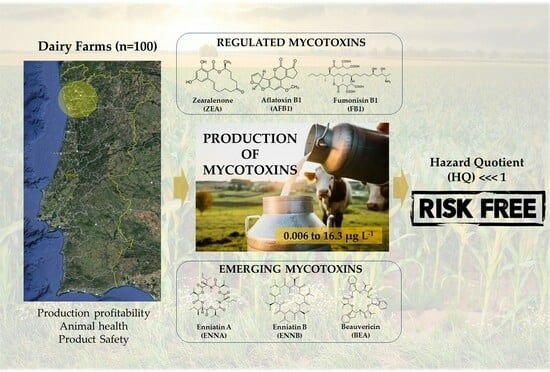
Regulated and Emerging Mycotoxins in Bulk Raw Milk: What Is the Human Risk?
Abstract
:1. Introduction
2. Results and Discussion
2.1. Occurrence of Mycotoxins in Raw Bulk Milk
2.1.1. Aflatoxins
2.1.2. Fumonisins
2.1.3. Trichothecenes
2.1.4. Zearalenone
2.1.5. Emerging & Non-Regulated Mycotoxins
2.2. Co-Occurrence of Mycotoxins in Raw Bulk Milk
2.3. Assessment of Human Exposure to Mycotoxins
2.4. Risk Characterization
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sampling
4.3. Mycotoxin Extraction
4.4. Mycotoxin Analysis
4.5. Descriptive & Statistical Analysis
4.6. Estimated Daily Intake (EDI) & Hazard Quotient (HQ)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilandžić, N.; Varga, I.; Varenina, I.; Kolanović, B.S.; Luburić, Ð.B.; Ðokić, M.; Sedak, M.; Cvetnić, L.; Cvetnić, Ž. Seasonal Occurrence of Aflatoxin M1 in Raw Milk during a Five-Year Period in Croatia: Dietary Exposure and Risk Assessment. Foods 2022, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; González-Peñas, E. Development and Validation of a High Performance Liquid Chromatographic–Mass Spectrometry Method for the Simultaneous Quantification of 10 Trichothecenes in Ultra-High Temperature Processed Cow Milk. J. Chromatogr. A 2015, 1419, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Becker-Algeri, T.A.; Castagnaro, D.; de Bortoli, K.; de Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO Dairy and Dairy Products. In OECD-FAO Agricultural Outlook 2019–2028; OECD Publishing: Paris, France, 2019; pp. 180–189. ISBN 78-92-64-31246-3.
- National Food Institute, Technical University of Denmark (DTU Food); Assunção, R.; Pires, S.; Nauta, M.J. Risk-Benefit Assessment of Foods. EFSA J. 2019, 17, e170917. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Public Health Risks Related to the Consumption of Raw Drinking Milk. EFSA J. 2015, 13, 3940. [Google Scholar] [CrossRef]
- Kalmus, P.; Kramarenko, T.; Roasto, M.; Meremae, K.; Viltrop, A. Quality of Raw Milk Intended for Direct Consumption in Estonia Quality of Raw Milk Intended for Direct Consumption in Estonia. Food Control 2015, 51, 135–139. [Google Scholar] [CrossRef]
- Berge, A.C.; Baars, T. Raw Milk Producers with High Levels of Hygiene and Safety. Epidemiol. Infect. 2020, 148, 1–7. [Google Scholar] [CrossRef]
- De Klerk, J.N.; Robinson, P.A. Drivers and Hazards of Consumption of Unpasteurised Bovine Milk and Milk Products in High-Income Countries. PeerJ 2022, 10, e13426. [Google Scholar] [CrossRef]
- Leone, C.; Thippareddi, H.; Ndiaye, C.; Niang, I.; Diallo, Y.; Singh, M. Safety and Quality of Milk and Milk Products in Senegal—A Review. Foods 2022, 11, 3479. [Google Scholar] [CrossRef]
- Signorini, M.L.; Gaggiotti, M.; Molineri, A.; Chiericatti, C.A.; Zapata de Basílico, M.L.; Basílico, J.C.; Pisani, M. Exposure Assessment of Mycotoxins in Cow’s Milk in Argentina. Food Chem. Toxicol. 2012, 50, 250–257. [Google Scholar] [CrossRef]
- Dagnac, T.; Latorre, A.; Fernández Lorenzo, B.; Llompart, M. Validation and Application of a Liquid Chromatography-Tandem Mass Spectrometry Based Method for the Assessment of the Co-Occurrence of Mycotoxins in Maize Silages from Dairy Farms in NW Spain. Food Addit. Contam. 2016, 33, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J. Mycotoxins in Cattle Feeds and Carry-over to Dairy Milk: A Review. Food Addit. Contam. 2008, 25, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Britzi, M.; Friedman, S.; Miron, J.; Solomon, R.; Cuneah, O.; Shimshoni, J.A.; Soback, S.; Ashkenazi, R.; Armer, S.; Shlosberg, A. Carry-over of Aflatoxin B1 to Aflatoxin M1 in High Yielding Israeli Cows in Mid- and Late-Lactation. Toxins 2013, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, J.A.; Cuneah, O.; Sulyok, M.; Krska, R.; Galon, N.; Sharir, B.; Shlosberg, A. Mycotoxins in Corn and Wheat Silage in Israel. Food Addit. Contam. Part A 2013, 30, 1614–1625. [Google Scholar] [CrossRef]
- Drejer Storm, I.M.L.; Rasmussen, R.R.; Rasmussen, P.H. Occurrence of Pre- and Post-Harvest Mycotoxins and Other Secondary Metabolites in Danish Maize Silage. Toxins 2014, 6, 2256–2269. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; van der Merwe, D. Mycotoxins in the Food Chain: Contamination of Foods of Animal Origin. In Food Safety Assurance and Veterinary Public Health: Vol. 7 Chemical Hazards in Foods of Animal Origin; Smulders, F.J.M., Rietjens, I.M.C.M., Rose, M.D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; pp. 241–261. ISBN 978-90-8686-326-6. [Google Scholar]
- European Commission Consolidated Text. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2020, 364, 5–24. [Google Scholar]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-Mycotoxin Occurrence in Feed, Metabolism and Carry-over to Animal-Derived Food Products: A Review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef]
- Pattono, D.; Gallo, P.F.; Civera, T. Detection and Quantification of Ochratoxin A in Milk Produced in Organic Farms. Food Chem. 2011, 127, 374–377. [Google Scholar] [CrossRef]
- Boudra, H.; Barnouin, J.; Dragacci, S.; Morgavi, D.P. Aflatoxin M1 and Ochratoxin a in Raw Bulk Milk from French Dairy Herds. J. Dairy Sci. 2007, 90, 3197–3201. [Google Scholar] [CrossRef]
- Rocchetti, G.; Ghilardelli, F.; Masoero, F.; Gallo, A. Screening of Regulated and Emerging Mycotoxins in Bulk Milk Samples by High-Resolution Mass Spectrometry. Foods 2021, 10, 2025. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize Food Chain and Mycotoxins: A Review on Occurrence Studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- Hof, H. Mycotoxins in Milk for Human Nutrition: Cow, Sheep and Human Breast Milk. GMS Infect. Dis. 2016, 4, Doc03. [Google Scholar] [CrossRef] [PubMed]
- Turna, N.; Wu, F. Aflatoxin M1 in Milk: A Global Occurrence, Intake, & Exposure Assessment. Trends Food Sci. Technol. 2021, 110, 183–192. [Google Scholar] [CrossRef]
- Maragos, C.M.; Richard, J.L. Quantitation and Stability of Fumonisins B1 and B2 in Milk. J. AOAC Int. 1994, 77, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Bertero, A.; Fossati, P.; Tedesco, D.; Caloni, F. Beauvericin and Enniatins: In Vitro Intestinal Effect. Toxins 2020, 12, 686. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-Occurrence of Regulated, Masked and Emerging Mycotoxins and Secondary Metabolites in Finished Feed and Maize–An Extensive Survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Dvořáčková, M.; Kašparovský, T. Feedborne Mycotoxins Beauvericin and Enniatins and Livestock Animals. Toxins 2021, 13, 32. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of Alternaria Toxins in Feed and Food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Risks for Public and Animal Health Related to the Presence of Citrinin in Food and Feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on Risks for Animal and Public Health Related to the Presence of Nivalenol in Food and Feed. EFSA J. 2013, 11, 3262. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Risks to Human and Animal Health Related to the Presence of Beauvericin and Enniatins in Food and Feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Mycotoxins in Raw Bovine Milk: UHPLC-QTrap-MS/MS Method as a Biosafety Control Tool. Toxins 2023, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Zheng, N.; Zheng, B.Q.; Wen, F.; Cheng, J.B.; Han, R.W.; Xu, X.M.; Li, S.L.; Wang, J.Q. Simultaneous Determination of Aflatoxin M1, Ochratoxin A, Zearalenone and a-Zearalenol in Milk by UHPLC–MS/MS. Food Chem. 2014, 146, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zheng, N.; Wen, F.; Guo, L.; Fu, C.; Ouyang, H.; Zhong, L.; Wang, J.; Lei, S. Multi-Mycotoxins Analysis in Raw Milk by Ultra High Performance Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Food Control 2018, 84, 305–311. [Google Scholar] [CrossRef]
- Debevere, S.; De Baere, S.; Haesaert, G.; Rychlik, M.; Fievez, V.; Croubels, S. Development of an UPLC-MS/MS Method for the Analysis of Mycotoxins in Rumen Fluid with and without Maize Silage Emphasizes the Importance of Using Matrix-Matched Calibration. Toxins 2019, 11, 519. [Google Scholar] [CrossRef]
- Monbaliu, S.; Poucke, C.; Detavernier, C.; Dumoulin, F.; De Velde, M.; Schoeters, E.; Van Dyck, S.; Averkieva, O.; Van Peteghem, C.; De Saeger, S. Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method. J. Agric. Food Chem. 2010, 58, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Braun, D.; Windisch, P.; Warth, B.; Ezekiel, C.N. Assessment of Multiple Mycotoxins in Raw Milk of Three Different Animal Species in Nigeria. Food Control 2022, 131, 108258. [Google Scholar] [CrossRef]
- De Roma, A.; Rossini, C.; Ritieni, A.; Gallo, P.; Esposito, M. A Survey on the Aflatoxin M1 Occurrence and Seasonal Variation in Buffalo and Cow Milk from Southern Italy. Food Control 2017, 81, 30–33. [Google Scholar] [CrossRef]
- Rahimi, E.; Bonyadian, M.; Rafei, M.; Kazemeini, H.R. Occurrence of Aflatoxin M1 in Raw Milk of Five Dairy Species in Ahvaz, Iran. Food Chem. Toxicol. 2010, 48, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.C.; Almeida, A.M.; Teixeira, A.S.; Pereira, A.L.; Falcão, A.C.; Pena, A.; Lino, C.M. Aflatoxin M1 in Marketed Milk in Portugal: Assessment of Human and Animal Exposure. Food Control 2013, 30, 411–417. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Rodríguez-Canás, I.; Alfonso, A.; Sainz, M.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte Method for the Determination of Regulated, Emerging and Modified Mycotoxins in Milk: QuEChERS Extraction Followed by UHPLC–MS/MS Analysis. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, P.T.; Becker-algeri, T.; Drunkler, D.; Badiale-furlong, E. Aflatoxin B1 and M1 in Milk. Anal. Chim. Acta 2014, 829, 68–74. [Google Scholar] [CrossRef]
- Magnussen, A.; Parsi, M.A. Aflatoxins, Hepatocellular Carcinoma and Public Health. World J. Gastroenterol. 2013, 19, 1508–1512. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens—The IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- IARC. Risk Assessment and Risk Management of Mycotoxins. In IARC Scientific Publications; IARC: Lyon, France, 2012; pp. 105–117. [Google Scholar]
- BIOMIN. World Mycotoxin Survey 2020: Annual Report; BIOMIN: Inzersdorf-Getzersdorf, Austria, 2021; pp. 1–10. [Google Scholar]
- Grajewski, J.; Błajet-Kosicka, A.; Twaruzek, M.; Kosicki, R. Occurrence of Mycotoxins in Polish Animal Feed in Years. J. Anim. Physiol. Anim. Nutr. 2012, 96, 870–877. [Google Scholar] [CrossRef]
- Miller, J.D. Mycotoxins in Small Grains and Maize: Old Problems, New Challenges. Food Addit. Contam.-Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 219–230. [Google Scholar] [CrossRef]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and Levels of Regulated and Emerging Mycotoxins in Silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef]
- Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin Occurrence in Maize Silage—A Neglected Risk for Bovine Gut Health? Toxins 2019, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Romera, D.; Mateo, E.M. Study on Mycotoxin Contamination of Maize Kernels in Spain. Food Control 2020, 118, 107370. [Google Scholar] [CrossRef]
- Caloni, F.; Spotti, M.; Auerbach, H.; Camp, H.O.; Den; Gremmels, J.F.; Pompa, G. In Vitro Metabolism of Fumonisin B1 by Ruminal Microflora. Vet. Res. Commun. 2000, 24, 379–387. [Google Scholar] [CrossRef]
- Coffey, R.; Cummins, E.; Ward, S. Exposure Assessment of Mycotoxins in Dairy Milk. Food Control 2009, 20, 239–249. [Google Scholar] [CrossRef]
- Gazzotti, T.; Lugoboni, B.; Zironi, E.; Barbarossa, A.; Serraino, A.; Pagliuca, G. Determination of Fumonisin B1 in Bovine Milk by LC-MS/MS. Food Control 2009, 20, 1171–1174. [Google Scholar] [CrossRef]
- Winkler, J.; Kersten, S.; Valenta, H.; Meyer, U.; Engelhardt, U.H.; Dänicke, S. Development of a Multi-Toxin Method for Investigating the Carryover of Zearalenone, Deoxynivalenol and Their Metabolites into Milk of Dairy Cows. Food Addit. Contam. Part A 2015, 32, 371–380. [Google Scholar] [CrossRef]
- Keese, C.; Meyer, U.; Valenta, H.; Schollenberger, M.; Weber, I.; Rehage, J.; Breves, G.; Dänicke, S. No Carry over of Unmetabolised Deoxynivalenol in Milk of Dairy Cows Fed High Concentrate Proportions. Mol. Nutr. Food Res. 2008, 52, 1514–1529. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Elbæk, T.H. Determination of Mycotoxins in Bovine Milk by Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 820, 183–196. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Mirocha, C.J.; Behrens, J.C.; Swanson, S.P. Metabolic Fate of T-2 in a Lactating Cow. Food Cosmet. Toxicol. 1981, 19, 31–39. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risks for Animal Health Related to the Presence of Zearalenone and Its Modified Forms in Feed. EFSA J. 2017, 15, 4851. [Google Scholar] [CrossRef]
- Puga-Torres, B.; Cáceres-Chicó, M.; Alarcón-Vásconez, D.; Gómez, C. Determination of Zearalenone in Raw Milk from Different Provinces of Ecuador. Vet. World 2021, 14, 2048–2054. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple Mycotoxin Analysis in Nut Products: Occurrence and Risk Characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Fraeyman, S.; Meyer, E.; Devreese, M.; Antonissen, G.; Demeyere, K.; Haesebrouck, F.; Croubels, S. Comparative in Vitro Cytotoxicity of the Emerging Fusarium Mycotoxins Beauvericin and Enniatins to Porcine Intestinal Epithelial Cells. Food Chem. Toxicol. 2018, 121, 566–572. [Google Scholar] [CrossRef]
- Pietruszka, K.; Panasiuk, Ł.; Jedziniak, P. Survey of the Enniatins and Beauvericin in Raw and UHT Cow’s Milk in Poland. J. Vet. Res. 2023, 67, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, K.; Jestoi, M.; Eriksen, G.S. Health Effects of Moniliformin: A Poorly Understood Fusarium Mycotoxin. World Mycotoxin J. 2010, 3, 403–414. [Google Scholar] [CrossRef]
- Flores-Flores, M.E.; Lizarraga, E.; López de Cerain, A.; González-Peñas, E. Presence of Mycotoxins in Animal Milk: A Review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in Food: A Mycotoxin Concern for Human Health and Its Management Strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; González-Peñas, E. Short Communication: Analysis of Mycotoxins in Spanish Milk. J. Dairy Sci. 2018, 101, 113–117. [Google Scholar] [CrossRef]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twaruzek, M. Multiannual Mycotoxin Survey in Feed Materials and Feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. [Google Scholar] [CrossRef]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of Multiple Mycotoxins in European Feedingstuffs, Assessment of Dietary Intake by Farm Animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I.P. Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Management of Left-Censored Data in Dietary Exposure Assessment of Chemical Substances. EFSA J. 2010, 8, 1557. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística (INE). Consumo Humano de Leite e Produtos Lácteos per Capita (Kg/Hab.) Por Tipo de Leites e Produtos Lácteos. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000214&contexto=bd&selTab=tab2 (accessed on 25 May 2023).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Mycotoxins in Food: Fifty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; Joint FAO/WHO Expert Committee on Food Additives: San Francisco, CA, USA, 2001. [Google Scholar]
- Marin, S.; Ramos, A.J.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins Co-Contamination: Methodological Aspects and Biological Relevance of Combined Toxicity Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Comprehensive Assessment of Different Extraction Methodologies for Optimization and Validation of an Analytical Multi-Method for Determination of Emerging and Regulated Mycotoxins in Maize by UHPLC-MS/MS. Food Chem. Adv. 2023, 2, 100145. [Google Scholar] [CrossRef]

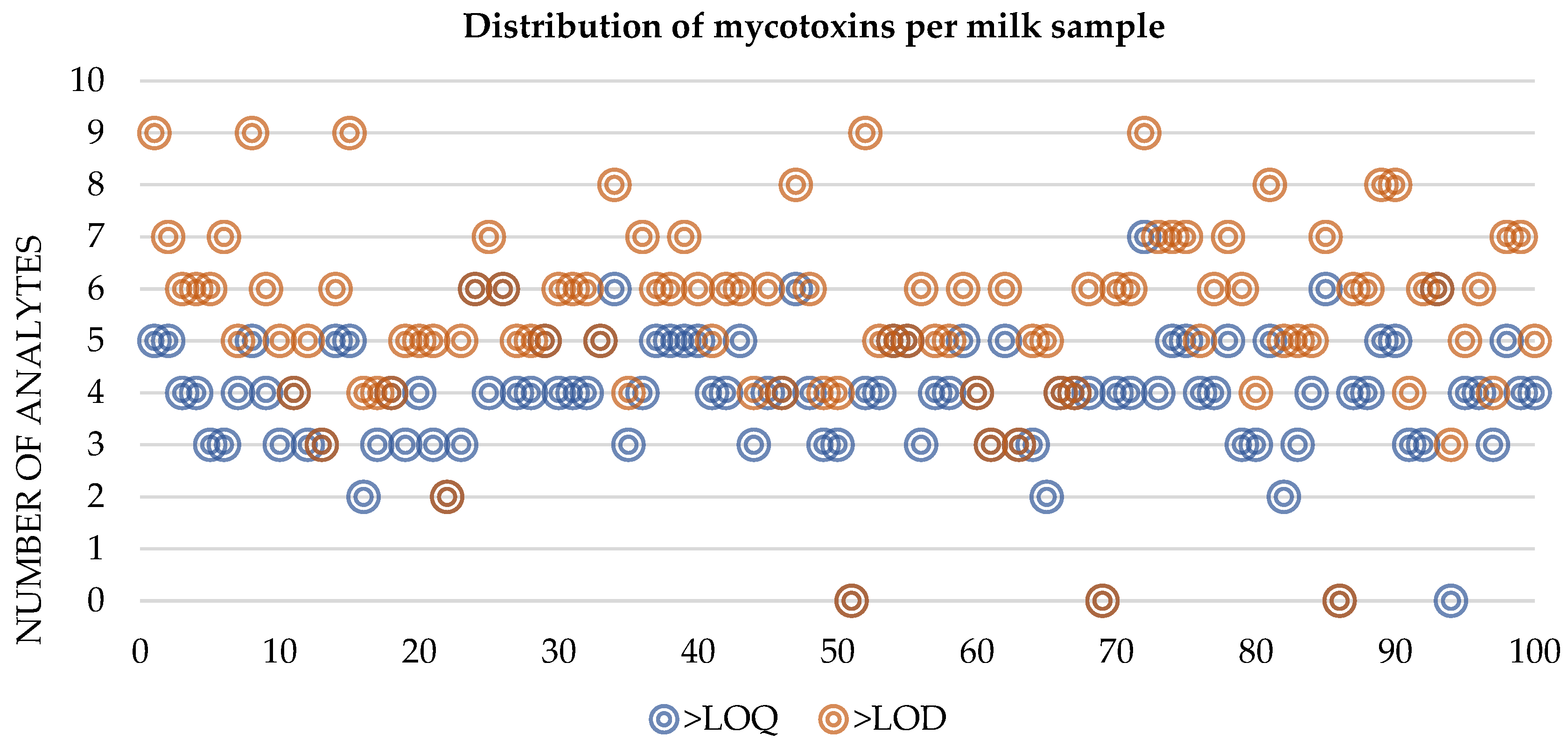
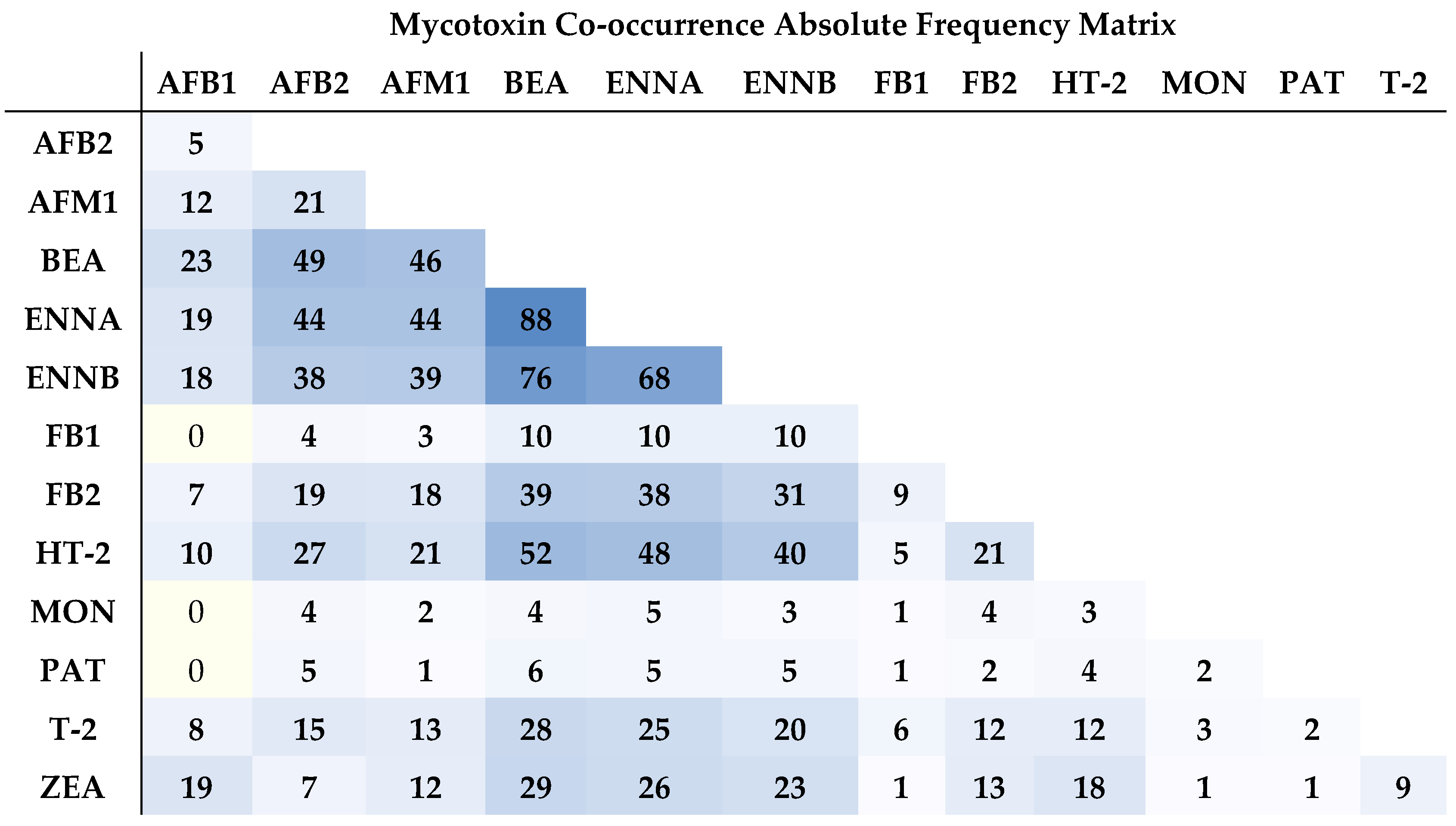
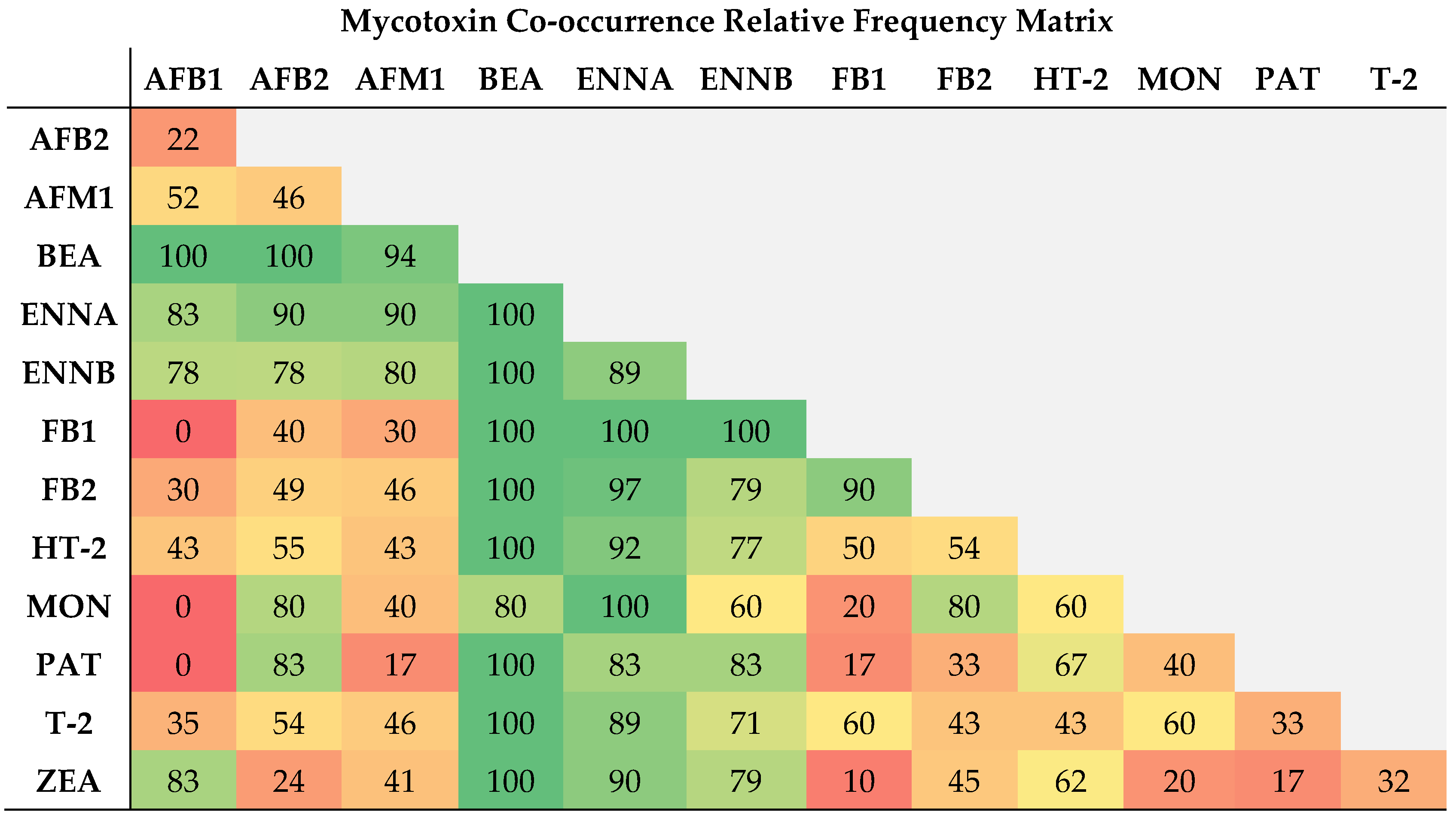
| Mycotoxins | % Positive Samples (>LOD) | % Positive Samples (>LOQ) | Maximum Value (µg L−1) | Range (µg L−1) | Mean Concentration of Positive Samples (µg L−1) |
|---|---|---|---|---|---|
| AFB1 | 23 | 19 | 0.20 | 0.018–0.20 | 0.08 |
| AFB2 | 49 | 47 | 0.23 | 0.006–0.23 | 0.06 |
| AFM1 | 46 | 13 | 0.02 | 0.011–0.02 | 0.02 |
| BEA | 97 | 92 | 0.81 | 0.09–0.81 | 0.30 |
| ENNA | 88 | 88 | 16.32 | 9.78–16.32 | 10.16 |
| ENNB | 76 | 75 | 10.60 | 4.36–10.60 | 5.97 |
| FB1 | 10 | 0 | - | - | - |
| FB2 | 39 | 0 | - | - | - |
| HT-2 | 52 | 44 | 1.91 | 0.086–1.91 | 0.50 |
| MON | 5 | 1 | 1.30 | - | - |
| PAT | 6 | 0 | - | - | - |
| T-2 | 28 | 11 | 6.39 | 0.90–6.39 | 1.70 |
| ZEA | 29 | 1 | 9.80 | - | - |
| Total Number of Positive Samples (ng kg−1 bw Day−1) | Total Number of Samples (ng kg−1 bw Day−1) | |||
|---|---|---|---|---|
| Mycotoxins | EDI (Mean Level) | EDI (Maximum Level) | EDI (LB) | EDI (UB) |
| AFB1 | 0.18 | 0.45 | 0.03 | 0.05 |
| AFB2 | 0.13 | 0.53 | 0.06 | 0.06 |
| AFM1 | 0.04 | 0.05 | 0.01 | 0.03 |
| BEA | 0.69 | 1.84 | 0.64 | 0.65 |
| ENNA | 23.12 | 37.11 | 20.34 | 22.83 |
| ENNB | 13.58 | 24.13 | 10.28 | 12.59 |
| FB1 | ** | ** | 0.15 | 2.09 |
| FB2 | ** | ** | 0.35 | 5.43 |
| HT-2 | 1.16 | 4.34 | 0.51 | 0.53 |
| MON | * | * | 0.09 | 1.57 |
| PAT | ** | ** | 0.08 | 2.41 |
| T-2 | 3.9 | 14.5 | 0.54 | 1.27 |
| ZEA | * | * | 1.71 | 9.51 |
| Sum of T-2 and HT-2 | 5.02 | 18.87 | 1.06 | 1.80 |
| Sum of FB1 and FB2 | - | - | 0.50 | 7.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Regulated and Emerging Mycotoxins in Bulk Raw Milk: What Is the Human Risk? Toxins 2023, 15, 605. https://doi.org/10.3390/toxins15100605
Leite M, Freitas A, Barbosa J, Ramos F. Regulated and Emerging Mycotoxins in Bulk Raw Milk: What Is the Human Risk? Toxins. 2023; 15(10):605. https://doi.org/10.3390/toxins15100605
Chicago/Turabian StyleLeite, Marta, Andreia Freitas, Jorge Barbosa, and Fernando Ramos. 2023. "Regulated and Emerging Mycotoxins in Bulk Raw Milk: What Is the Human Risk?" Toxins 15, no. 10: 605. https://doi.org/10.3390/toxins15100605
APA StyleLeite, M., Freitas, A., Barbosa, J., & Ramos, F. (2023). Regulated and Emerging Mycotoxins in Bulk Raw Milk: What Is the Human Risk? Toxins, 15(10), 605. https://doi.org/10.3390/toxins15100605