Domperidone Protects Cells from Intoxication with Clostridioides difficile Toxins by Inhibiting Hsp70-Assisted Membrane Translocation
Abstract
:1. Introduction
2. Results
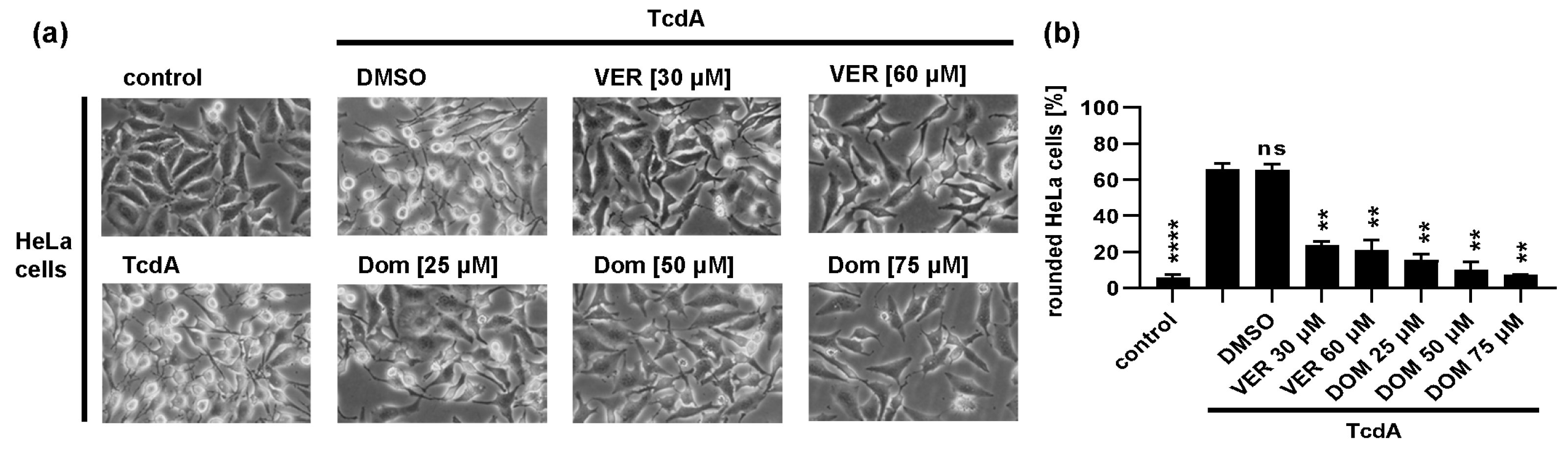
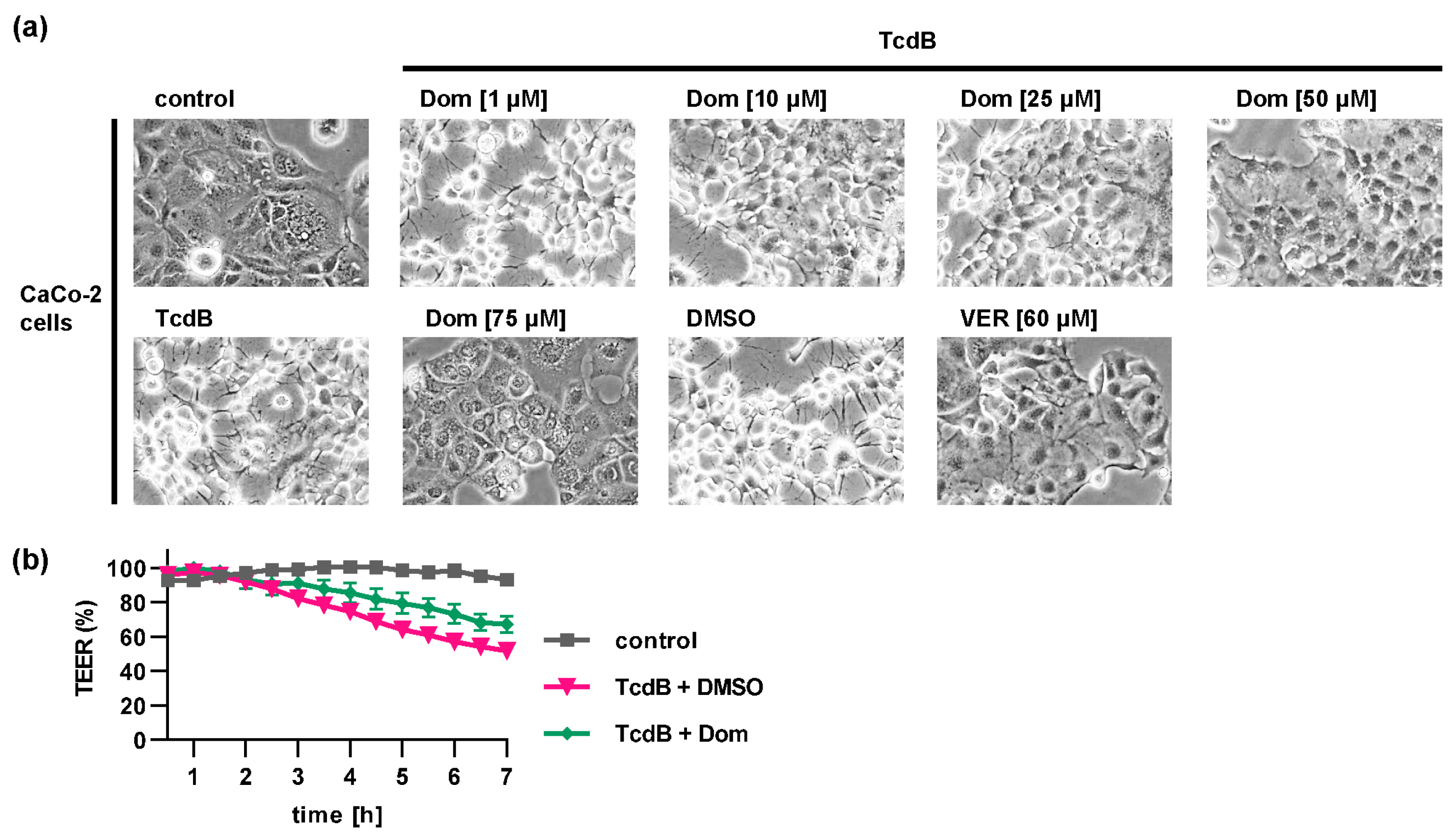
2.1. Pharmacological Inhibition of Hsp70 Reduces the TcdB Induced Cell Rounding in a Time- and Concentration-Dependent Manner
2.2. Pharmacological Inhibition of Hsp70 Leads to Reduced Glucosylation of Intracellular Rac1 by TcdB
2.3. Domperidone Has no Inhibitory Effect on TcdB Receptor Binding, Autoprotease Activity or In Vitro Glucosyltransferase Activity
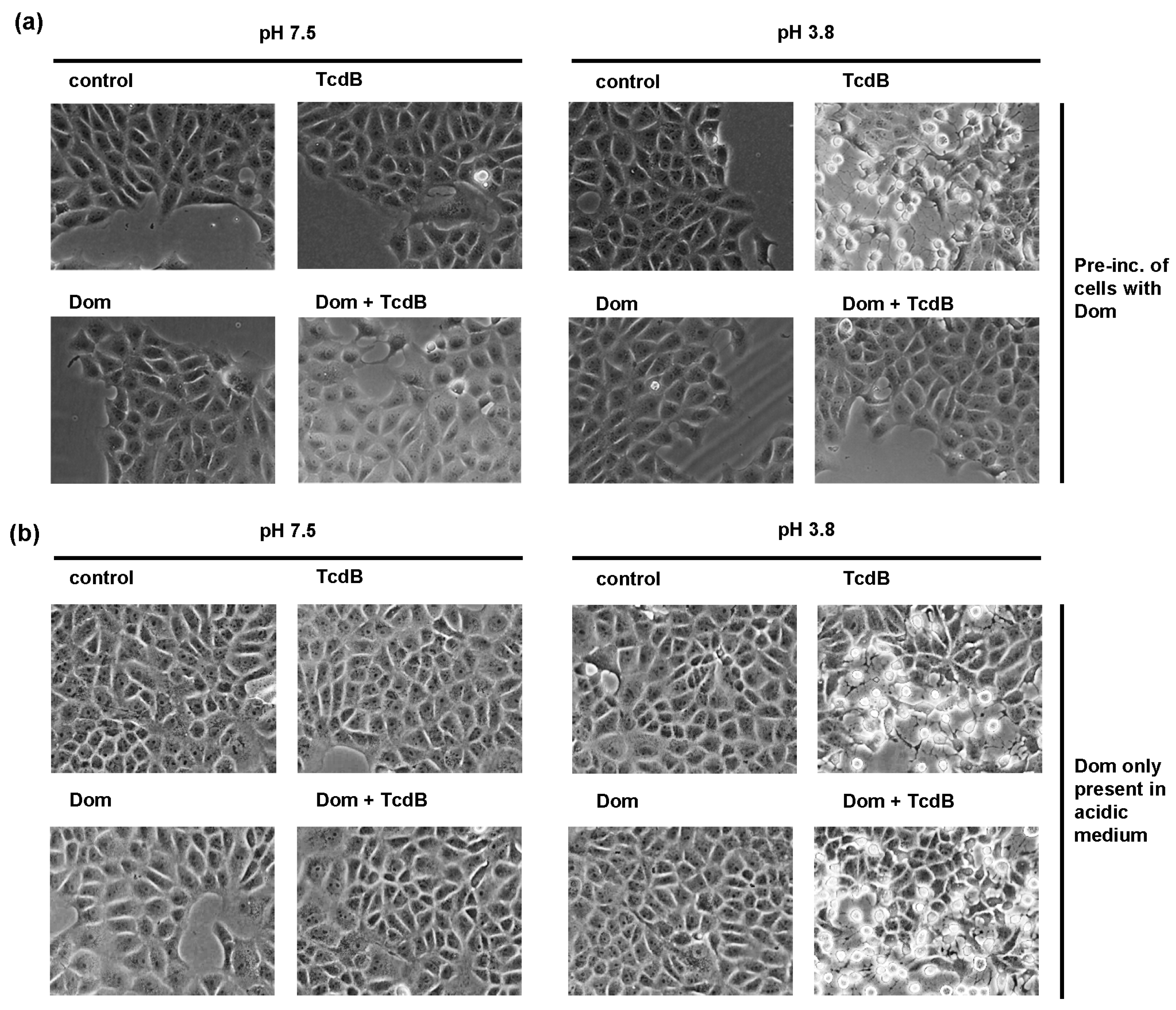
2.4. Domperidone Inhibits pH-Driven Membrane Translocation of the Enzyme Domain of TcdB
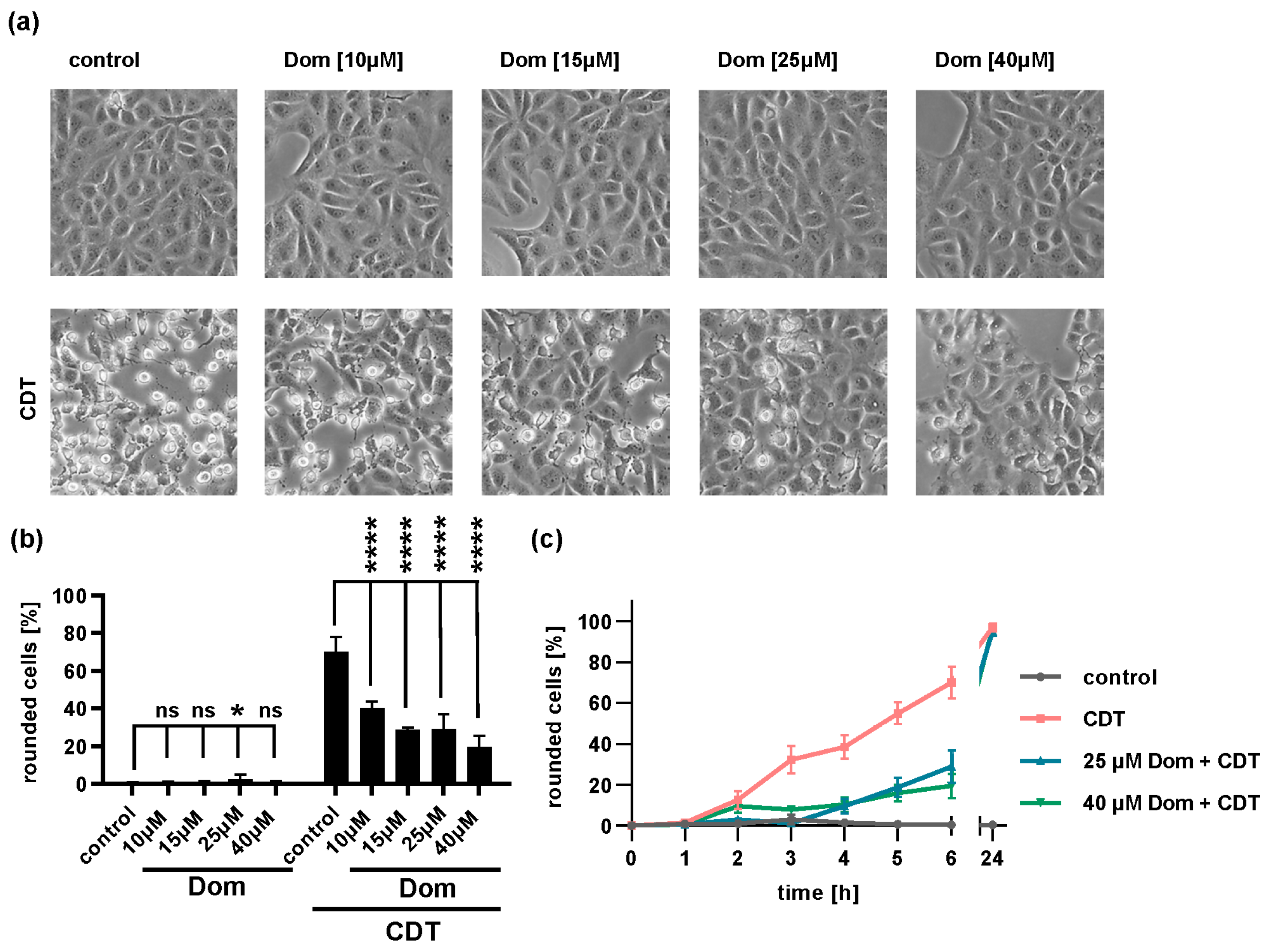
2.5. Domperidone Reduces Intoxication of Cells with Binary CDT
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Cell Culture and Intoxication Experiments
4.3. Glucosylation Status of Intracellular Rac1 after TcdB-Treatment
4.4. Cell-Binding Assay
4.5. In Vitro Autoprocessing of TcdB
4.6. UDP-GloTM Glucosylation Assay
4.7. In Vitro Glucosylation of Rac1 by TcdB
4.8. Toxin Translocation Assay
4.9. Transepithelial Electrical Resistance (TEER) Measurements
4.10. Reproducibility of Experiments and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef]
- Kordus, S.L.; Thomas, A.K.; Lacy, D.B. Clostridioides difficile Toxins: Mechanisms of Action and Antitoxin Therapeutics. Nat. Rev. Microbiol. 2022, 20, 285–298. [Google Scholar] [CrossRef]
- Kuehne, S.A.; Collery, M.M.; Kelly, M.L.; Cartman, S.T.; Cockayne, A.; Minton, N.P. Importance of Toxin A, Toxin B, and CDT in Virulence of an Epidemic Clostridium Difficile Strain. J. Infect. Dis. 2014, 209, 83–86. [Google Scholar] [CrossRef]
- Shen, A. Clostridioides difficile Spores: Bile Acid Sensors and Trojan Horses of Transmission. Clin. Colon Rectal Surg. 2020, 33, 58–66. [Google Scholar] [CrossRef]
- Abeyawardhane, D.L.; Godoy-Ruiz, R.; Adipietro, K.A.; Varney, K.M.; Rustandi, R.R.; Pozharski, E.; Weber, D.J. The Importance of Therapeutically Targeting the Binary Toxin from Clostridioides Difficile. Int. J. Mol. Sci. 2021, 22, 2926. [Google Scholar] [CrossRef]
- Qa’Dan, M.; Spyres, L.M.; Ballard, J.D. PH-Induced Conformational Changes InClostridium Difficile Toxin B. Infect. Immun. 2000, 68, 2470–2474. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, G.; Schirmer, J.; Leemhuis, J.; Busch, C.; Meyer, D.K.; Aktories, K.; Barth, H. Cellular Uptake of Clostridium Difficile Toxin B: Translocation of the N-terminal catalytic domain into the cytosol of eukaryotic cells. J. Biol. Chem. 2003, 278, 44535–44541. [Google Scholar] [CrossRef] [Green Version]
- Barth, H.; Pfeifer, G.; Hofmann, F.; Maier, E.; Benz, R.; Aktories, K. Low PH-Induced Formation of Ion Channels by Clostridium Difficile Toxin B in Target Cells. J. Biol. Chem. 2001, 276, 10670–10676. [Google Scholar] [CrossRef] [Green Version]
- Reineke, J.; Tenzer, S.; Rupnik, M.; Koschinski, A.; Hasselmayer, O.; Schrattenholz, A.; Schild, H.; von Eichel-Streiber, C. Autocatalytic Cleavage of Clostridium Difficile Toxin B. Nature 2007, 446, 415–419. [Google Scholar] [CrossRef]
- Egerer, M.; Giesemann, T.; Jank, T.; Satchell, K.J.F.; Aktories, K. Auto-Catalytic Cleavage of Clostridium Difficile Toxins A and B Depends on Cysteine Protease Activity. J. Biol. Chem. 2007, 282, 25314–25321. [Google Scholar] [CrossRef] [Green Version]
- Just, I.; Selzer, J.; Wilm, M.; Eichel-Streiber, C.; von Mann, M.; Aktories, K. Glucosylation of Rho Proteins by Clostridium Difficile Toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef]
- Just, I.; Wilm, M.; Selzer, J.; Rex, G.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. The Enterotoxin from Clostridium Difficile (ToxA) Monoglucosylates the Rho Proteins. J. Biol. Chem. 1995, 270, 13932–13936. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in Cell Biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Shiota, A.; Asai, N.; Koizumi, Y.; Yamagishi, Y.; Sakanashi, D.; Nakamura, A.; Suematsu, H.; Ohnishi, M.; Mikamo, H. Risk Factors of First Recurrence of Clostridioides difficile Infection. Anaerobe 2022, 75, 102556. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium Difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Ernst, K. Requirement of Peptidyl-Prolyl Cis/Trans Isomerases and Chaperones for Cellular Uptake of Bacterial AB-Type Toxins. Front. Cell. Infect. Microbiol. 2022, 12, 938015. [Google Scholar] [CrossRef]
- Haug, G.; Leemhuis, J.; Tiemann, D.; Meyer, D.K.; Aktories, K.; Barth, H. The Host Cell Chaperone Hsp90 Is Essential for Translocation of the Binary Clostridium Botulinum C2 Toxin into the Cytosol. J. Biol. Chem. 2003, 278, 32266–32274. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Kroll, C.; Ernst, K.; Schwan, C.; Popoff, M.; Fischer, G.; Buchner, J.; Aktories, K.; Barth, H. Membrane Translocation of Binary Actin-ADP-Ribosylating Toxins from Clostridium Difficile and Clostridium Perfringens Is Facilitated by Cyclophilin A and Hsp90. Infect. Immun. 2011, 79, 3913–3921. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Böhm, N.; Ernst, K.; Langer, S.; Schwan, C.; Aktories, K.; Popoff, M.; Fischer, G.; Barth, H. FK506-Binding Protein 51 Interacts with Clostridium Botulinum C2 Toxin and FK506 Inhibits Membrane Translocation of the Toxin in Mammalian Cells. Cell. Microbiol. 2012, 14, 1193–1205. [Google Scholar] [CrossRef]
- Ernst, K.; Langer, S.; Kaiser, E.; Osseforth, C.; Michaelis, J.; Popoff, M.R.; Schwan, C.; Aktories, K.; Kahlert, V.; Malesevic, M.; et al. Cyclophilin-Facilitated Membrane Translocation as Pharmacological Target to Prevent Intoxication of Mammalian Cells by Binary Clostridial Actin ADP-Ribosylated Toxins. J. Mol. Biol. 2015, 427, 1224–1238. [Google Scholar] [CrossRef]
- Ernst, K.; Schmid, J.; Beck, M.; Hägele, M.; Hohwieler, M.; Hauff, P.; Ückert, A.K.; Anastasia, A.; Fauler, M.; Jank, T.; et al. Hsp70 Facilitates Trans-Membrane Transport of Bacterial ADP-Ribosylating Toxins into the Cytosol of Mammalian Cells. Sci. Rep. 2017, 7, 2724. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.; Liebscher, M.; Mathea, S.; Granzhan, A.; Schmid, J.; Popoff, M.R.; Ihmels, H.; Barth, H.; Schiene-Fischer, C. A Novel Hsp70 Inhibitor Prevents Cell Intoxication with the Actin ADP-Ribosylating Clostridium Perfringens Iota Toxin. Sci. Rep. 2016, 6, 20301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, K.; Eberhardt, N.; Mittler, A.-K.; Sonnabend, M.; Anastasia, A.; Freisinger, S.; Schiene-Fischer, C.; Malešević, M.; Barth, H. Pharmacological Cyclophilin Inhibitors Prevent Intoxication of Mammalian Cells with Bordetella pertussis Toxin. Toxins 2018, 10, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, K.; Mittler, A.-K.; Winkelmann, V.; Kling, C.; Eberhardt, N.; Anastasia, A.; Sonnabend, M.; Lochbaum, R.; Wirsching, J.; Sakari, M.; et al. Pharmacological Targeting of Host Chaperones Protects from Pertussis Toxin in Vitro and in Vivo. Sci. Rep. 2021, 11, 5429. [Google Scholar] [CrossRef]
- Kaiser, E.; Pust, S.; Kroll, C.; Barth, H. Cyclophilin A Facilitates Translocation of the Clostridium Botulinum C2 Toxin across Membranes of Acidified Endosomes into the Cytosol of Mammalian Cells. Cell. Microbiol. 2009, 11, 780–795. [Google Scholar] [CrossRef]
- Burress, H.; Kellner, A.; Guyette, J.; Tatulian, S.A.; Teter, K. HSC70 and HSP90 Chaperones Perform Complementary Roles in Translocation of the Cholera Toxin A1 Subunit from the Endoplasmic Reticulum to the Cytosol. J. Biol. Chem. 2019, 294, 12122–12131. [Google Scholar] [CrossRef]
- Burress, H.; Taylor, M.; Banerjee, T.; Tatulian, S.A.; Teter, K. Co- and Post-Translocation Roles for HSP90 in Cholera Intoxication. J. Biol. Chem. 2014, 289, 33644–33654. [Google Scholar] [CrossRef] [Green Version]
- Kellner, A.; Taylor, M.; Banerjee, T.; Britt, C.B.T.; Teter, K. A Binding Motif for Hsp90 in the A Chains of ADP-Ribosylating Toxins That Move from the Endoplasmic Reticulum to the Cytosol. Cell. Microbiol. 2019, 21, e13074. [Google Scholar] [CrossRef]
- Kellner, A.; Cherubin, P.; Harper, J.K.; Teter, K. Proline Isomerization as a Key Determinant for Hsp90-Toxin Interactions. Front. Cell. Infect. Microbiol. 2021, 11, 771653. [Google Scholar] [CrossRef]
- Taylor, M.; Navarro-Garcia, F.; Huerta, J.; Burress, H.; Massey, S.; Ireton, K.; Teter, K. Hsp90 Is Required for Transfer of the Cholera Toxin A1 Subunit from the Endoplasmic Reticulum to the Cytosol. J. Biol. Chem. 2010, 285, 31261–31267. [Google Scholar] [CrossRef] [Green Version]
- Dmochewitz, L.; Lillich, M.; Kaiser, E.; Jennings, L.D.; Lang, A.E.; Buchner, J.; Fischer, G.; Aktories, K.; Collier, R.J.; Barth, H. Role of CypA and Hsp90 in Membrane Translocation Mediated by Anthrax Protective Antigen. Cell. Microbiol. 2011, 13, 359–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinemann, M.; Schlosser, A.; Jank, T.; Aktories, K. The Chaperonin TRiC/CCT Is Essential for the Action of Bacterial Glycosylating Protein Toxins like Clostridium Difficile Toxins A and B. Proc. Natl. Acad. Sci. USA 2018, 115, 9580–9585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, D.S.; Borgognoni, J.; Clay, A.; Daniels, Z.; Dokurno, P.; Drysdale, M.J.; Foloppe, N.; Francis, G.L.; Graham, C.J.; Howes, R.; et al. Novel Adenosine-Derived Inhibitors of 70 KDa Heat Shock Protein, Discovered through Structure-Based Design. J. Med. Chem. 2009, 52, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Nillegoda, N.B.; Mayer, M.P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell. Biol. 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Schnell, L.; Feigl, P.; Birkhofer, C.; Mohr, K.; Roeder, M.; Carle, S.; Langer, S.; Tippel, F.; Buchner, J.; et al. The Hsp90 Machinery Facilitates the Transport of Diphtheria Toxin into Human Cells. Sci. Rep. 2017, 7, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Concilli, M.; Petruzzelli, R.; Parisi, S.; Catalano, F.; Sirci, F.; Napolitano, F.; Renda, M.; Galietta, L.J.V.; Di Bernardo, D.; Polishchuk, R.S. Pharmacoproteomics Pinpoints HSP70 Interaction for Correction of the Most Frequent Wilson Disease-Causing Mutant of ATP7B. Proc. Natl. Acad. Sci. USA 2020, 117, 32453–32463. [Google Scholar] [CrossRef]
- Clerico, E.M.; Tilitsky, J.M.; Meng, W.; Gierasch, L.M. How Hsp70 Molecular Machines Interact with Their Substrates to Mediate Diverse Physiological Functions. J. Mol. Biol. 2015, 427, 1575–1588. [Google Scholar] [CrossRef] [Green Version]
- Finka, A.; Sharma, S.K.; Goloubinoff, P. Multi-Layered Molecular Mechanisms of Polypeptide Holding, Unfolding and Disaggregation by HSP70/HSP110 Chaperones. Front. Mol. Biosci. 2015, 2, 29. [Google Scholar] [CrossRef] [Green Version]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In Vivo Aspects of Protein Folding and Quality Control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Yam, A.Y.; Xia, Y.; Lin, H.-T.J.; Burlingame, A.; Gerstein, M.; Frydman, J. Defining the TRiC/CCT Interactome Links Chaperonin Function to Stabilization of Newly Made Proteins with Complex Topologies. Nat. Struct. Mol. Biol. 2008, 15, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Howe, M.K.; Bodoor, K.; Carlson, D.A.; Hughes, P.F.; Alwarawrah, Y.; Loiselle, D.R.; Jaeger, A.M.; Darr, D.B.; Jordan, J.L.; Hunter, L.M.; et al. Identification of an Allosteric Small-Molecule Inhibitor Selective for the Inducible Form of Heat Shock Protein 70. Chem. Biol. 2014, 21, 1648–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddymasu, S.C.; Soykan, I.; McCallum, R.W. Domperidone: Review of Pharmacology and Clinical Applications in Gastroenterology. Am. J. Gastroenterol. 2007, 102, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.B.; Moriello, C.; Douros, A.; Filion, K.B. Domperidone and the Risks of Sudden Cardiac Death and Ventricular Arrhythmia: A Systematic Review and Meta-Analysis of Observational Studies. Br. J. Clin. Pharm. 2021, 87, 3649–3658. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Gerding, D.N. Bezlotoxumab. Clin. Infect. Dis. 2019, 68, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, K.; Sailer, J.; Braune, M.; Barth, H. Intoxication of Mammalian Cells with Binary Clostridial Enterotoxins Is Inhibited by the Combination of Pharmacological Chaperone Inhibitors. Naunyn Schmiedebergs Arch. Pharm. 2021, 394, 941–954. [Google Scholar] [CrossRef]
- Ernst, K.; Kling, C.; Landenberger, M.; Barth, H. Combined Pharmacological Inhibition of Cyclophilins, FK506-Binding Proteins, Hsp90, and Hsp70 Protects Cells from Clostridium Botulinum C2 Toxin. Front. Pharmacol. 2018, 9, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papatheodorou, P.; Zamboglou, C.; Genisyuerek, S.; Guttenberg, G.; Aktories, K. Clostridial Glucosylating Toxins Enter Cells via Clathrin-Mediated Endocytosis. PLoS ONE 2010, 5, e10673. [Google Scholar] [CrossRef] [Green Version]
- von Eichel-Streiber, C.; Harperath, U.; Bosse, D.; Hadding, U. Purification of Two High Molecular Weight Toxins of Clostridium Difficile Which Are Antigenically Related. Microb. Pathog. 1987, 2, 307–318. [Google Scholar] [CrossRef]
- Heber, S.; Barthold, L.; Baier, J.; Papatheodorou, P.; Fois, G.; Frick, M.; Barth, H.; Fischer, S. Inhibition of Clostridioides difficile Toxins TcdA and TcdB by Ambroxol. Front. Pharmacol. 2022, 12, 809595. [Google Scholar] [CrossRef]
- Jank, T.; Ziegler, M.O.P.; Schulz, G.E.; Aktories, K. Inhibition of the Glucosyltransferase Activity of Clostridial Rho/Ras-Glucosylating Toxins by Castanospermine. FEBS Lett. 2008, 582, 2277–2282. [Google Scholar] [CrossRef] [Green Version]
- Barth, H.; Blocker, D.; Behlke, J.; Bergsma-Schutter, W.; Brisson, A.; Benz, R.; Aktories, K. Cellular Uptake of Clostridium Botulinum C2 Toxin Requires Oligomerization and Acidification. J. Biol. Chem. 2000, 275, 18704–18711. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braune-Yan, M.; Jia, J.; Wahba, M.; Schmid, J.; Papatheodorou, P.; Barth, H.; Ernst, K. Domperidone Protects Cells from Intoxication with Clostridioides difficile Toxins by Inhibiting Hsp70-Assisted Membrane Translocation. Toxins 2023, 15, 384. https://doi.org/10.3390/toxins15060384
Braune-Yan M, Jia J, Wahba M, Schmid J, Papatheodorou P, Barth H, Ernst K. Domperidone Protects Cells from Intoxication with Clostridioides difficile Toxins by Inhibiting Hsp70-Assisted Membrane Translocation. Toxins. 2023; 15(6):384. https://doi.org/10.3390/toxins15060384
Chicago/Turabian StyleBraune-Yan, Maria, Jinfang Jia, Mary Wahba, Johannes Schmid, Panagiotis Papatheodorou, Holger Barth, and Katharina Ernst. 2023. "Domperidone Protects Cells from Intoxication with Clostridioides difficile Toxins by Inhibiting Hsp70-Assisted Membrane Translocation" Toxins 15, no. 6: 384. https://doi.org/10.3390/toxins15060384
APA StyleBraune-Yan, M., Jia, J., Wahba, M., Schmid, J., Papatheodorou, P., Barth, H., & Ernst, K. (2023). Domperidone Protects Cells from Intoxication with Clostridioides difficile Toxins by Inhibiting Hsp70-Assisted Membrane Translocation. Toxins, 15(6), 384. https://doi.org/10.3390/toxins15060384






