1. Introduction
Peripheral nerve injury is an essential medical condition because it can impair patient function by increasing the risk of secondary disabilities [
1]. These injuries are frequently caused by trauma, lacerations, or fractures. Moreover, ischemia, traction, electric shock, and vibration can play a role [
2]. An estimated 2.8–5.0% of trauma admissions in North America and Europe involve these injuries [
3]. As neural tissue injuries have a significant impact on patients’ quality of life, their regeneration has long been of great interest in medicine [
4]. Numerous attempts have been made to improve the healing of injured peripheral nerves [
5]. Peripheral nerve injuries are typically treated surgically. Primary end-to-end repair and nerve grafts are two surgical treatment options [
6]. Nonsurgical therapeutic methods, such as physical therapy, hydrotherapy, electrical stimulation, and medicine, have received the most attention. Recently, several studies have reported that botulinum neurotoxin A (BoNT/A) is effective in nerve regeneration [
7,
8,
9,
10].
BoNT/A is used for several indications including cosmetics, muscle spasticity, migraines, dystonia, and axillary hyperhidrosis. It blocks acetylcholine from being released by cholinergic nerve terminals through the cleavage and degradation of a synaptosomal-associated protein of 25 kDa, which reduces muscle contractility [
3]. BoNT/A has well-established therapeutic applications that are constantly growing, particularly in the management of peripheral nerve damage. It is assumed that BoNT/A directly stimulates axonal regeneration in injured peripheral nerves, leading to neurological recovery [
7]. Following complete compression injury to the sciatic nerve, Cobianchi showed that a single injection of botulinum toxin into the nerve accelerated axonal growth and increased the number of regenerated myelinated fibers [
7]. Previous studies have shown that BoNT/A affects the improvement of electrophysiological recovery and promotes neural sprouting [
3,
11]. A recent study by Luvisetto et al. reported that BoNT/A can modulate astrocyte activity, which plays a crucial role in regulating neuronal function. BoNT/A can inhibit the release of proinflammatory molecules from astrocytes, which may have implications for the treatment of neuroinflammatory disorders [
8].
However, there has been no research on the most appropriate dose of BoNT/A that produces optimal neurological recovery. This study aimed to investigate and compare neural recovery after treatment with various doses of onabotulinum neurotoxin type A in rats with sciatic nerve injury produced experimentally. An enzyme-linked immunosorbent assay (ELISA), immunofluorescence staining, and toluidine blue staining were used in the experiments. The sciatic functional index (SFI) and electrophysiological tests were used to assess functional improvement.
3. Discussion
Our study is the first to investigate the neuroregenerative effects of varying doses of BoNT/A. The overall purpose of this investigation was to determine the optimal doses of BoNT/A for neural regeneration. Our previous study showed a superior effect of BoNT/A than the normal saline control group in nerve regeneration and functional recovery after peripheral nerve injury in rats [
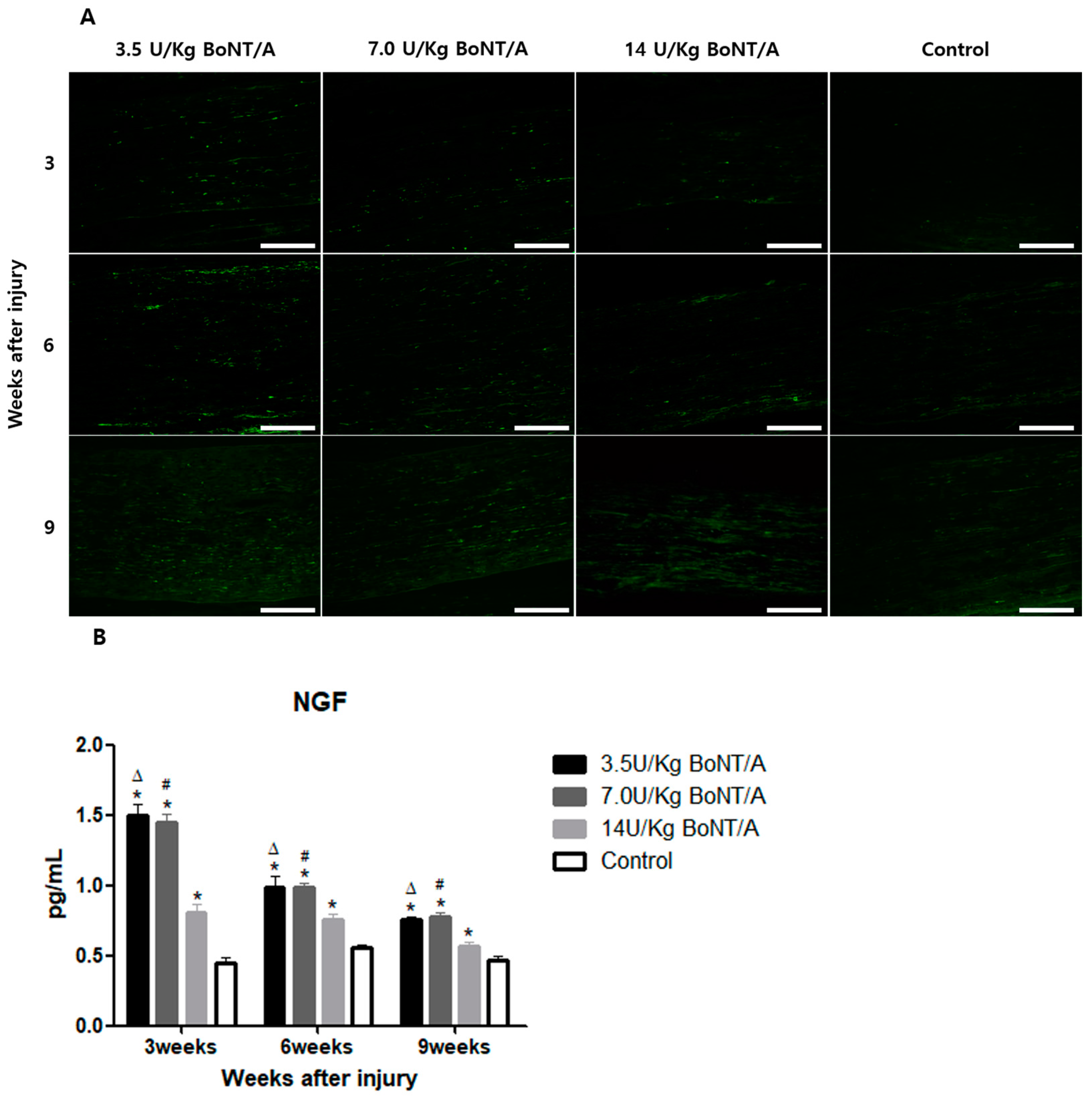
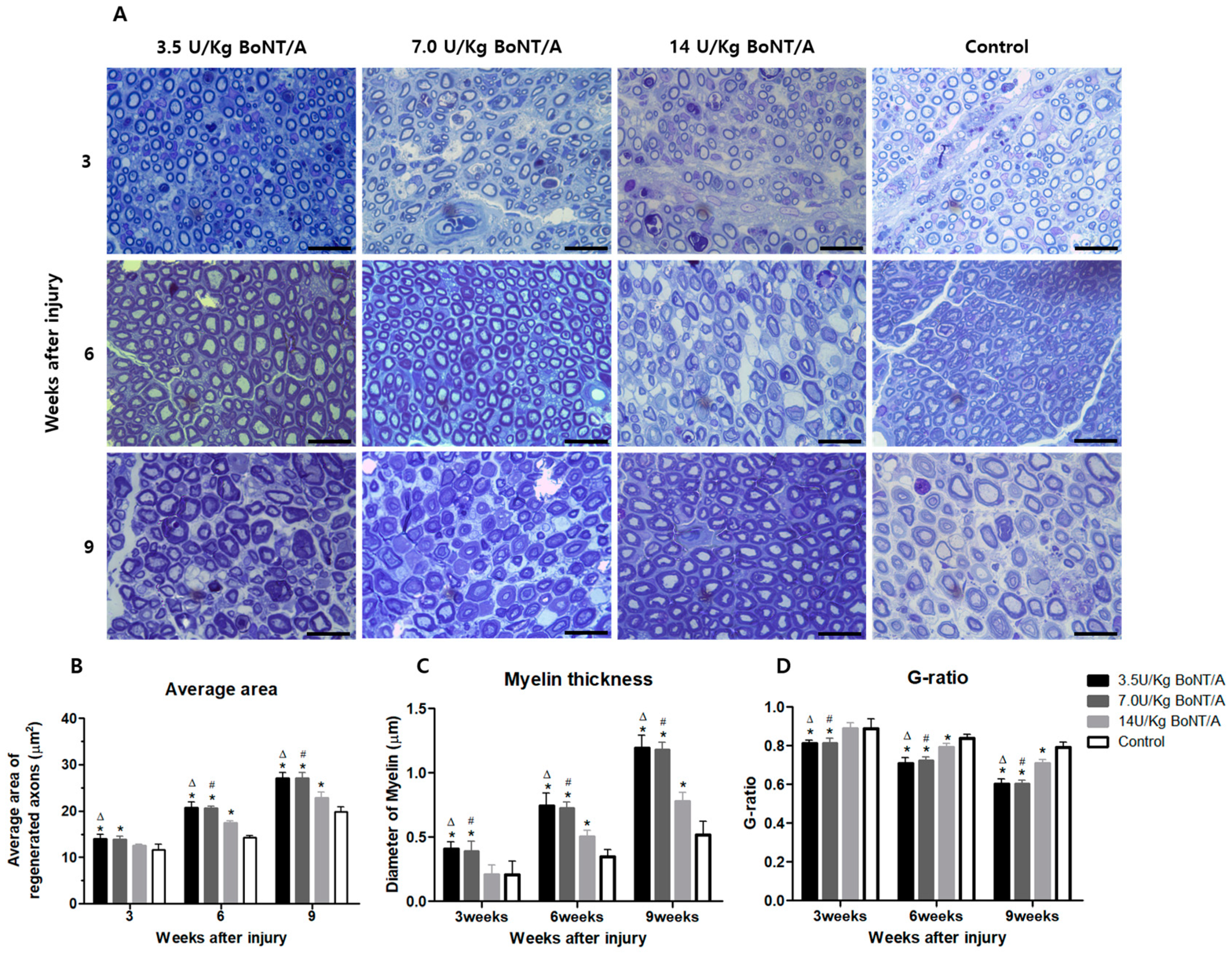
10]. The stimulation of Schwann cells and axonal regrowth after BoNT/A appeared to improve functional recovery, according to the electrophysiological, clinical behavioral data, and neural tissue analysis results presented in this study. Higher expression levels of proteins for Schwann cell activities, axonal regeneration, and nerve growth were more pronounced in the 3.5 and 7.0 U/kg BoNT/A groups compared to the other groups. Furthermore, we found that the 3.5 and 7.0 U/kg BoNT/A groups showed a larger area and thicker myelin sheath through toluidine blue staining. Moreover, by week 9, the G-ratio was nearly 0.6, which is the optimal value [
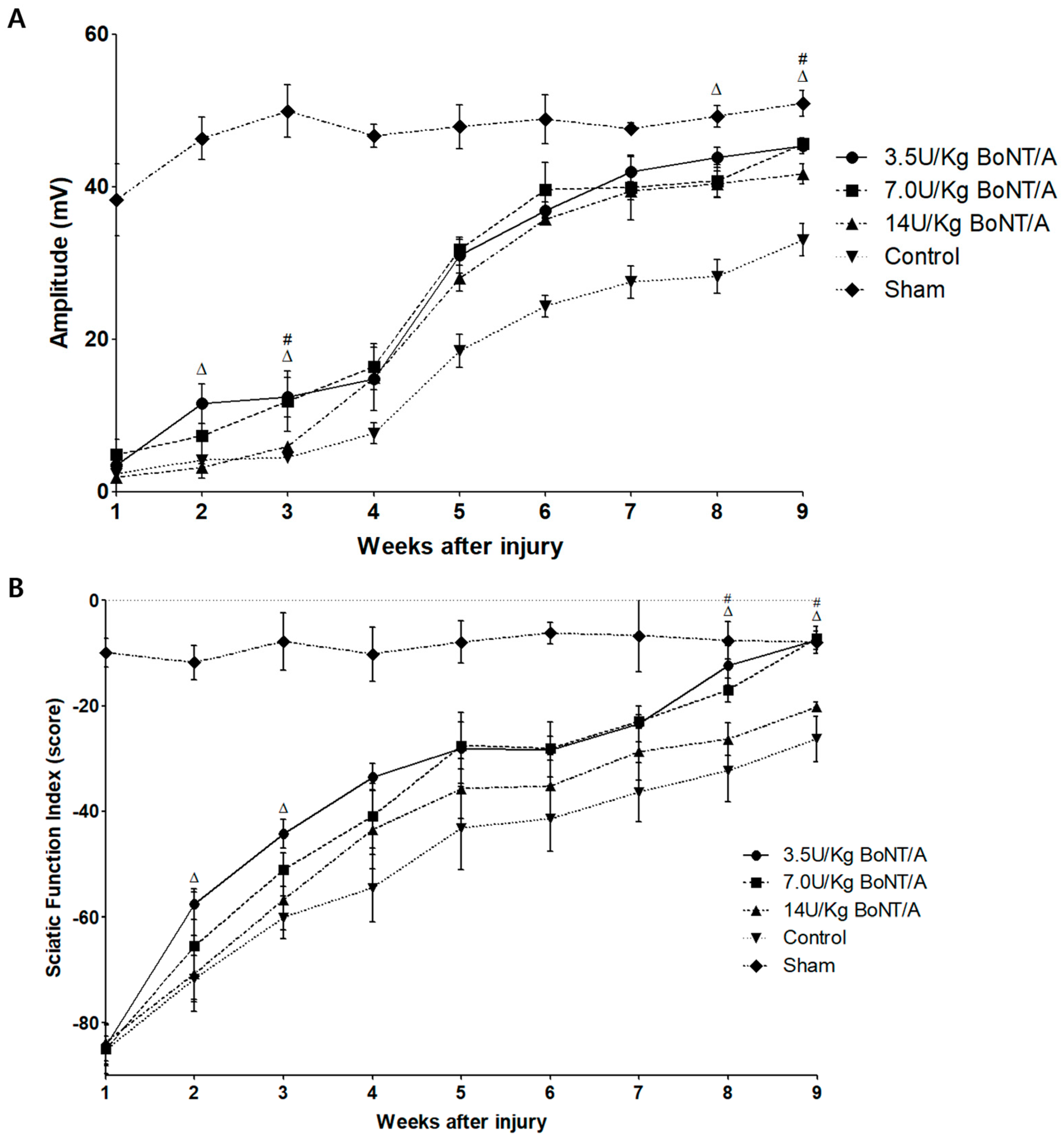
12]. In addition to morphology, the electrophysiological findings confirmed that the 3.5 and 7.0 U/kg BoNT/A groups showed a larger CMAP than the control group. In the functional evaluation, the SFI showed trends identical to those of the other results. Although the 3.5 and 7.0 U/kg BoNT/A groups did not reach the CMAP amplitude of the sham group on the NCS at week 9, the SFI fully recovered and was similar to the normal gait pattern of the sham group. These results indicate that the 3.5 and 7.0 U/kg BoNT/A groups have a greater neuroregenerative effect morphologically, electrophysiologically, and functionally than the 14 U/kg BoNT/A and control groups.
Botulinum toxins are produced by anaerobic bacteria of the genus Clostridium under seven different serotypes, referred to as BoNT/A to BoNT/G, and include several variants [
13,
14]. Among those serotypes, the clinically used botulinum toxin is type A and type B. In a 2018 study by Finocchiaro et al., botulinum toxin type B did not elicit functional recovery after peripheral nerve injury. Therefore, botulinum toxin type A was used in this study [
15].
Recent research has elucidated the mechanisms underlying the neuroregenerative effects of BoNT/A after peripheral nerve injury. Some of BoNT/A’s less well-known functions have been shown in earlier research, such as its ability to speed up nerve regeneration after axotomy, nerve crush, and chronic constriction injury. Franz et al. reported the effects of BoNT/A preconditioning on reinnervation [
16]. Adlet et al. suggested that BoNT/A may accelerate nerve regeneration by improving blood flow and upregulating angiogenesis [
17]. A study by Finocchiaro et al. describes that mast cells and macrophages are further activated to speed up the removal of myelin debris and promotes nerve regeneration after BoNT/A injection [
15]. We focused on the role of Schwann cells in peripheral nerve regeneration. Nerve regeneration is predominantly linked to enhanced Schwann cell proliferation, transformation to the repair phenotype, and mast cell and macrophage activation [
17]. Spinal glial cell activation and the consequent release of pro-inflammatory factors can be inhibited by BoNT/A [
8]. BoNT/A can interact with glial cells such as Schwann cells and oligodendrocytes, which are responsible for rebuilding the myelin sheath of neural axons, possibly resulting in a gradual recovery of myelin function following injury [
8]. Schwann cells produce neurotrophic factors to provide a favorable environment for axon regeneration and create guidance tracks (bands of Büngner) that help regenerated axons reach their intended targets [
18].
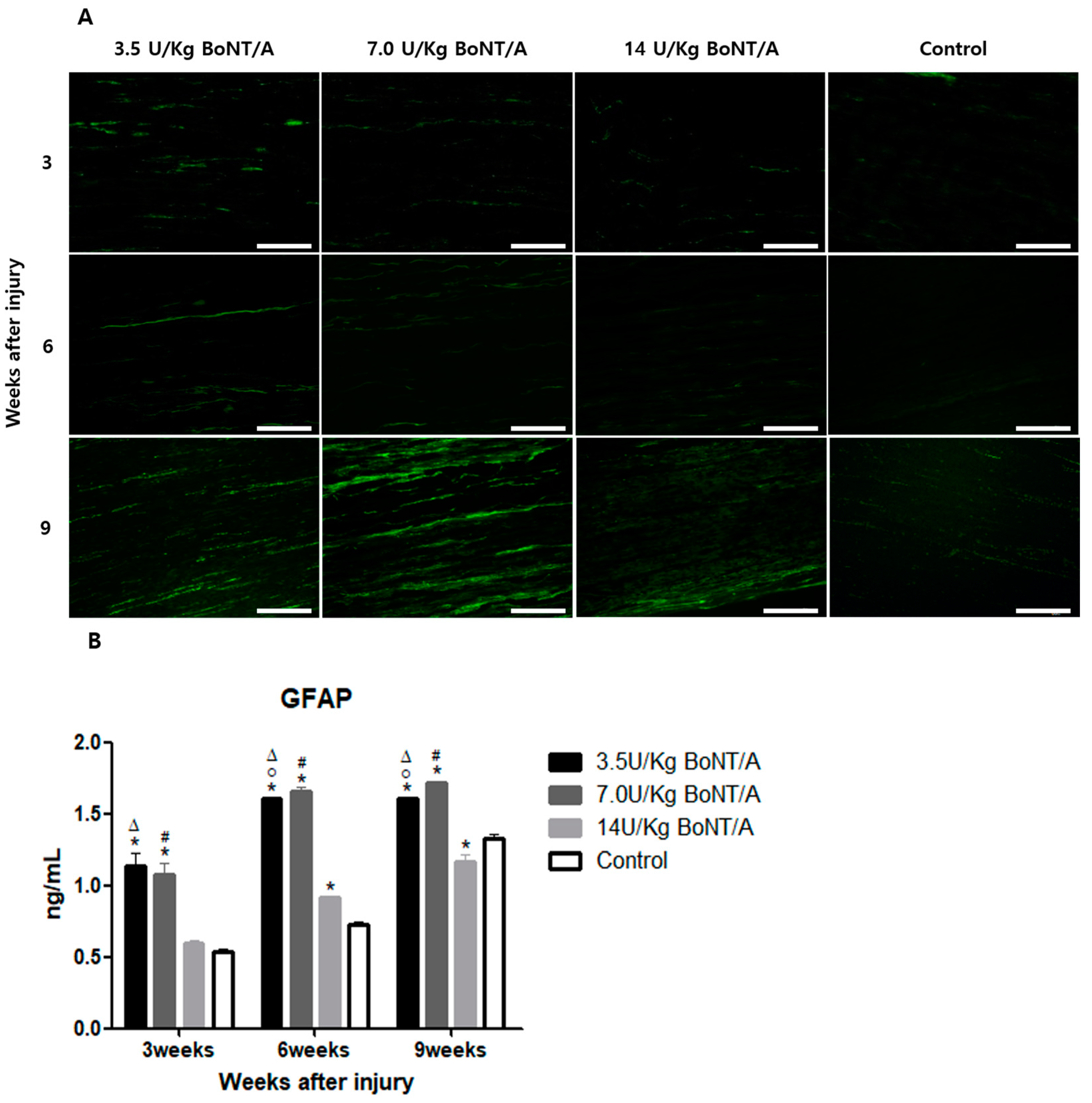
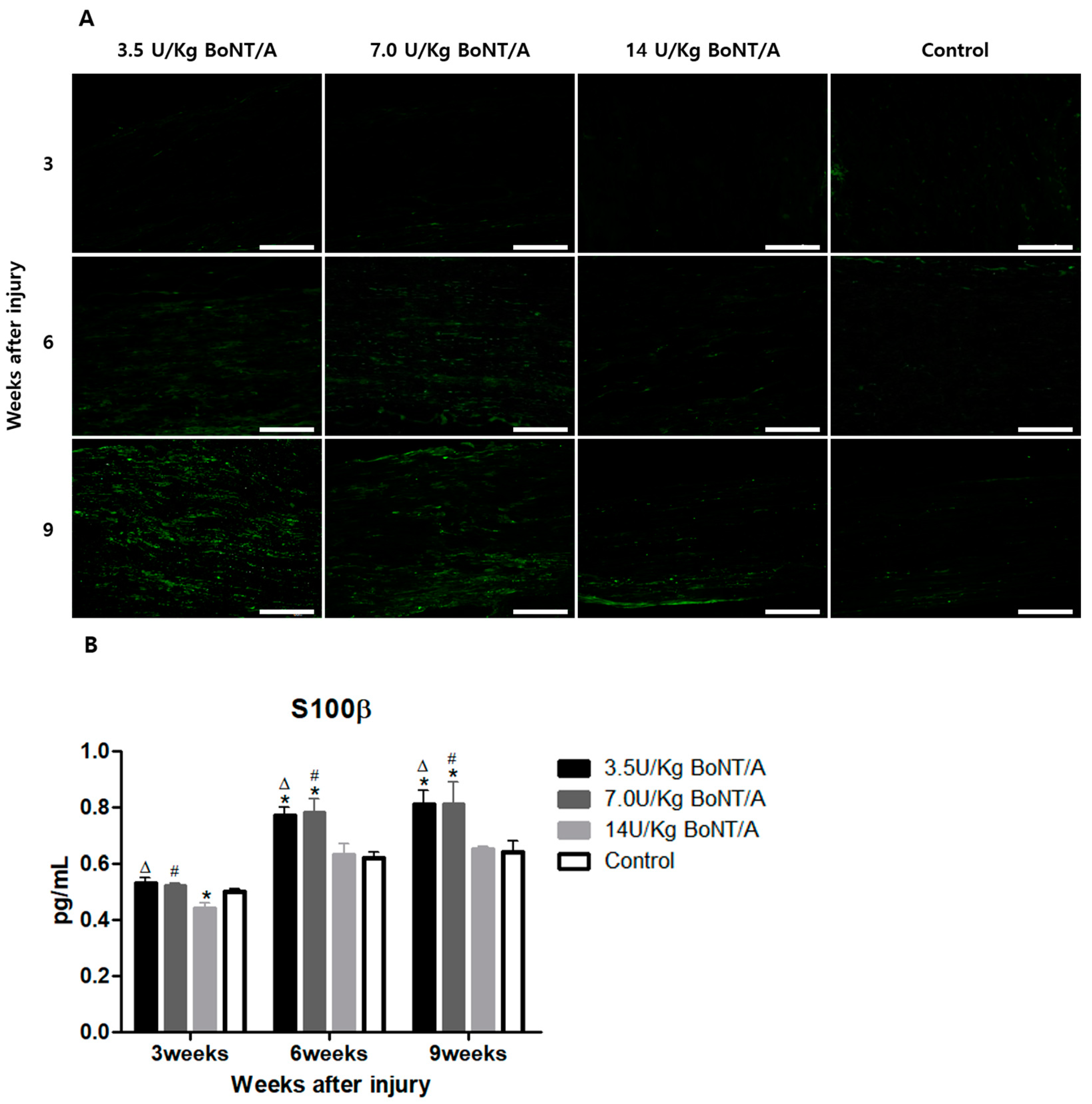
Schwann cells play a crucial role after Wallerian degeneration, beginning to proliferate and gradually enhancing the expression of GFAP and S100β, which are proteins expressed by non-myelinating and myelinating Schwann cells, respectively [
19,
20,
21,
22]. GFAP, which is the cytoskeletal constituent of Schwann cells, is expressed by immature, dedifferentiated non-myelinating cells and mature non-myelinating cells. Peripheral nerve damage causes Schwann cells to lose contact with axons, upregulating GFAP expression and developing an immature, dedifferentiated phenotype similar to that of non-myelin-forming Schwann cells [
15]. Similar to previously reported data from in vitro studies, our results revealed a direct interaction between BoNT/A and Schwann cells [
23]. The interaction between Schwann cells in damaged nerves is also essential for creating a beneficial environment for axon maturation, sprouting, and regrowth [
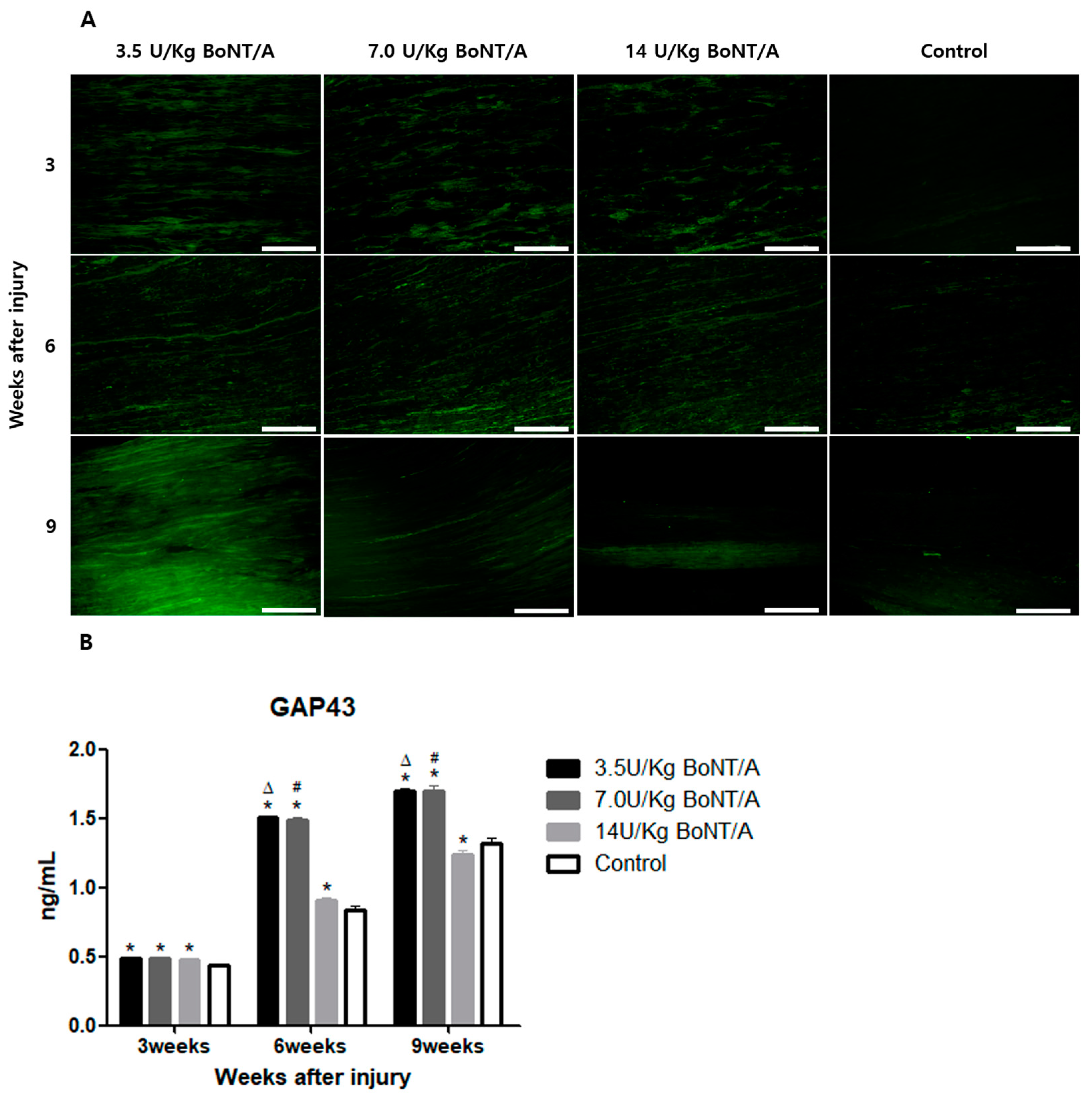
24]. In our study, significantly higher expression levels of GAP43 and NF200 were observed in the BoNT/A groups than in the control group. GAP43 and NF200 expression can be used to identify the reactivation of neural regeneration programs [
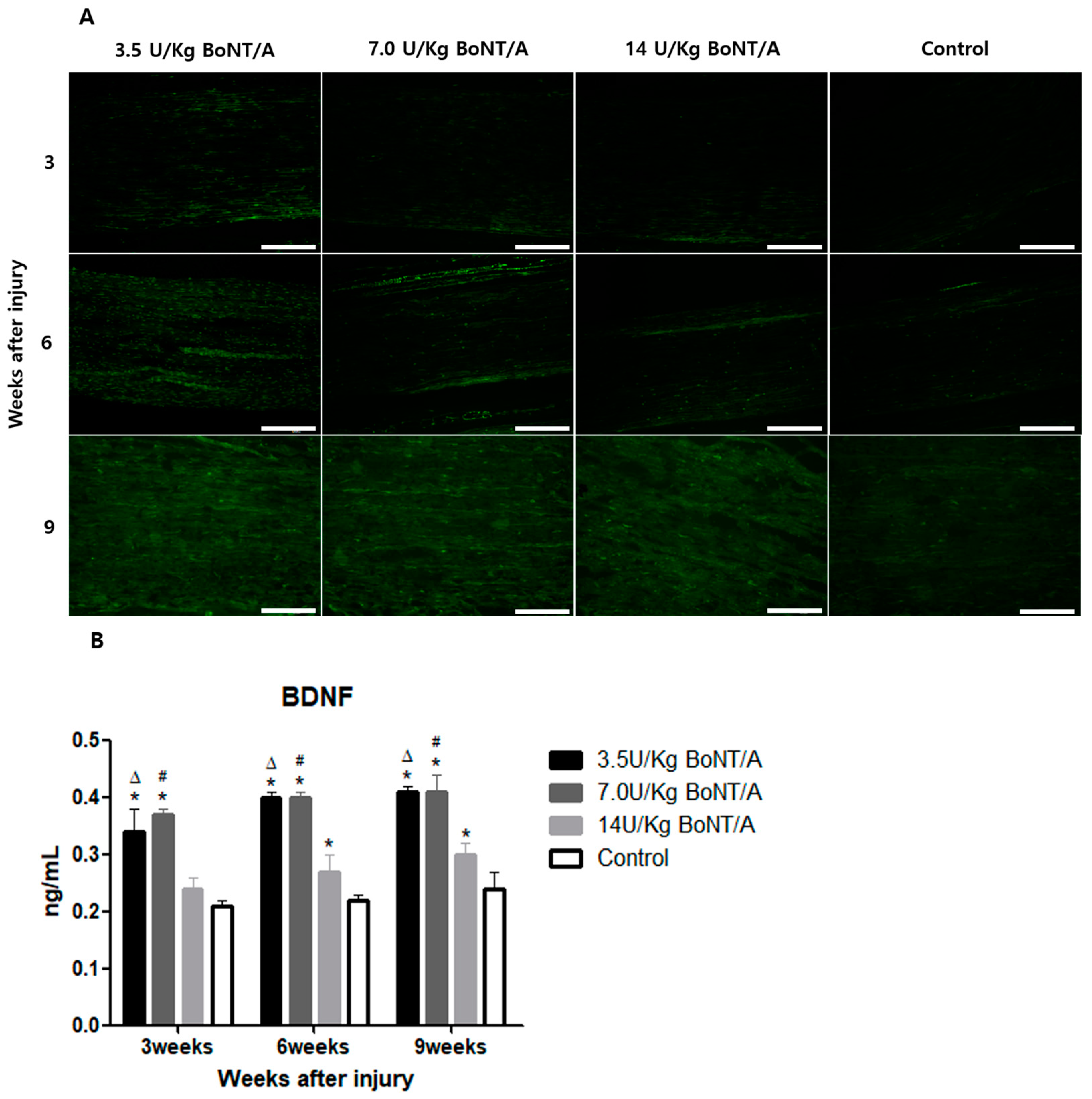
25]. BDNF and NGF stimulate axonal regeneration in dorsal root ganglion neurons [
26]. The expression of BDNF and NGF showed similar trends to the other proteins studied. All of these results suggest that the 3.5 and 7.0 U/kg BoNT/A groups showed a superior nerve regeneration ability compared to the 14 U/kg BoNT/A and control groups.
There are several methods for injecting BoNT/A (including intramuscular, perineural, and intraneural) [
3,
7,
16]. In this study, we attempted to determine the effect of BoNT/A on nerve regeneration, and intramuscular injection was judged to be inappropriate for functional evaluation. Perineural injection was also judged to be inappropriate because BoNT/A could not be placed in the desired location for a long period. Intraneural injection has the advantage of being able to accurately place BoNT/A at the desired location. A previous study showed that, after the complete crushing of peripheral nerves, a single intra-nerve injection of BoNT/A increased the number of regenerated myelin fibers and the rate of axonal elongation [
7]. In contrast to trials of other injectants, like local anesthetics, a previous study showed that the direct intraneural injection of BoNT/A generated minimal harm to nerves [
27]. Furthermore, no rats were lost during the study due to death or significant adverse events following intraneural BoNT/A injections.
Very little research has been conducted on intraneural injections of BoNT/A. Previous studies have analyzed the effects of different concentrations of BoNT/A using different injection methods. Subcutaneous injections at concentrations of 30 U/kg have been reported to render rats lethargic [
28], and intramuscular injections of high doses of BoNT/A have been reported to impair skeletal muscle function and destroy structure [
29]. There is a report that the direct intraneural injection of BoNT/A is not neurotoxic, but the study only used 3.5 U/kg of BoNT/A [
27]. Whether it is not toxic at higher concentrations and the mechanism of its inhibitory effect on nerve regeneration needs to be elucidated. Additional tests such as the MTT assay in Schwann cells, which measures cell viability and proliferation, may help to explain why intraneural injections of high concentrations of BoNT/A contribute less to nerve regeneration.
Previous studies were reviewed to establish appropriate comparative doses of BoNT/A. The study of Cobianchi used BoNT/A (15 pg) in 2 μL of saline intraneurally, which corresponds to a concentration of approximately 10.9 U/kg [
7]. This study reported that BoNT/A accelerates sensorimotor recovery by stimulating myelinated axonal regeneration. Seo et al. used 7 U/kg of BoNT/A to report successful nerve regeneration compared to a control group [
10]. Based on earlier investigations showing minimal side effects and great effectiveness at low doses, a single session of intra-nerve 7.0 U/kg BoNT/A was set as the median value in this study. According to our experimental hypothesis, the 3.5 and 14 U/kg BoNT/A groups were set up for comparison.
This study had some limitations. First, our results were based on animal studies. It is crucial to remember that the BoNT/A doses used in animal models cannot be translated into therapeutic doses for humans based on weight ratios; rather, they must be carefully selected based on toxicity factors [
8]. Furthermore, it would be of considerable interest to extend these studies to larger animals, where the magnitude of change observed in rodents may have more profound consequences owing to their longer peripheral nerves and slower rate of spontaneous regeneration. Second, there are more factors to consider when injecting BoNT/A, based on the aforementioned study on the use of BoNT/A causing injury, serotype/subtype selection, and the interval between toxin injection and injury. Our study used onabotulinum neurotoxin A, which means that the dosage we recommend cannot be extrapolated to other brands or other serotypes. In particular, it is difficult to perform a precise intraneural injection of BoNT/A without an opening. Therefore, perineural injections may be more suitable for clinical use. In addition, new preclinical studies on other injection methodologies and the timing of injections are required. As for timing, we expect that injecting BoNT/A immediately after nerve injury will be more effective than delaying it. After the point of proliferation of Schwann cells following Wallerian degeneration, we believe that injections of BoNT/A that enhance the activity of Schwann cells will not have a significant impact. However, patients do not visit the clinic immediately after injury, so further studies are definitely needed, including how delayed injection differs in outcomes from no treatment or normal saline injection. Third, because we observed favorable neural regeneration at the lowest concentration, further research on the effectiveness of lower concentrations is also warranted. Finally, there have been no clear reports of BoNT/A to injure nerves when injected intraneurally, but further research is needed to determine if there is a difference in the mechanism of action between BoNT/A injections intraneurally and other methodologies.
5. Materials and Methods
5.1. Animals and Surgical Procedure
In this study, sixty-five SPF Sprague Dawley (SD) rats were used. The rats were housed in five groups of standard cages (22 × 22 × 13 cm) with unlimited access to food and water upon arrival. The temperature (23 ± 1 °C) was kept constant under a 12 h light/dark cycle (06:30–18:30). At the time of the operation, the mice were approximately six weeks old and weighed 187–213 g. Over the course of two days, the experiment took place between 13:30 and 17:30. The guiding principles of the “Guide for the Care and Use of Laboratory Animals” were followed in all aspects of animal experimentation (Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care and Use of Laboratory Animals, National Research Council of The National Academies, Washington, DC, USA; The National Academies Press: Washington, DC, USA, 2011) and approved by the “Institutional Animal Care and Use Committee” of Dongguk University (protocol code 202209239; approval date: 30 September 2022).
Surgery was carried out under the influence of 1–2% isoflurane. A skin incision was made beneath the hip to expose the right sciatic nerve at the mid-thigh level. Fine surgical scissors and forceps were then used to bluntly cut the muscle. After that, the sciatic nerve was crushed 1 cm above the division of its tibial, common peroneal, and sural branches. The sciatic nerve was crushed for 30 s using a Halsey needle holder (AE 064/13, NOPA, Tuttlingen, Germany) [
30]. After applying VICRYL 3-0 (W9114, Ethicon LLC, Bridgewater Township, NJ, USA) to seal the wound, the rats were returned to their heated cages until all reflexes returned to normal.
The sciatic nerve-crushed rats were divided into five groups: (1) rats (
n = 15) injected with 3.5 U/kg BoNT/A through the crushed sciatic nerve location immediately before the incision was sutured; (2) rats (
n = 15) injected with 7.0 U/kg BoNT/A through the crushed sciatic nerve location immediately before the incision was sutured; (3) rats (
n = 15) injected with 14.0 U/kg BoNT/A through the crushed sciatic nerve location immediately before the incision was sutured; (4) rats (
n = 15, control) injected with 0.9% saline (70 μL/kg) after sciatic nerve injury; (5) rats (
n = 5, sham) after exposing the sciatic nerve whose skin and muscle were sutured immediately. At weeks 3, 6, and 9, sciatic nerves were harvested from all groups, except the sham group. In the sham group, neurohistology was not performed, and only weekly neurophysiological and behavioral testing were performed for nine weeks. All intraneural injections were performed extrafascicularly by a medical professional to avoid potential mechanical nerve injury [
31].
5.2. Experimental Groups
Before suturing the lesion, onabotulinum neurotoxin A (Allergan, Irvine, CA, USA) was intraneurally administered into the nerve-crush site using a 50 μL Hamilton Syringe (HAMILTON CO., NO706, Hamilton, OH, USA). The BoNT/A dose was based on our previous study that demonstrated efficient neural regeneration without side effects [
10]. One hundred U of botulinum toxin is equivalent to 4.8 ngtox [
28]. Stock solutions of BoNT/A (100 U/mL) were freshly prepared by dilution in 0.5 mL of normal saline (0.9% NaCl). To compare the effect of neural regeneration, several doses of intra-nerve BoNT/A (3.5 U/kg, 168 pgtox/kg body weight; 7.0 U/kg, 336 pgtox/kg body weight; 14 U/kg, 672 pgtox/kg body weight) were used. A single session of intraneural BoNT/A was administered as planned.
5.3. Neurophysiology Test
Serial electrophysiological studies were performed weekly for nine weeks. The CMAP amplitude was measured in animals under deep anesthesia (1–2% isoflurane). Single pulses of a 0.3 ms duration were applied to a pair of bar electrodes positioned at the sciatic notch to percutaneously stimulate the sciatic nerve. Microneedle electrodes were used to record the CMAP of the gastrocnemius medialis muscle. The insertion site of the Achilles tendon served as the reference electrode location. To measure the amplitude, all potentials were amplified and presented on an electromyography machine (Synergy; Viasys Healthcare, PA, USA) at the appropriate settings. Once supramaximal stimulation was reached, the CMAP amplitudes were recorded by progressively increasing the stimulus intensity [
32].
5.4. Behavioral Tests
The gradual recovery of motor function was observed by analyzing individual gait patterns. The SFI was calculated by measuring multiple footstep parameters. The hind paws of the rats were dipped in black ink and allowed to walk along a Perspex runway corridor (12 × 12 × 60 cm) to record their footsteps. A minimum of five footsteps were recorded on each of three different tracks to calculate footstep parameters. The rats were examined weekly from weeks 1 to 9. An analysis was performed on the steps taken to measure the distances between the heel and third toe (print length, PL), the first and fifth toes (toe spread, TS) and the second and fourth toes (intermediate toe spread, IT). For both left and right footsteps, the experimental (E) and normal (N) data were recorded. The SFI was evaluated using the following equation: [SFI = −38.3 × (EPL-NPL)/NPL + 109.5 × (ETS-NTS)/NTS + 13.3 × (EIT-NIT)/NIT − 8.8] [
33]. The SFI of a normal rat typically hovers around 0, whereas that of a seriously damaged rat is nearly −100.
5.5. Immunostaining of Sciatic Nerves
The rats were anesthetized at weeks 3, 6, and 9 using 1–2% isoflurane (Sigma-Aldrich, St. Louis, MO, USA). Distal to the crushed nerve location, a 10 mm section of the sciatic nerve was cut. The base mold was filled with a cryo-embedding medium (OCT compound), and the entire tissue was placed inside. To ensure that the tissue was completely frozen, the base mold containing the tissue block was submerged in liquid nitrogen until it reached the top of the mold. Until it was ready to section the frozen tissue block, it was kept at a temperature of −80 °C.
For immunofluorescence staining, 8 μm thick frozen tissue sections were used, and they were then transferred onto glass slides. After 30 min of room-temperature drying, the tissue sections were fixed for 1 h at 4 °C using a 4% paraformaldehyde solution (Sigma-Aldrich, Milan, Italy). The tissues upon which the antibodies were applied were permeabilized using a 0.5% Triton X-100 solution. Afterwards, the fixed sections were rinsed in phosphate-buffered saline (PBS) to achieve blocking. The slides were incubated in a 5% bovine serum albumin solution (Sigma-Aldrich, Milan, Italy) at room temperature for an hour after the fixed sections were washed in PBS. The sections were incubated with diluted primary antibodies overnight at 4 °C in a humidified room. The antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). BoNT/A’s influence on the activity of Schwann cells in an injured sciatic nerve was evaluated through the expression of two proteins, GFAP and S100β, which are expressed by non-myelinating and myelinating Schwann cells, respectively [
15]. Axonal regrowth was assessed based on the expression of GAP43, NF200, BDNF, and NGF [
7,
26,
34,
35]. The primary antibodies utilized were GFAP (1:500 dilution), S100β (1:200 dilution), GAP43 (1:250 dilution), NF200 (1:500 dilution), BDNF (1:250 dilution), and NGF (1:250 dilution). Secondary antibodies (anti-mouse IgG and anti-rabbit IgG2 at 1:500–1:1000 dilution) that had been diluted were applied to the slides the following day, rinsed with PBS, and incubated for an hour at room temperature in a humid environment. The samples were rinsed in PBS, coverslipped, and observed under a confocal microscope (STELLARIS, Leica Microsystems, Wetzlar, Germany).
5.6. ELISA
Using an ELISA kit (CUSABIO, Houston, TX, USA) the levels of GFAP, S100β, GAP43, NF200, BDNF, and NGF were assessed in order to evaluate the expression of proteins linked to nerve regeneration in the frozen sciatic nerve tissue that was collected. The manufacturer’s instructions were followed.
5.7. Toluidine Blue Staining
One 5 mm sample from each naive nerve was fixed for 48 h at 4 °C using a mixture of 2% glutaraldehyde (16216, EMS), 4% paraformaldehyde (15754-S, EMS), and 0.1 M Sorensen’s phosphate buffer pH 7.2 solution. The specimens were embedded in Araldite1502 resin (Polyscience, IL, USA) after being post-fixed in 2% osmium tetroxide and dehydrated using increasing alcohol series (starting at 50%). Using an Ultracut E microtome (Reichert Inc., Buffalo, NY, USA), semi-thin (1 μm) tissue sections were cut and stained with 1% toluidine blue. Five sample sites per section were randomly selected at 2 mm intervals on the microscope stage. These sites were examined at 1000× magnification under an optical microscope (Eclipse Ci-L, Nikon, Tokyo, Japan), and axonal regeneration was estimated based on (1) the average area of regenerated nerves, (2) the mean diameter of the myelin sheath, and (3) the axon to fiber diameter ratio (G-ratio).
For analysis, non-overlapping micrograph segments were imported into the ImageJ software (FIJI Package, version 2.0, NIH, Bethesda, MD, USA). Axons touching the borders were included in the measurements. Thresholding was performed automatically to create a black-and-white image. For fibers where the myelin touched, lines were drawn between them so that they could be segmented individually. For particle analysis, the minimum size and circularity were set at 2 μm2 and 0.2, respectively, to remove small motes from statistics
5.8. Statistical Analysis
In both graphic and textual forms, the results are presented as the mean values ± standard error of the mean or standard deviation. Using GraphPad Prism version 9 for Windows, a one-way ANOVA and Tukey’s multiple comparison test were used to assess differences between the groups at each time point (GraphPad Prism 5.01, San Diego, CA, USA). The threshold for statistical significance was a p-value < 0.05.