCTTAC | [44] |
| Dorsal antisense | GGAAAGGGTCCACTCTAATC | |
| ALF8 sense | GGGGGAAGCGATGACGAG | [45] |
| ALF8 antisense | GACGGGTTGGCACAAGAGC | |
| gst sense | ACTTAGAGACGGACTTCCAG | [46] |
| gst antisense | CGAGGGCGAACTTCACGG | |
| PcCru4 sense | CTCTGACTGCCAGGTGTTT | [47] |
| PcCru4 antisense | TGCGAGCTGTGATGGTTAG | |
| akp sense | CCACACTACGTGGCAGCAGCGAC | [48] |
| akp antisense | GCCAGTGAAGAGGTGGGCATGG | |
| cat sense | GCTGAGGTGGAACAGATGGCA | [49] |
| cat antisense | AAGGGAATCAGACCGTGAGTGATC | |
| sod sense | CAAATCAGTGGCAGGCTGGAAA | [49] |
| sod antisense | CAAATCAGTGGCAGGCTGGAAA | |
| PcCTSL sense | CGGATCACTGGAGGGTCAAACACTT | [35] |
| PcCTSL antisense | GCAATTTTCATCCTCGGCATCAT | |
| 18S sense | TCTTCTTAGAGGGATTAGCGG | [50] |
| 18S antisense | AAGGGGATTGAACGGGTTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Z.; Xu, X.; Xiang, D.; Xu, J.; Yang, Q.; Wang, X.; Liu, J.; Luo, M.; Wei, W. Effects of Lipopolysaccharide and Deoxynivalenol on the Survival, Antioxidant and Immune Response, and Histopathology of Crayfish (Procambarus clarkii). Toxins 2023, 15, 479. https://doi.org/10.3390/toxins15080479
Wen Z, Xu X, Xiang D, Xu J, Yang Q, Wang X, Liu J, Luo M, Wei W. Effects of Lipopolysaccharide and Deoxynivalenol on the Survival, Antioxidant and Immune Response, and Histopathology of Crayfish (Procambarus clarkii). Toxins. 2023; 15(8):479. https://doi.org/10.3390/toxins15080479
Chicago/Turabian StyleWen, Zhengrong, Xiaoli Xu, Dan Xiang, Junfeng Xu, Qiufeng Yang, Xiaofu Wang, Jiashou Liu, Mingzhong Luo, and Wei Wei. 2023. "Effects of Lipopolysaccharide and Deoxynivalenol on the Survival, Antioxidant and Immune Response, and Histopathology of Crayfish (Procambarus clarkii)" Toxins 15, no. 8: 479. https://doi.org/10.3390/toxins15080479
APA StyleWen, Z., Xu, X., Xiang, D., Xu, J., Yang, Q., Wang, X., Liu, J., Luo, M., & Wei, W. (2023). Effects of Lipopolysaccharide and Deoxynivalenol on the Survival, Antioxidant and Immune Response, and Histopathology of Crayfish (Procambarus clarkii). Toxins, 15(8), 479. https://doi.org/10.3390/toxins15080479