Cardiac Toxicity Induced by Long-Term Environmental Levels of MC-LR Exposure in Mice
Abstract
1. Introduction
2. Results
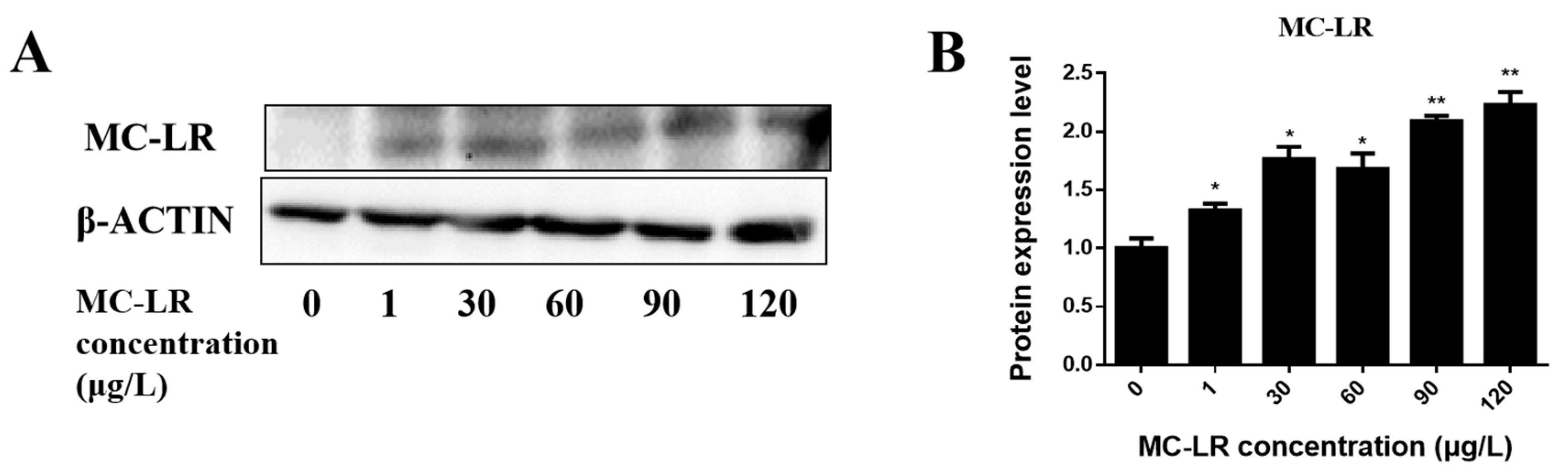
2.1. Detection of Endogenous Exposure to MC-LR in Cardiac Tissue
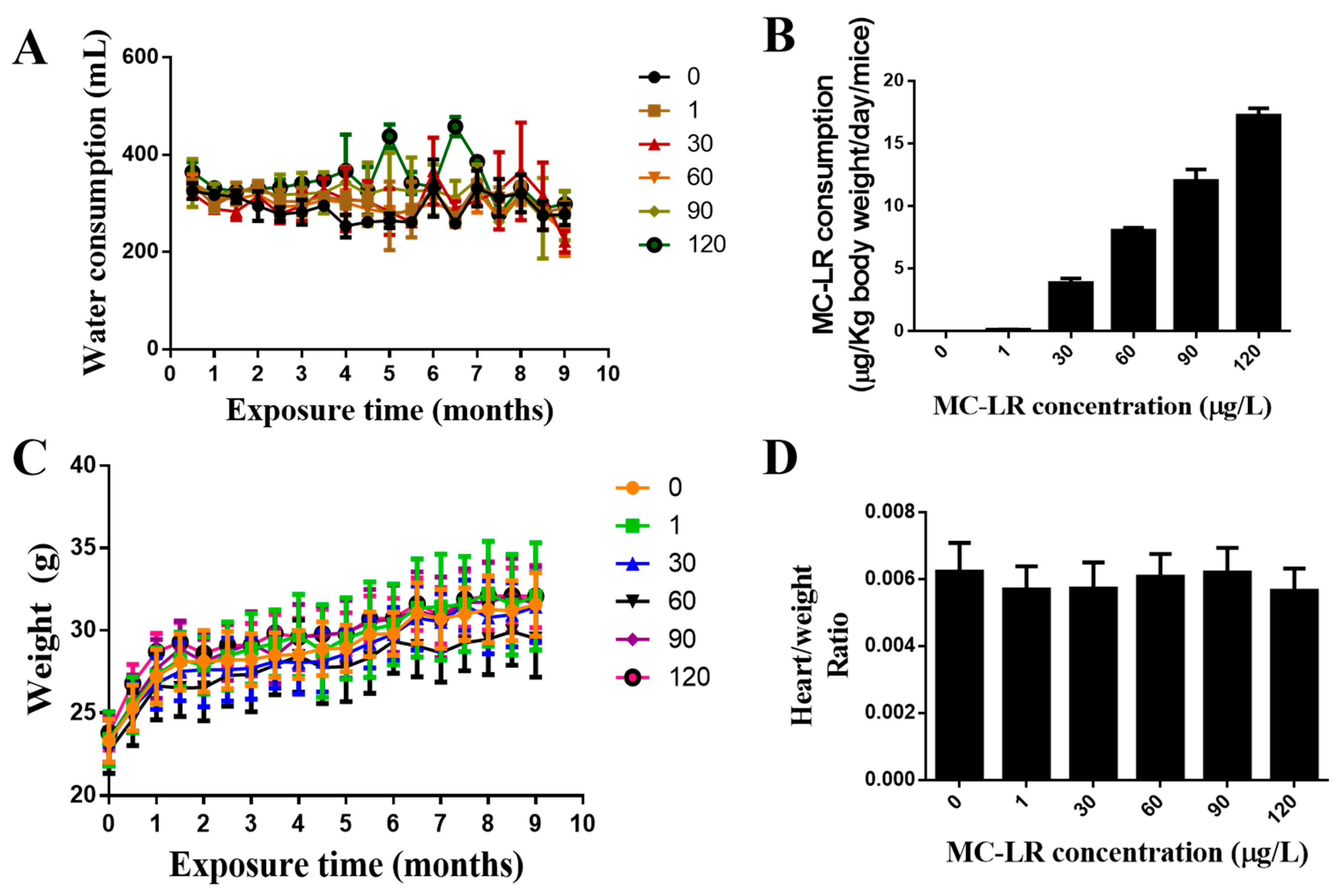
2.2. The Effect of MC-LR on the General Condition and the Weight Index of the Mouse Heart
2.3. Effects of MC-LR Treatment on Mouse Cardiac Function
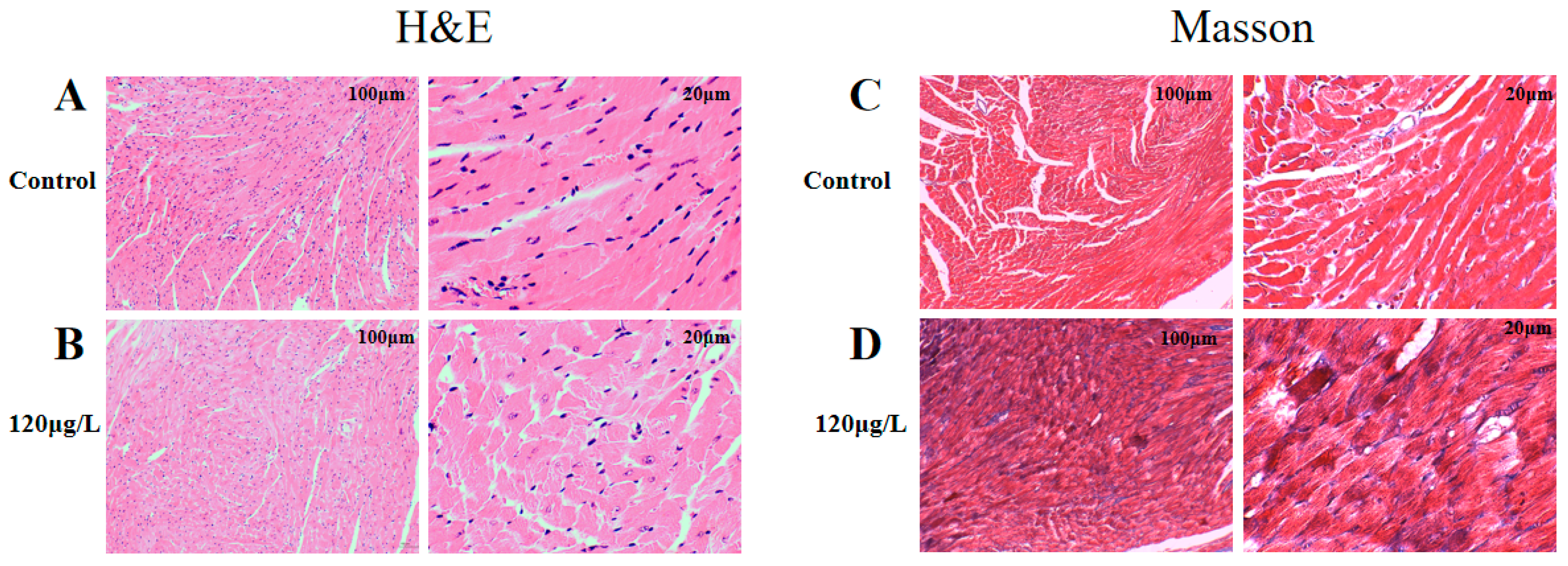
2.4. Effects of MC-LR Treatment on the Histological Morphology of Mouse Heart
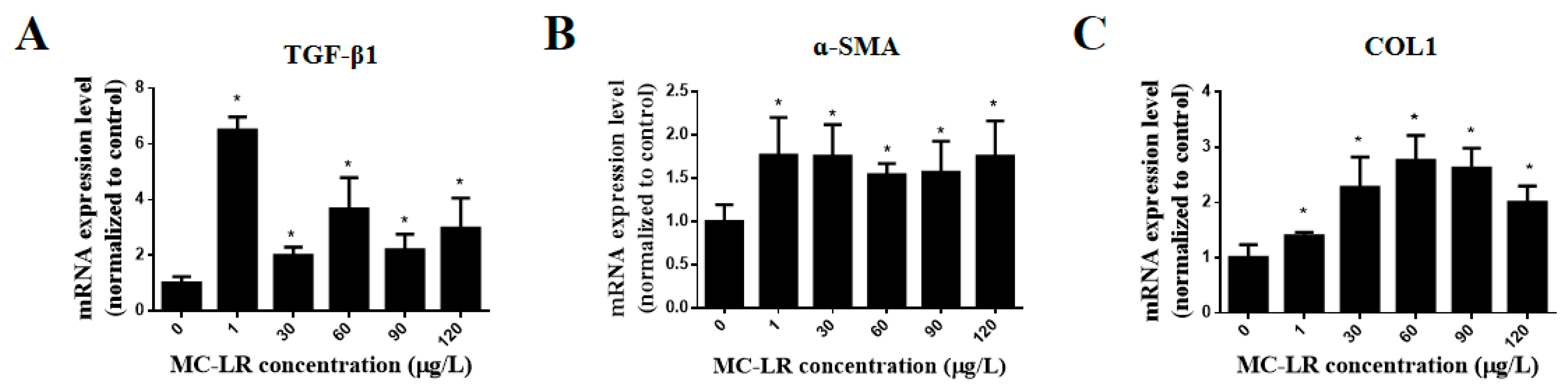
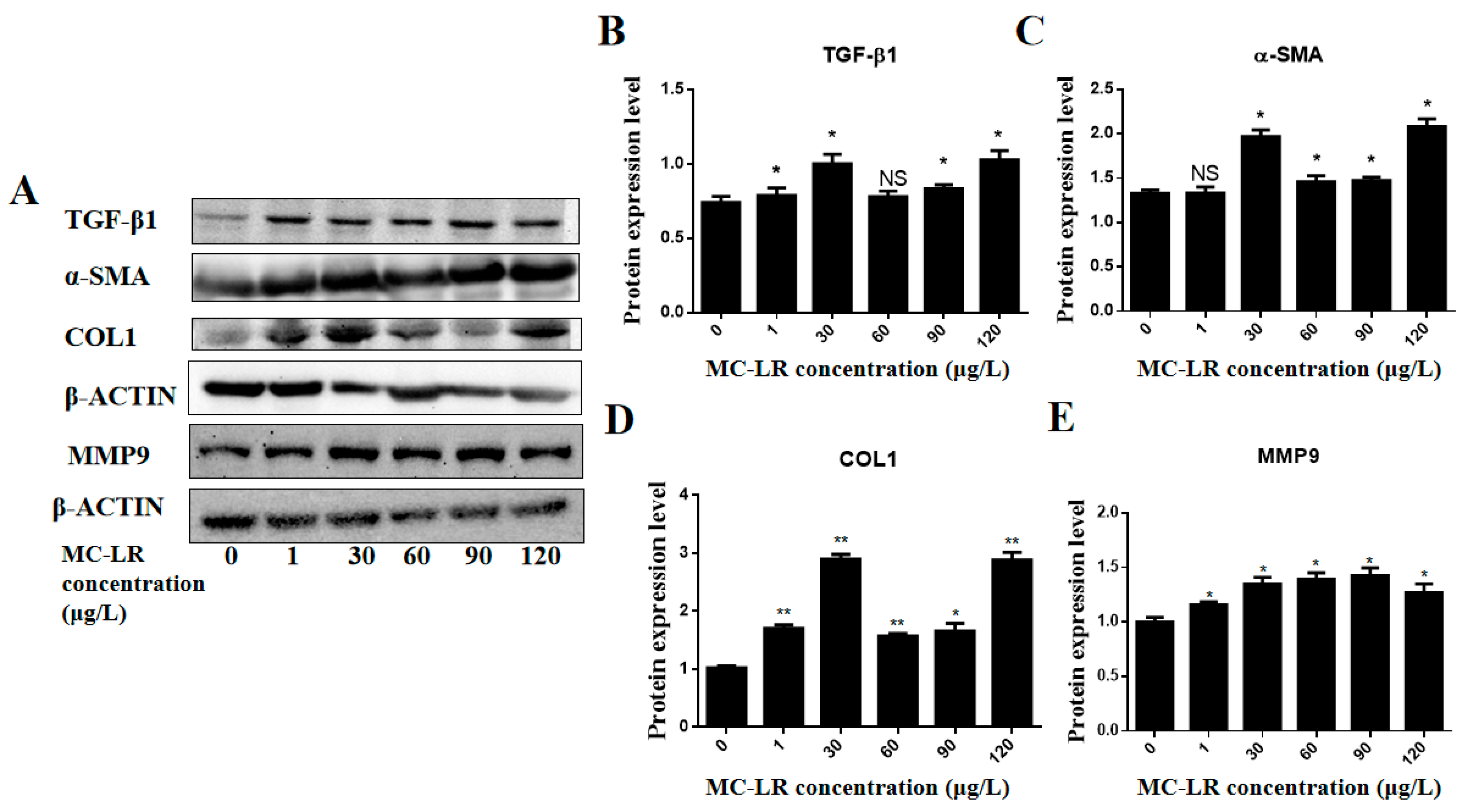
2.5. Effects of MC-LR on the Expression of Myocardial Fibrosis Marker Factors in Mouse Heart Tissue
2.6. MC-LR Expression of PI3K/AKT/mTOR Pathway-Related Proteins in the Mouse Heart
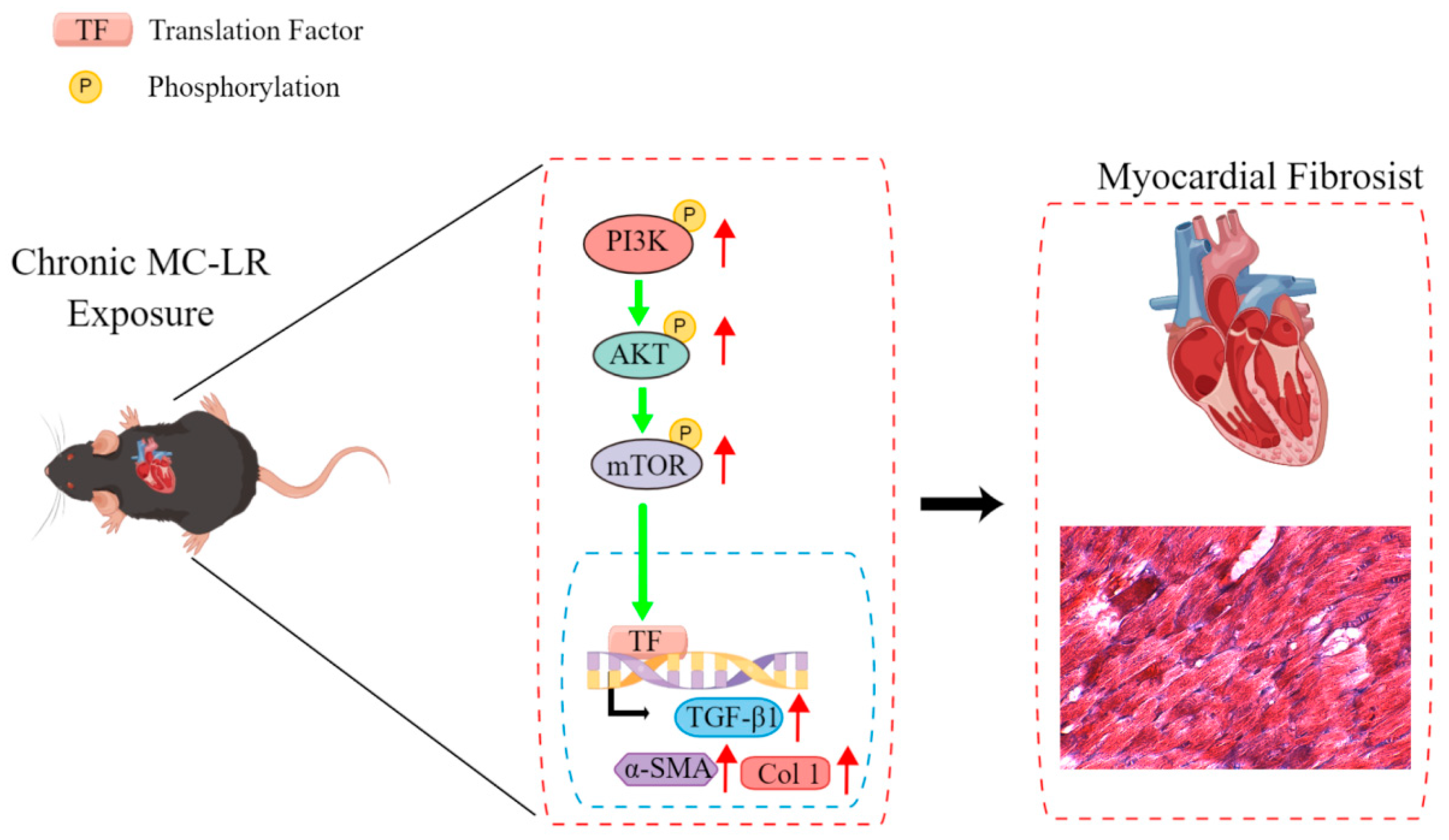
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
Grouping and Handling of Experimental Mice
5.2. Experimental Method
5.2.1. H&E Staining
5.2.2. Masson Staining
5.2.3. Echocardiography
5.2.4. qPCR
5.2.5. Western Blot
5.2.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baures, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, S.; Wen, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Luo, D.; Liu, R.; Yang, F. Regulation of Microcystin-LR-Induced DNA Damage by miR-451a in HL7702 Cells. Toxins 2019, 11, 164. [Google Scholar] [CrossRef]
- Yang, F.; Huang, F.; Feng, H.; Wei, J.; Massey, I.Y.; Liang, G.; Zhang, F.; Yin, L.; Kacew, S.; Zhang, X.; et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res. 2020, 174, 115638. [Google Scholar] [CrossRef]
- Wei, J.; Pengji, Z.; Zhang, J.; Peng, T.; Luo, J.; Yang, F. Biodegradation of MC-LR and its key bioactive moiety Adda by Sphingopyxis sp. YF1: Comprehensive elucidation of the mechanisms and pathways. Water Res. 2023, 229, 119397. [Google Scholar] [CrossRef]
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V.L. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wei, J.; Huang, F.; Feng, H.; Peng, T.; Luo, J.; Yang, F. The detoxification activities and mechanisms of microcystinase towards MC-LR. Ecotoxicol. Environ. Saf. 2022, 236, 113436. [Google Scholar] [CrossRef]
- Ait Abderrahim, L.; Taibi, K.; Boussaid, M.; Al-Shara, B.; Ait Abderrahim, N.; Ait Abderrahim, S. Allium sativum mitigates oxidative damages induced by Microcystin-LR in heart and liver tissues of mice. Toxicon 2021, 200, 30–37. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Y.; Wen, C.; Liu, W.; Cao, L.; Feng, X.; Chen, J.; Wang, H.; Tang, Y.; Tian, L.; et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ. Int. 2021, 154, 106555. [Google Scholar] [CrossRef]
- La-Salete, R.; Oliveira, M.M.; Palmeira, C.A.; Almeida, J.; Peixoto, F.P. Mitochondria a key role in microcystin-LR kidney intoxication. J. Appl. Toxicol. 2008, 28, 55–62. [Google Scholar] [CrossRef]
- Piyathilaka, M.A.; Pathmalal, M.M.; Tennekoon, K.H.; De Silva, B.G.; Samarakoon, S.R.; Chanthirika, S. Microcystin-LR-induced cytotoxicity and apoptosis in human embryonic kidney and human kidney adenocarcinoma cell lines. Microbiology 2015, 161, 819–828. [Google Scholar] [CrossRef]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. Int. 2016, 23, 7211–7219. [Google Scholar] [CrossRef]
- Ito, E.; Kondo, F.; Harada, K. First report on the distribution of orally administered microcystin-LR in mouse tissue using an immunostaining method. Toxicon 2000, 38, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Wang, X.; Chen, L.; Liu, W.; Cai, D.; Deng, S.; Chu, H.; Liu, Y.; Feng, X.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, H.; Yang, H.; Xue, W.; Wang, L.; Wei, W. Microcystin-LR disturbs testicular development of giant freshwater prawn Macrobrachium rosenbergii. Chemosphere 2019, 222, 584–592. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Ma, Y.; Chen, X.; Losiewicz, M.D.; Du, X.; Tian, Z.; Zhang, S.; Shi, L.; Zhang, H.; et al. Long-term exposure to low concentrations of MC-LR induces blood-testis barrier damage through the RhoA/ROCK pathway. Ecotoxicol. Environ. Saf. 2022, 236, 113454. [Google Scholar] [CrossRef]
- Cao, L.; Massey, I.Y.; Feng, H.; Yang, F. A Review of Cardiovascular Toxicity of Microcystins. Toxins 2019, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, J.; Zhang, M. Microcystins Induces Vascular Inflammation in Human Umbilical Vein Endothelial Cells via Activation of NF-kappaB. Mediat. Inflamm. 2015, 2015, 942159. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cyanobacterial Toxins: Microcystin-LR Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Komatsu, M.; Furukawa, T.; Ikeda, R.; Takumi, S.; Nong, Q.; Aoyama, K.; Akiyama, S.; Keppler, D.; Takeuchi, T. Involvement of mitogen-activated protein kinase signaling pathways in microcystin-LR-induced apoptosis after its selective uptake mediated by OATP1B1 and OATP1B3. Toxicol. Sci. 2007, 97, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Kock, K.; Oswald, S.; Draber, K.; Meissner, K.; Eckel, L.; Bohm, M.; Felix, S.B.; Vogelgesang, S.; Jedlitschky, G.; et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin. Pharmacol. Ther. 2006, 80, 607–620. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, P.; Tang, R.; Zhang, X.; Li, L.; Li, D. In vivo studies on the toxic effects of microcystins on mitochondrial electron transport chain and ion regulation in liver and heart of rabbit. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Xie, P. The dose makes the poison. Sci. Total Environ. 2018, 621, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Li, W.; Li, J.; Wu, W.; Tao, J.; Liu, H. Vitamin C Protects Porcine Oocytes From Microcystin-LR Toxicity During Maturation. Front. Cell Dev. Biol. 2020, 8, 582715. [Google Scholar] [CrossRef]
- Milutinović, A.; Zorc-Pleskovic, R.; Petrovic, D.; Zorc, M.; Suput, D. Microcystin-LR induces alterations in heart muscle. Folia Biol. 2006, 52, 116–118. [Google Scholar]
- Alosman, M.; Cao, L.; Massey, I.Y.; Yang, F. The lethal effects and determinants of microcystin-LR on heart: A mini review. Toxin Rev. 2020, 40, 517–526. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- Qiu, T.; Xie, P.; Liu, Y.; Li, G.; Xiong, Q.; Hao, L.; Li, H. The profound effects of microcystin on cardiac antioxidant enzymes, mitochondrial function and cardiac toxicity in rat. Toxicology 2009, 257, 86–94. [Google Scholar] [CrossRef]
- Martins, N.D.; Yunes, J.S.; McKenzie, D.J.; Rantin, F.T.; Kalinin, A.L.; Monteiro, D.A. Microcystin—LR exposure causes cardiorespiratory impairments and tissue oxidative damage in trahira, Hoplias malabaricus. Ecotoxicol. Environ. Saf. 2019, 173, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.R.; Frenkel, A.; Harke, M.J.; Jankowiak, J.G.; Gobler, C.J.; McElroy, A.E. Effects of Microcystis on development of early life stage Japanese medaka (Oryzias latipes): Comparative toxicity of natural blooms, cultured Microcystis and microcystin-LR. Aquat. Toxicol. 2018, 194, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Dang, Y.; Xu, Q.; Yu, L.; Liu, C.; Yuan, Y.; Wang, J. Microcystin-LR induced developmental toxicity and apoptosis in zebrafish (Danio rerio) larvae by activation of ER stress response. Chemosphere 2016, 157, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Suput, D.; Zorc-Pleskovic, R.; Petrovic, D.; Milutinović, A. Cardiotoxic injury caused by chronic administration of microcystin-YR. Folia Biol. 2010, 56, 14–18. [Google Scholar]
- Ma, Z.G.; Yuan, Y.P.; Wu, H.M.; Zhang, X.; Tang, Q.Z. Cardiac fibrosis: New insights into the pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Medeiros, N.I.; Gomes, J.A.S.; Correa-Oliveira, R. Synergic and antagonistic relationship between MMP-2 and MMP-9 with fibrosis and inflammation in Chagas’ cardiomyopathy. Parasite Immunol. 2017, 39, e12446. [Google Scholar] [CrossRef]
- Zhabyeyev, P.; McLean, B.; Patel, V.B.; Wang, W.; Ramprasath, T.; Oudit, G.Y. Dual loss of PI3Kalpha and PI3Kgamma signaling leads to an age-dependent cardiomyopathy. J. Mol. Cell. Cardiol. 2014, 77, 155–159. [Google Scholar] [CrossRef]
- Yang, K.C.; Ku, Y.C.; Lovett, M.; Nerbonne, J.M. Combined deep microRNA and mRNA sequencing identifies protective transcriptomal signature of enhanced PI3Kalpha signaling in cardiac hypertrophy. J. Mol. Cell. Cardiol. 2012, 53, 101–112. [Google Scholar] [CrossRef]
- McMullen, J.R.; Amirahmadi, F.; Woodcock, E.A.; Schinke-Braun, M.; Bouwman, R.D.; Hewitt, K.A.; Mollica, J.P.; Zhang, L.; Zhang, Y.; Shioi, T.; et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA 2007, 104, 612–617. [Google Scholar] [CrossRef]
- Ma, Z.G.; Yuan, Y.P.; Zhang, X.; Xu, S.C.; Wang, S.S.; Tang, Q.Z. Piperine Attenuates Pathological Cardiac Fibrosis Via PPAR-gamma/AKT Pathways. EBioMedicine 2017, 18, 179–187. [Google Scholar] [CrossRef]
- Wei, W.Y.; Ma, Z.G.; Xu, S.C.; Zhang, N.; Tang, Q.Z. Pioglitazone Protected against Cardiac Hypertrophy via Inhibiting AKT/GSK3beta and MAPK Signaling Pathways. PPAR Res. 2016, 2016, 9174190. [Google Scholar] [CrossRef] [PubMed]
- Sekulić, A.; Hudson, C.C.; Homme, J.L.; Yin, P.; Otterness, D.M.; Karnitz, L.M.; Abraham, R.T. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000, 60, 3504–3513. [Google Scholar]
- Yano, N.; Tseng, A.; Zhao, T.C.; Robbins, J.; Padbury, J.F.; Tseng, Y.T. Temporally controlled overexpression of cardiac-specific PI3Kalpha induces enhanced myocardial contractility—A new transgenic model. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1690–H1694. [Google Scholar] [CrossRef]
- Abeyrathna, P.; Su, Y. The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 2015, 74, 38–48. [Google Scholar] [CrossRef]
- Nagoshi, T.; Matsui, T.; Aoyama, T.; Leri, A.; Anversa, P.; Li, L.; Ogawa, W.; del Monte, F.; Gwathmey, J.K.; Grazette, L.; et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J. Clin. Investig. 2005, 115, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Luna, J.I.; Marusina, A.I.; Merleev, A.; Kundu-Raychaudhuri, S.; Fiorentino, D.; Raychaudhuri, S.P.; Maverakis, E. Dual mTOR Inhibition Is Required to Prevent TGF-beta-Mediated Fibrosis: Implications for Scleroderma. J. Investig. Dermatol. 2015, 135, 2873–2876. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, B.; Chen, T.; Wang, X.; Huang, P.; Xu, L.; Guo, Z. Microcystin-LR promotes proliferation by activating Akt/S6K1 pathway and disordering apoptosis and cell cycle associated proteins phosphorylation in HL7702 cells. Toxicol. Lett. 2016, 240, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Pan, C.; Li, D.; Han, X. Microcystin-leucine-arginine causes blood-testis barrier disruption and degradation of occludin mediated by matrix metalloproteinase-8. Cell. Mol. Life Sci. 2018, 75, 1117–1132. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, M.; Tang, Y.; Chen, Y.; Zhu, C.; Yang, Y.; Wang, C.; Yu, G.; Tang, Z. Responses of the proteome in testis of mice exposed chronically to environmentally relevant concentrations of Microcystin-LR. Ecotoxicol. Environ. Saf. 2020, 187, 109824. [Google Scholar] [CrossRef]

| Groups | Control (µg/L) | MC-LR Treated (120 µg/L) |
|---|---|---|
| LVEF (%) | 53.88 ± 3.96 | 36.28 ± 4.32 *** |
| LVFS (%) | 27.36 ± 2.38 | 17.24 ± 2.39 *** |
| LVESD(mm) | 29.4 ± 7.55 | 49.58 ± 2.90 ** |
| LVEDD (mm) | 63.02 ± 12.22 | 77.95 ± 4.43 * |
| LVPWd (mm) | 0.57 ± 0.10 | 0.72 ± 0.07 |
| LVPWs (mm) | 0.88 ± 0.17 | 0.89 ± 0.11 |
| IDd (mm) | 3.81 ± 0.33 | 4.18 ± 0.10 |
| LVIDs (mm) | 2.78 ± 0.32 | 3.46 ± 0.08 |
| HR (beat/min) | 403.28 ± 43.69 | 388.00 ± 26.03 |
| Primer Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | CTAAGGCCAACCGTGAAAAG | AGGGTCCCAGACAGAAGTTG |
| Col1 | TGTTCAGCTTTGTGGACCTC | TCAAGCATACCTCGGGTTTC |
| α-SMA | CCTGAAGAGCATCCGACACT | TACGTCCAGAGGCATAGAGG |
| TGF-β | ACCGGAGAGCCCTGGATA | CCACGTAGTAGACGATGG |
| Antibody Name | Source | Dilution Ratios |
|---|---|---|
| TGF-β | Proteintech, Wuhan, China | 1:1000 |
| α-SMA | Proteintech, Wuhan, China | 1:1000 |
| COL1 | Proteintech, Wuhan, China | 1:1000 |
| PI3K | Proteintech, Wuhan, China | 1:1000 |
| P-PI3K | Cell Signaling Technology Company, Danvers, MA, USA | 1:1000 |
| AKT | Proteintech, Wuhan, China | 1:1000 |
| P-AKT | Cell Signaling Technology Company, Danvers, MA, USA | 1:1000 |
| mTOR | Proteintech, Wuhan, China | 1:1000 |
| p-mTOR | Proteintech, Wuhan, China | 1:1000 |
| β-ACTIN | Cell Signaling Technology Company, Danvers, MA, USA | 1:1000 |
| Rabbit secondary antibody | Abbkine company, Wuhan, China | 1:3000 |
| MC-LR | Alexis Corporation (Lausen, Switzerland) | 1:3000 |
| MMP9 | Proteintech, Wuhan, China | 1:1500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Liu, Y.; Yang, Y.; Massey, I.Y.; Cao, L.; Osman, M.A.; Yang, F. Cardiac Toxicity Induced by Long-Term Environmental Levels of MC-LR Exposure in Mice. Toxins 2023, 15, 427. https://doi.org/10.3390/toxins15070427
Yan C, Liu Y, Yang Y, Massey IY, Cao L, Osman MA, Yang F. Cardiac Toxicity Induced by Long-Term Environmental Levels of MC-LR Exposure in Mice. Toxins. 2023; 15(7):427. https://doi.org/10.3390/toxins15070427
Chicago/Turabian StyleYan, Canqun, Ying Liu, Yue Yang, Isaac Yaw Massey, Linghui Cao, Muwaffak Al Osman, and Fei Yang. 2023. "Cardiac Toxicity Induced by Long-Term Environmental Levels of MC-LR Exposure in Mice" Toxins 15, no. 7: 427. https://doi.org/10.3390/toxins15070427
APA StyleYan, C., Liu, Y., Yang, Y., Massey, I. Y., Cao, L., Osman, M. A., & Yang, F. (2023). Cardiac Toxicity Induced by Long-Term Environmental Levels of MC-LR Exposure in Mice. Toxins, 15(7), 427. https://doi.org/10.3390/toxins15070427






