IoT for Monitoring Fungal Growth and Ochratoxin A Development in Grapes Solar Drying in Tunnel and in Open Air
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stochastic Analysis of Drying Data Collected during the Drying Period
2.2. Deterministic Analysis of the Drying Process
- -
- The Deff should be lower than those for self-diffusion of water (3.6 × 10−9 m2/s (at 45 °C), 4.37 × 10−9 m2/s (at 55 °C) and 5.09 × 10−9 m2/s (at 65 °C)) [12].
- -
- The Deff should be in the range of 10−11–10−9 m2/s [13].
- -
- The mean relative error (MRE) between the estimated and experimental water content should be less than 10%.
3. Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Fungal Inoculumn and Treatment Samples
4.3. Fungal Infection of Grapes and Detection of Ochratoxin A
4.4. Hygrothermal Measurements during Drying Experiments
4.5. Statistical Analysis and Weibull Distribution
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, A.; Anand, A.; Shukla, A.; Kumar, A.; Buddhi, D.; Atul Sharma, A. A Comprehensive Overview on Solar Grapes Drying: Modelling, Energy, Environmental and Economic Analysis. Sustain. Energy Technol. Assess. 2021, 47, 101513. [Google Scholar] [CrossRef]
- Gomes, I.A.; Markova, E.; da Silvá, J.P.L.; Venâncio, A.; Freitas-Silva, O. Global Trends for Patulin Adsorption: A Review. Res. Soc. Dev. 2021, 10, e58310616162. [Google Scholar] [CrossRef]
- Freire, L.; Braga, P.A.C.; Furtado, M.M.; Delafiori, J.; Dias-Audibert, F.L.; Pereira, G.E.; Reyes, F.G.; Catharino, R.R.; Sant’Ana, A.S. From Grape to Wine: Fate of Ochratoxin A during Red, Rose, and White Winemaking Process and the Presence of Ochratoxin Derivatives in the Final Products. Food Contr. 2020, 113, 107167. [Google Scholar] [CrossRef]
- Mondani, L.; Palumbo, R.; Tsitsigiannis, D.; Perdikis, D.; Mazzoni, E.; Battilani, P. Pest Management and Ochratoxin A Contamination in Grapes: A Review. Toxins 2020, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Pieristè, M.; Forey, E.; Lounès-Hadj Sahraoui, A.; Meglouli, H.; Laruelle, F.; Delporte, P.; Robson, T.M.; Chauvat, M. Spectral Composition of Sunlight Affects the Microbial Functional Structure of Beech Leaf Litter during the Initial Phase of Decomposition. Plant Soil 2020, 451, 515–530. [Google Scholar] [CrossRef]
- Raghda, A.E.S.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2020, 2, 91–102. [Google Scholar] [CrossRef]
- Da Costa, K.A.P.; Papa, J.P.; Lisboa, C.O.; Munoz, R.; De Albuquerque, V.H.C. Internet of Things: A survey on Machine Learning-based Intrusion Detection Approaches. Comput. Netw. 2019, 151, 147–157. [Google Scholar] [CrossRef]
- Leong, A.L. Black Aspergillus Species: Implications for Ochratoxin A in Australian Grapes and Wine. Ph.D. Thesis, University of Adelaide, Adelaide, SA, Australia, 2005. [Google Scholar]
- González-Curbelo, M.Á.; Kabak, B. Occurrence of Mycotoxins in Dried Fruits Worldwide, with a Focus on Aflatoxins and Ochratoxin A: A Review. Toxins 2023, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- International Association of Certified Thermographers (IACT): Clinical Thermography Standards & Guidelines. Available online: https://iactthermography.org/ (accessed on 27 September 2023).
- Templalexis, C.; Giorni, P.; Lentzou, D.; Mesisca, S.; Tsitsigiannis, D.I.; Battilani, P.; Xanthopoulos, G. Environmental Conditions Affecting Ochratoxin A during Solar Drying of Grapes: The Case of Tunnel and Open-air Drying. Toxins 2021, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Saravacos, G.D.; Maroulis, Z.B. Transport Properties of Foods; Marcel Decker: New York, NY, USA, 2001. [Google Scholar]
- Ramla, K.; Hassène, Z.; Daoued, M. Raisin Processing: Physicochemical, Nutritional and Microbiological Quality Characteristics as Affected by Drying Process. Food Rev. Int. 2019, 35, 246–298. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists 1997. In Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Rinne, H. The Weibull Distribution: A Handbook; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Mason, R.L.; Gunst, R.F.; Hess, J.L. Statistical Design and Analysis of Experiments: With Applications to Engineering and Science; Wiley-Interscience: New York, NY, USA, 2003. [Google Scholar]

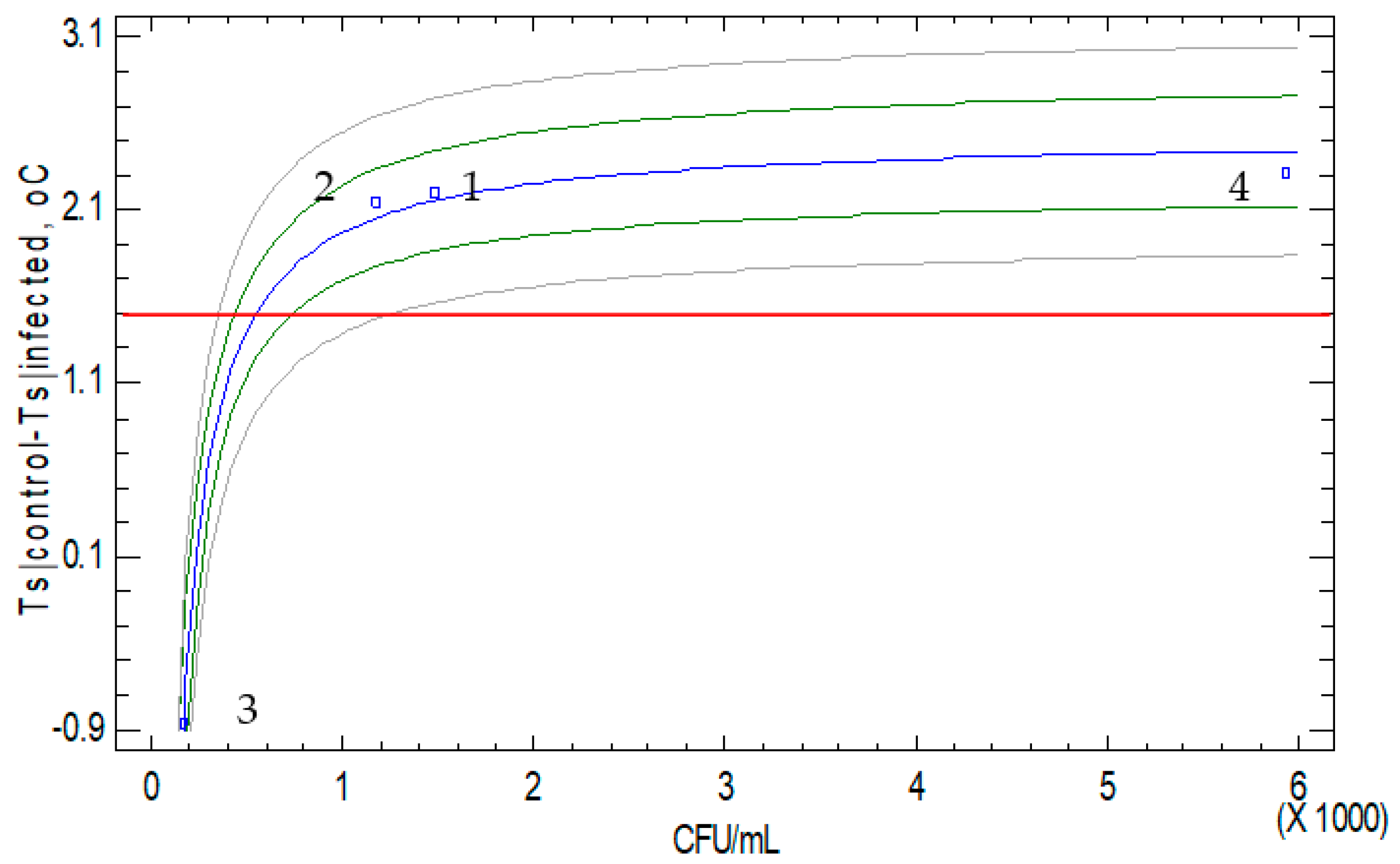
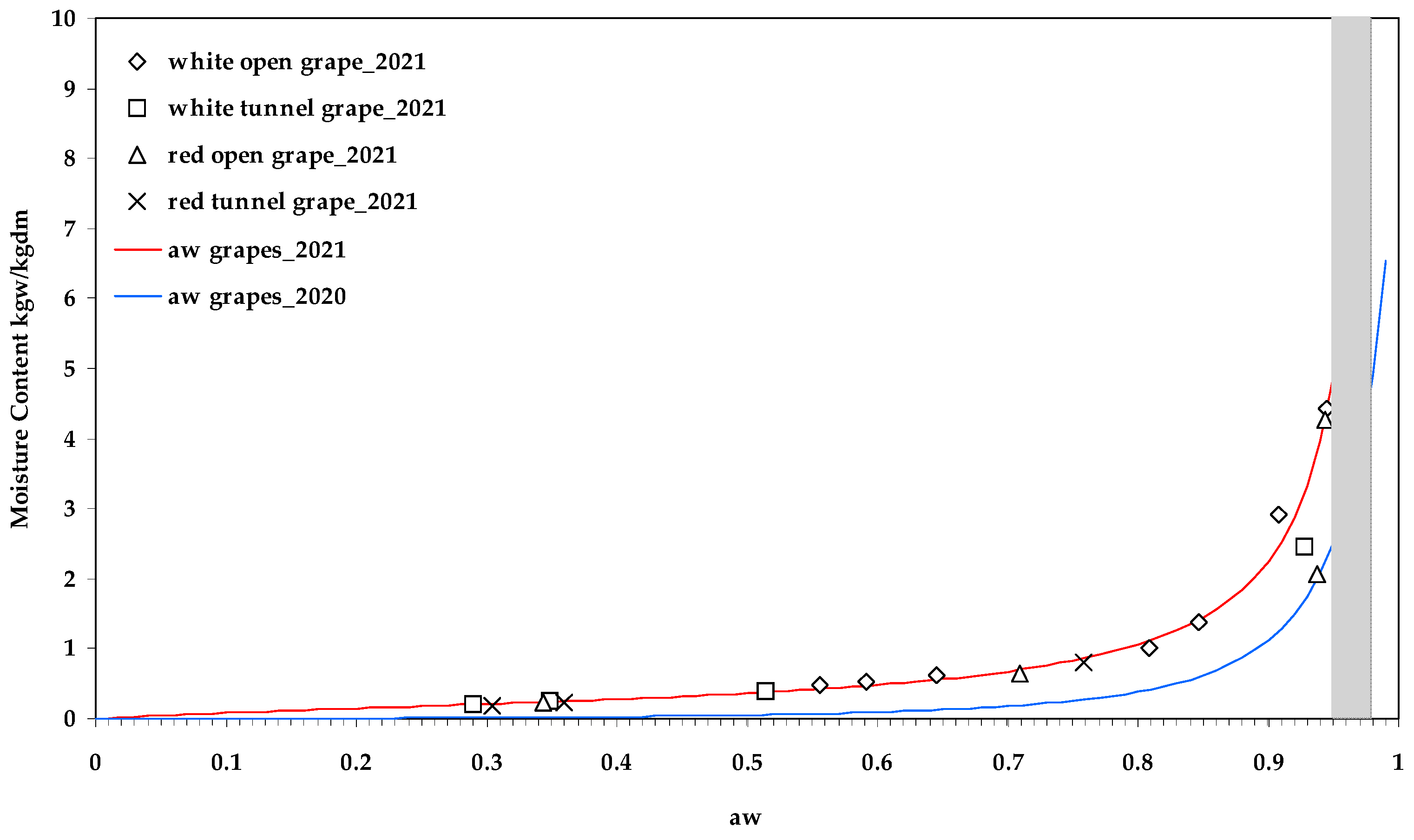


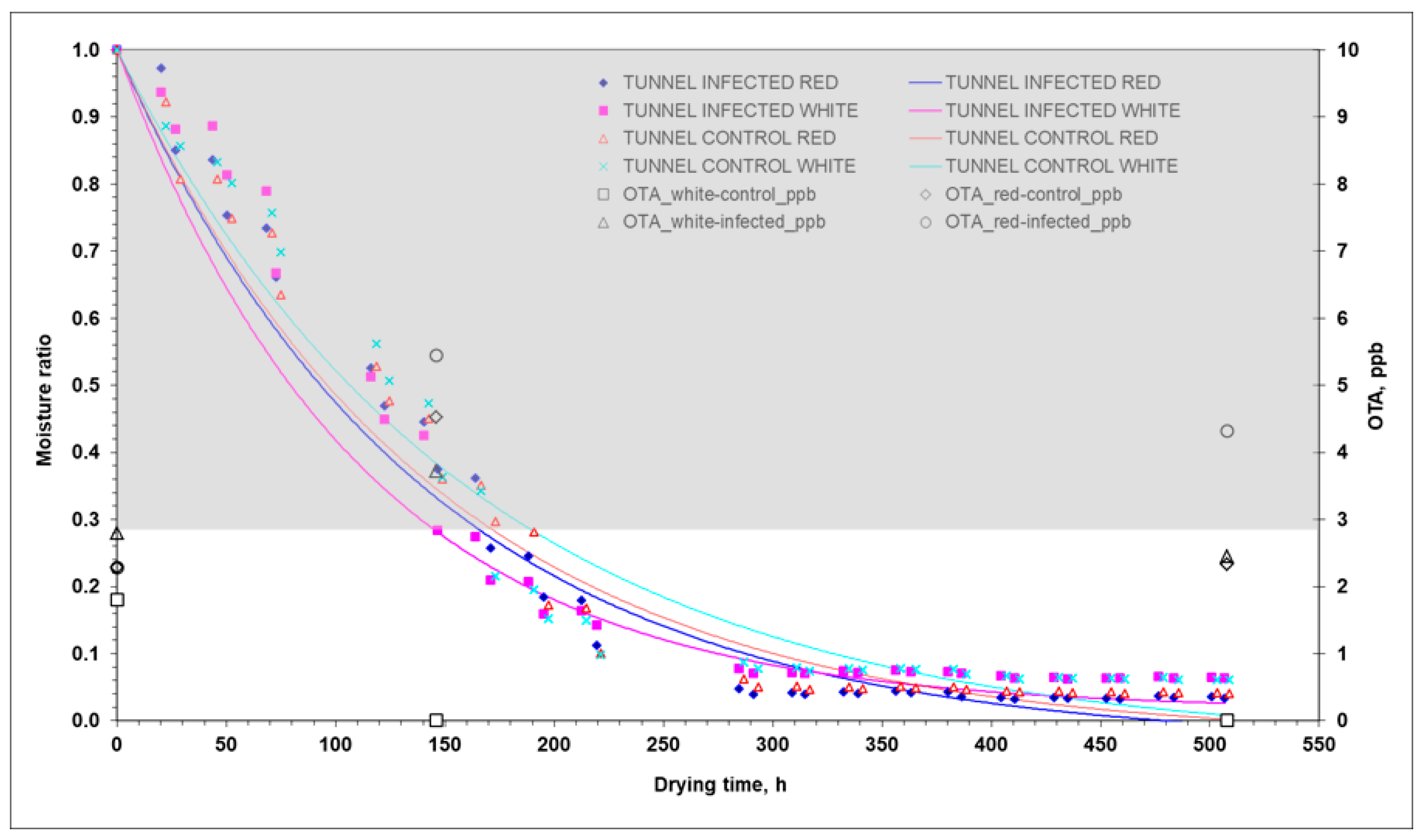
| Property | Drying Method | Sample Treatment | Grape Variety | Min | Max | Average | Homogeneous Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grapes surface temperature (°C) | Tunnel | Infected | Red | 7.23 | 65.61 | 24.63 | X | X | X | ||||
| White | 3.65 | 65.40 | 23.98 | X | X | ||||||||
| Control | Red | 6.61 | 65.07 | 25.72 | X | X | |||||||
| White | 4.34 | 62.16 | 24.76 | X | X | ||||||||
| Open | Infected | Red | 6.99 | 51.66 | 22.40 | X | |||||||
| White | 8.18 | 51.02 | 22.92 | X | X | ||||||||
| Control | Red | 9.97 | 50.73 | 24.36 | X | X | |||||||
| White | 7.38 | 55.93 | 23.34 | X | X | X | |||||||
| Air temperature inside the plastic crates (°C) | Tunnel | Control | Red | 4.83 | 75.24 | 25.24 | X | X | |||||
| White | 5.32 | 75.52 | 25.51 | X | |||||||||
| Open | Control | Red | 8.18 | 55.38 | 24.36 | X | |||||||
| White | 8.12 | 59.23 | 24.55 | X | X | ||||||||
| Air RH inside the plastic crates (%) | Tunnel | Red | 10.62 (5) | 93.97 (360) | 49.79 (26) | X | |||||||
| White | 9.025 (6) | 87.97 (396) | 47.93 (27) | X | X | ||||||||
| Open | Red | 12.24 (7) | 94.06 (117) | 46.69 (21) | X | ||||||||
| White | 16.76 (7) | 91.08 (146) | 48.35 (22) | X | |||||||||
| Air RH in distance from the plastic crates (%) | 14.29 (13) | 70.30 (78) | 41.77 (24) | X | |||||||||
| Solar diffusive radiation, W/m2 | 0.0 | 425 | 56.78 | ||||||||||
| Solar total radiation, W/m2 | 0.0 | 871 | 210.5 | ||||||||||
| Hour | Open-Air Drying/ White Grapes | Open-Air Drying/ Red Grapes | Tunnel Drying/ White Grapes | Tunnel Drying/ Red Grapes | Average Total Solar Radiation (W/m2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | SE | Average | SE | Average | SE | Average | SE | 2021 | 2020 | |
| 1 | −0.58 | 0.16 | 1.48 | 0.19 | 1.74 | 0.18 | 0.95 | 0.23 | 0.0 | 0.0 |
| 2 | −0.72 | 0.17 | 1.41 | 0.17 | 1.44 | 0.17 | 0.66 | 0.22 | 0.0 | 0.0 |
| 3 | −0.71 | 0.17 | 1.37 | 0.18 | 1.16 | 0.15 | 0.43 | 0.20 | 0.0 | 0.0 |
| 4 | −0.64 | 0.18 | 1.45 | 0.19 | 0.98 | 0.14 | 0.26 | 0.19 | 0.0 | 0.0 |
| 5 | −0.77 | 0.19 | 1.45 | 0.19 | 0.91 | 0.14 | 0.14 | 0.16 | 0.0 | 0.0 |
| 6 | −0.81 | 0.18 | 1.41 | 0.19 | 0.81 | 0.12 | 0.05 | 0.17 | 0.0 | 0.0 |
| 7 | −0.93 | 0.19 | 1.32 | 0.21 | 0.67 | 0.12 | −0.03 | 0.16 | 21.0 | 20.64 |
| 8 | −0.85 | 0.21 | 1.27 | 0.23 | 0.51 | 0.13 | −0.05 | 0.14 | 168.2 | 174.3 |
| 9 | 0.30 | 0.17 | 1.59 | 0.18 | −0.87 | 0.15 | −0.69 | 0.20 | 347.4 | 345.9 |
| 10 | 3.56 | 0.61 | 3.17 | 0.57 | −6.13 | 0.73 | −1.86 | 0.77 | 538.3 | 514.7 |
| 11 | 4.31 | 0.63 | 4.47 | 0.67 | −8.26 | 0.84 | −0.63 | 1.03 | 661.9 | 659.3 |
| 12 | 3.82 | 0.58 | 3.03 | 0.76 | −7.03 | 0.85 | 0.40 | 0.99 | 696.1 | 748.7 |
| 13 | 3.10 | 0.62 | 1.39 | 0.54 | −4.01 | 0.69 | 1.82 | 0.64 | 687.9 | 731.8 |
| 14 | 2.03 | 0.58 | −0.50 | 0.45 | 1.36 | 0.76 | 3.46 | 0.65 | 642.3 | 684.2 |
| 15 | 0.90 | 0.53 | 0.74 | 0.38 | 1.98 | 0.80 | 4.93 | 0.44 | 550.6 | 596.3 |
| 16 | 2.57 | 0.46 | 1.17 | 0.36 | −0.89 | 0.72 | 5.77 | 0.41 | 414.5 | 455.0 |
| 17 | 3.26 | 0.46 | 1.86 | 0.34 | 0.08 | 0.58 | 5.14 | 0.33 | 270.6 | 271.8 |
| 18 | 2.97 | 0.32 | 3.20 | 0.34 | 3.45 | 0.36 | 4.50 | 0.32 | 90.35 | 102.8 |
| 19 | 1.68 | 0.20 | 3.46 | 0.37 | 4.73 | 0.41 | 4.37 | 0.36 | 2.21 | 2.96 |
| 20 | 0.99 | 0.17 | 3.00 | 0.37 | 4.23 | 0.30 | 3.01 | 0.30 | 0.0 | 0.0 |
| 21 | 0.26 | 0.15 | 2.27 | 0.32 | 3.87 | 0.27 | 2.22 | 0.28 | 0.0 | 0.0 |
| 22 | −0.27 | 0.15 | 1.78 | 0.27 | 3.41 | 0.25 | 1.77 | 0.23 | 0.0 | 0.0 |
| 23 | −0.47 | 0.16 | 1.55 | 0.23 | 2.70 | 0.23 | 1.47 | 0.23 | 0.0 | 0.0 |
| 24 | −0.56 | 0.17 | 1.52 | 0.21 | 2.09 | 0.20 | 1.14 | 0.22 | 0.0 | 0.0 |
| Parameter | Estimate | Asymptotic SE | Asymptotic Confidence 95.0% | |
|---|---|---|---|---|
| Lower Interval | Upper Interval | |||
| Mo | 0.2176 | 0.003 | 0.2106 | 0.2246 |
| C | 4.6244 | 1.337 | 1.7360 | 7.5129 |
| Kb | 1.0060 | 0.0002 | 1.0055 | 1.0065 |
| Variety | Infection | Drying | Sampling (h) | CFU (CFU/mL) | OTA (µg/kg) |
|---|---|---|---|---|---|
| White | Control | Open | 0 | 133 | 1.81 |
| 146 | 700 | 3.59 | |||
| 508 | 5417 | 4.02 | |||
| Tunnel | 0 | 133 | 1.81 | ||
| 146 | 22 | 0.00 | |||
| 508 | 0 | 0.00 | |||
| Infected | Open | 0 | 133 (40) | 2.80 (0.00) | |
| 146 | 1783 (364.4) | 1.66 (2.14) | |||
| 508 | 709 (6472.6) | 3.54 (4.39) | |||
| Tunnel | 0 | 133 (38.9) | 2.80 (0.00) | ||
| 146 | 633 (5.6) | 3.73 (4.04) | |||
| 508 | 78 (4.4) | 2.45 (12.51) | |||
| Red | Control | Open | 0 | 244 | 2.28 |
| 146 | 1783 | 2.80 | |||
| 508 | 2673 | 4.02 | |||
| Tunnel | 0 | 244 | 2.28 | ||
| 146 | 17,219 | 4.52 | |||
| 508 | 108 | 2.33 | |||
| Infected | Open | 0 | 244 (264.4) | 2.28 (0.00) | |
| 146 | 300 (14,539.4) | 2.21 (6.21) | |||
| 508 | 1823 (8249.6) | 4.34 (7.00) | |||
| Tunnel | 0 | 244 (233.9) | 2.28 (0.00) | ||
| 146 | 13,875 (112.8) | 5.45 (430) | |||
| 508 | 3876 (56.7) | 4.32 (8.57) |
| CFU (CFU/mL) | OTA (µg/kg) | |
|---|---|---|
| [1]: Variety | n.s. | * |
| White | 822.97 (1154.32) | 2.34 a (4.08) |
| Red | 3552.94 (3909.48) | 3.26 b (4.53) |
| [2]: Infection | n.s. | * |
| Control | 2389.84 | 2.5 a |
| Infected | 1986.07 | 3.15 b |
| [3]: Drying | n.s. | n.s. |
| Open-air | 1328.67 (4988.43) | 2.94 (3.50) |
| In tunnel | 3047.25 (75.37) | 2.66 (5.11) |
| [4]: Sampling | n.s. | n.s. |
| Start | 188.92 (144.31) | 2.28 (0.58) |
| Middle | 4539.46 (3755.56) | 2.99 (4.17) |
| End | 1835.49 (3695.83) | 3.13 (8.16) |
| Interactions | ||
| [1] × [2] | n.s. | n.s. |
| [1] × [3] | * | * |
| [1] × [4] | n.s | n.s. |
| [2] × [3] | n.s | * |
| [2] × [4] | n.s | n.s. |
| [3] × [4] | n.s | * |
| [1] × [2] × [3] | n.s | n.s. |
| [1] × [2] × [4] | n.s | n.s. |
| [1] × [3] × [4] | n.s | n.s. |
| [2] × [3] × [4] | n.s | n.s. |
| [1] × [2] × [3] × [4] | n.s | n.s. |
| Variety | Infection | Drying | CFU (CFU/mL) | OTA (µg/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| Shape Factor | Predicted Average | D-Value | Shape Factor | Predicted Average | D-Value | |||
| White | Control | Open | 0.531 | 1094.41 | 0.225 | 3.662 | 3.522 | 0.216 |
| Tunnel | 2.619 | 117.50 | 0.385 | 10.752 | 2.835 | 0.385 | ||
| Infected | Open | 0.657 | 1063.40 | 0.225 | 4.227 | 3.411 | 0.216 | |
| Tunnel | 1.056 | 281.91 | 0.385 | 5.554 | 2.991 | 0.385 | ||
| Red | Control | Open | 0.779 | 2031.18 | 0.301 | 5.189 | 3.899 | 0.262 |
| Tunnel | 0.382 | 4938.97 | 0.236 | 2.625 | 3.435 | 0.295 | ||
| Infected | Open | 1.275 | 981.41 | 0.301 | 8.376 | 3.800 | 0.262 | |
| Tunnel | 0.704 | 6787.15 | 0.236 | 5.489 | 4.524 | 0.295 | ||
| Drying | Infection | Variety | Shrinkage Velocity × 10−9 (m/s) | |
|---|---|---|---|---|
| x-Axis | y-Axis | |||
| Open-air | Control | White | 2.449 | 1.669 |
| Red | 2.090 | 1.483 | ||
| Infected | White | 3.165 | 2.625 | |
| Red | 2.888 | 2.051 | ||
| Tunnel | Control | White | 4.009 | 3.235 |
| Red | 2.476 | 1.576 | ||
| Infected | White | 4.007 | 2.431 | |
| Red | 3.859 | 1.704 | ||
| Drying | Infection | Variety | kc × 10−9 (m/s) | rpeel × 108 (s/m) | MRE (%) | × 10−11 (m2/s) |
|---|---|---|---|---|---|---|
| Open-air | Control | White | 5.05 (2.75) | 1.98 (3.64) | 3.42 (2.17) | 0.70 (14.8) |
| Red | 2.94 (3.33) | 3.40 (3.00) | 4.65 (3.64) | 1.80 (29.9) | ||
| Infected | White | 4.18 (3.16) | 2.39 (3.16) | 3.05 (2.55) | 3.60 (50.3) | |
| Red | 3.31 (2.61) | 3.02 (3.83) | 3.36 (5.53) | 2.10 (21.5) | ||
| Tunnel | Control | White | 5.18 (6.14) | 1.93 (1.63) | 3.88 (6.20) | 4.50 (10.6) |
| Red | 5.17 (6.37) | 1.93 (1.57) | 5.72 (6.74) | 3.40 (39.5) | ||
| Infected | White | 5.85 (8.31) | 1.71 (1.20) | 4.38 (6.72) | 8.60 (53.8) | |
| Red | 5.17 (7.79) | 1.93 (1.28) | 4.15 (8.30) | 3.50 (151) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Templalexis, C.; Giorni, P.; Lentzou, D.; Mozzoni, F.; Battilani, P.; Tsitsigiannis, D.I.; Xanthopoulos, G. IoT for Monitoring Fungal Growth and Ochratoxin A Development in Grapes Solar Drying in Tunnel and in Open Air. Toxins 2023, 15, 613. https://doi.org/10.3390/toxins15100613
Templalexis C, Giorni P, Lentzou D, Mozzoni F, Battilani P, Tsitsigiannis DI, Xanthopoulos G. IoT for Monitoring Fungal Growth and Ochratoxin A Development in Grapes Solar Drying in Tunnel and in Open Air. Toxins. 2023; 15(10):613. https://doi.org/10.3390/toxins15100613
Chicago/Turabian StyleTemplalexis, Charalampos, Paola Giorni, Diamanto Lentzou, Francesco Mozzoni, Paola Battilani, Dimitrios I. Tsitsigiannis, and Georgios Xanthopoulos. 2023. "IoT for Monitoring Fungal Growth and Ochratoxin A Development in Grapes Solar Drying in Tunnel and in Open Air" Toxins 15, no. 10: 613. https://doi.org/10.3390/toxins15100613
APA StyleTemplalexis, C., Giorni, P., Lentzou, D., Mozzoni, F., Battilani, P., Tsitsigiannis, D. I., & Xanthopoulos, G. (2023). IoT for Monitoring Fungal Growth and Ochratoxin A Development in Grapes Solar Drying in Tunnel and in Open Air. Toxins, 15(10), 613. https://doi.org/10.3390/toxins15100613








