Cyanobacteria Microcystis aeruginosa Contributes to the Severity of Fish Diseases: A Study on Spring Viraemia of Carp
Abstract
:1. Introduction
2. Results
2.1. Body Size Measurements
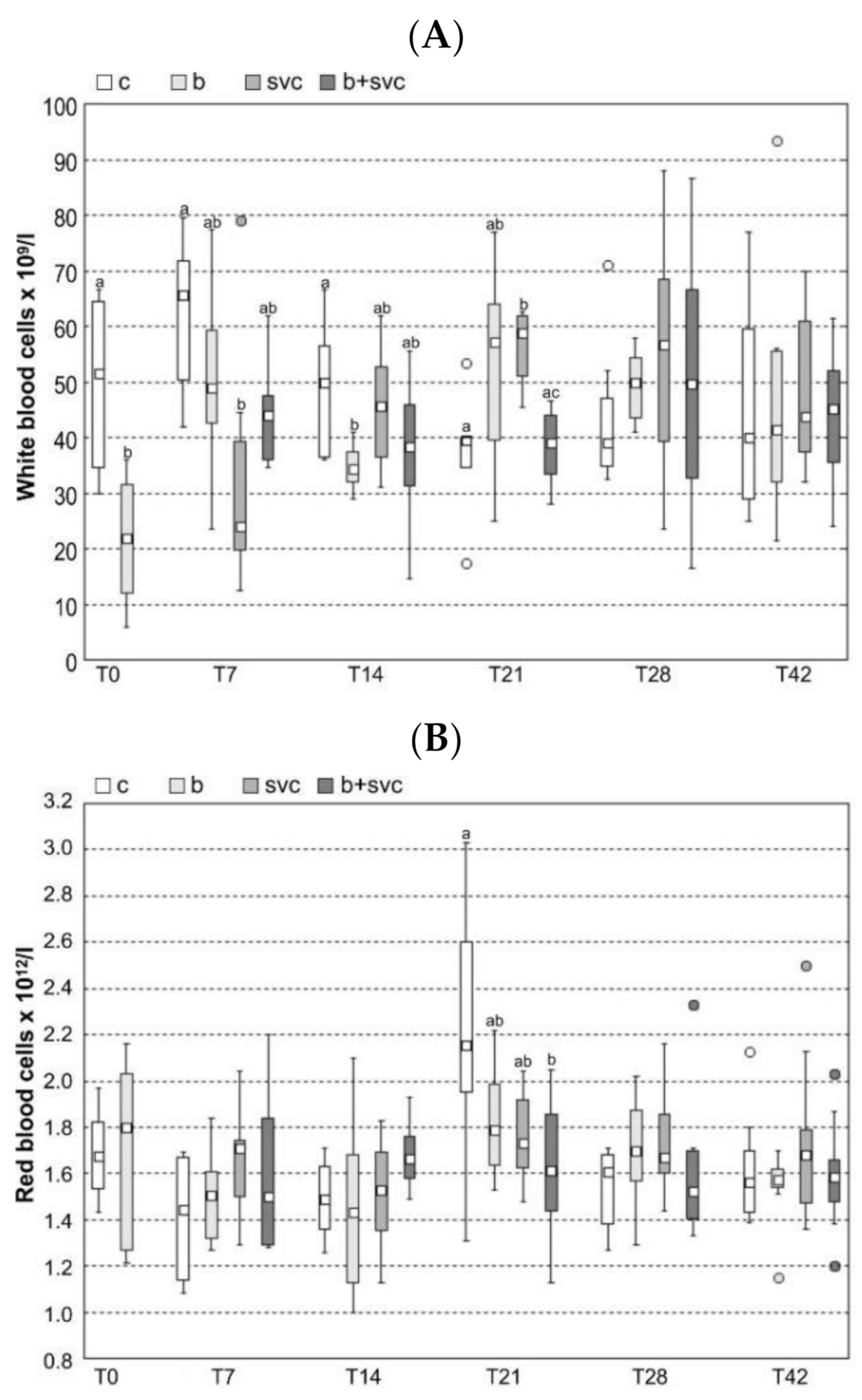
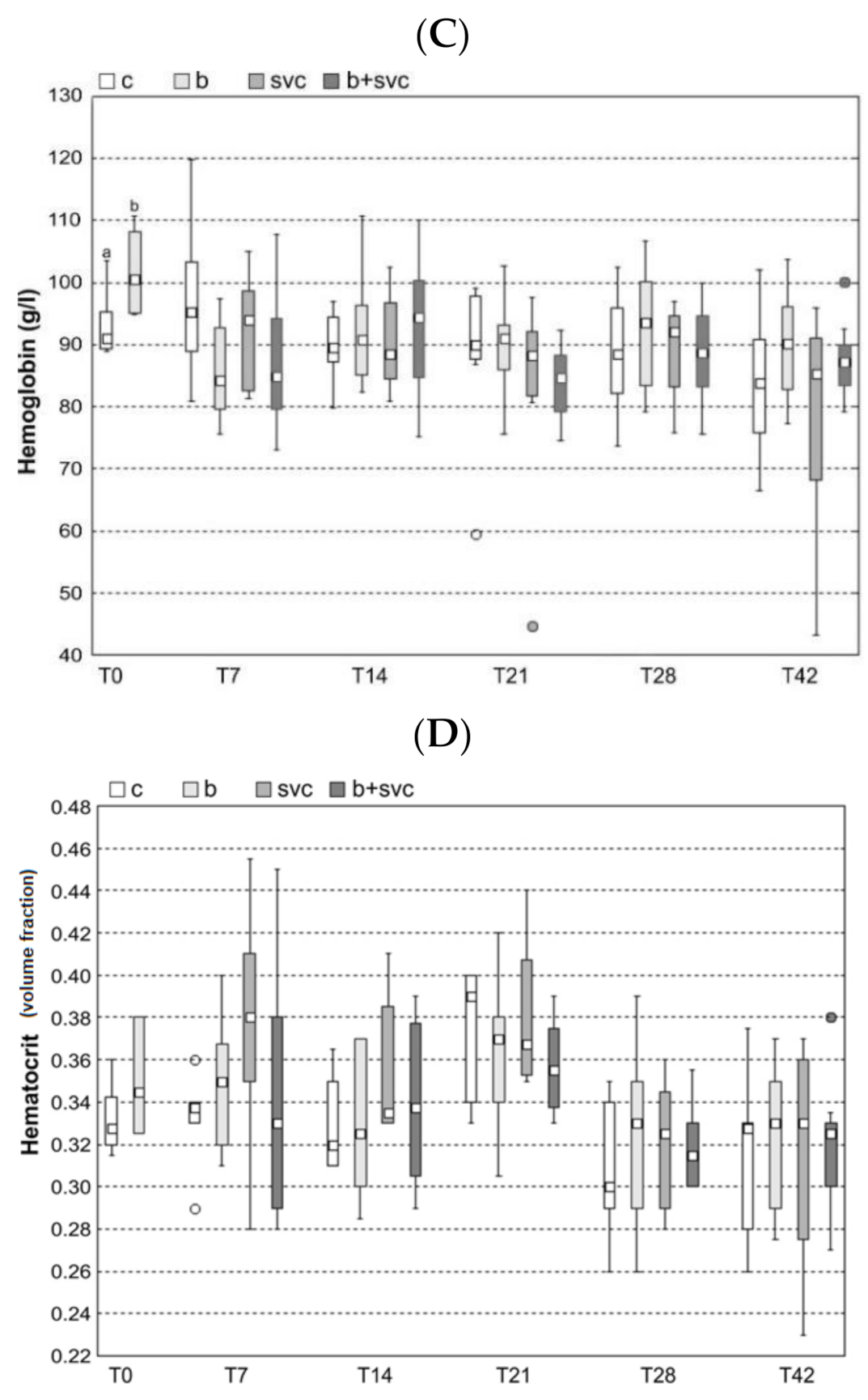
2.2. Hematology
2.3. Total Immunoglobulin Level
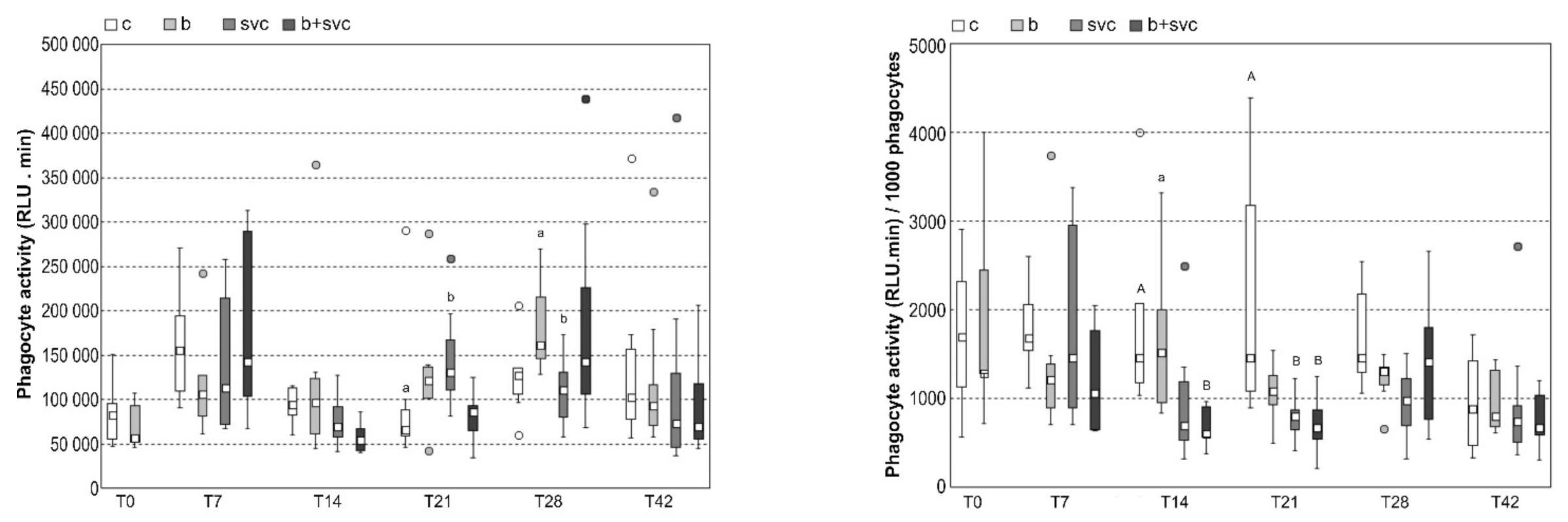
2.4. Respiratory Burst
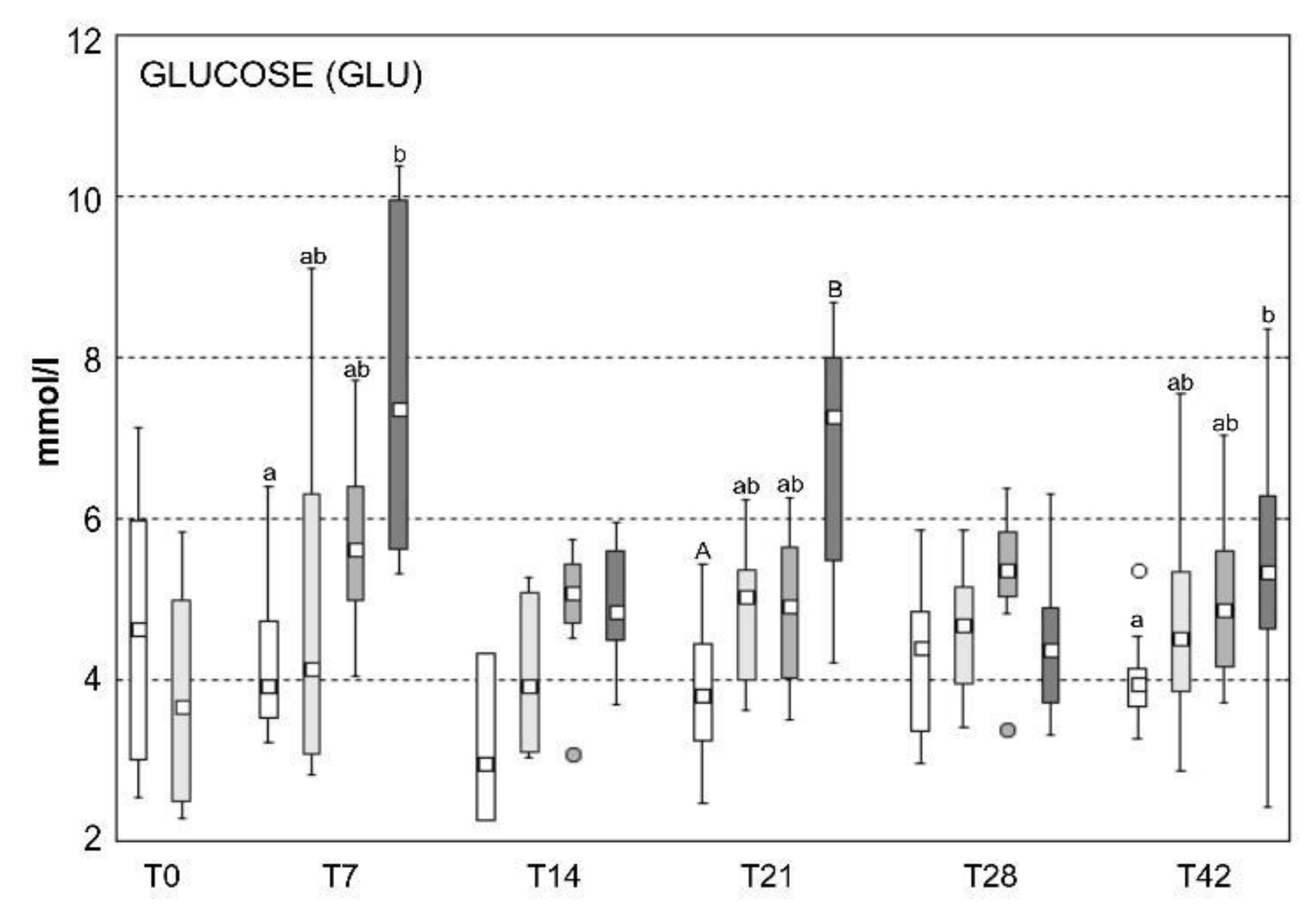
2.5. Blood Biochemistry
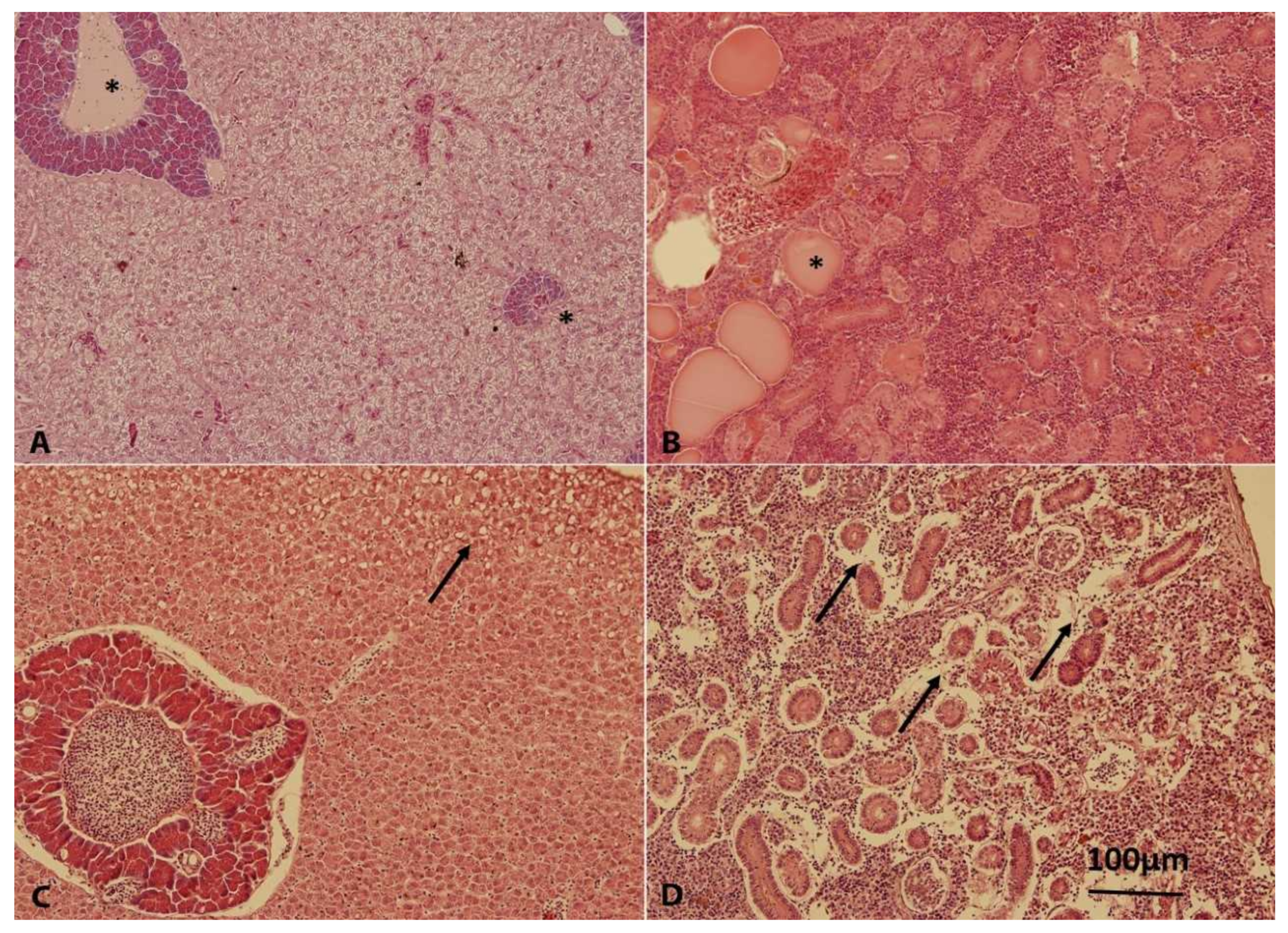
2.6. Pathological Evaluation and Histopathology
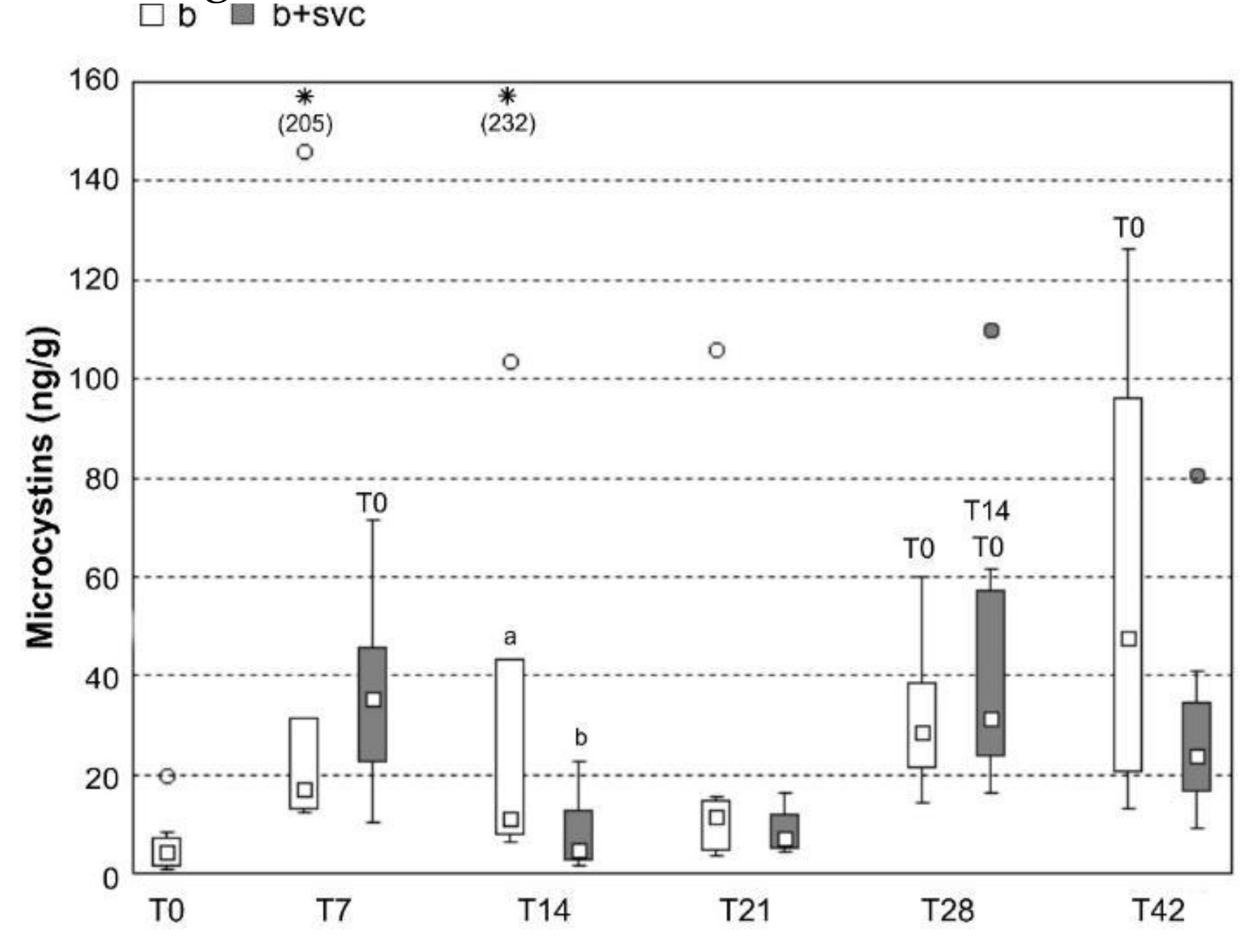
2.7. Burden of Microcystins in the Liver
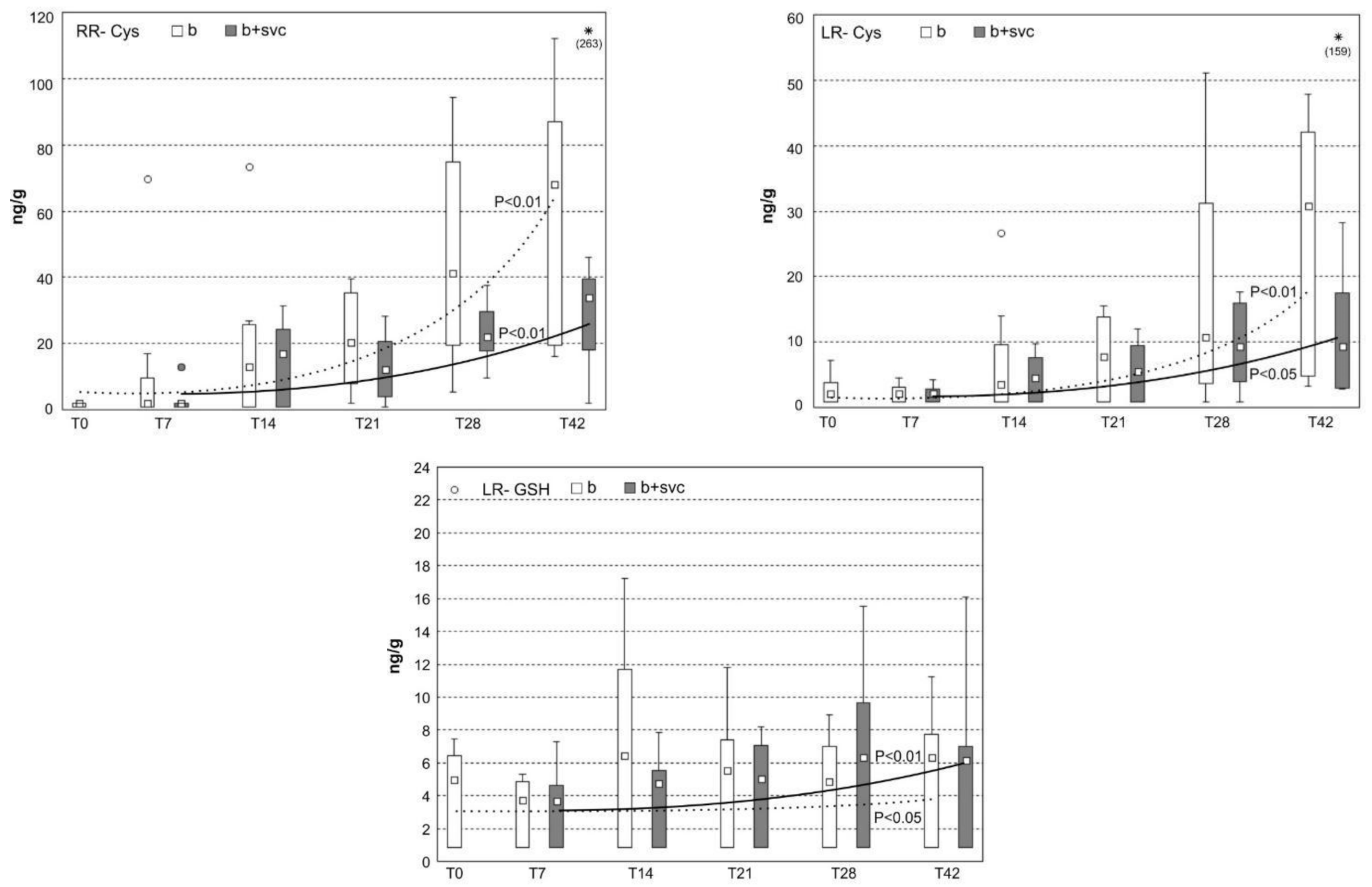
2.8. Concentration of Microcystin Conjugates in the Liver
2.9. Level of Specific Antibodies against SVCV
3. Discussion
4. Materials and Methods
4.1. Animal and Experimental Design
4.2. Experimental Timepoints and Tissue Sampling
4.3. Body size Measurements
4.4. Pathological Evaluation and Histopathology
4.5. Hematology
4.6. Blood Biochemistry
4.7. Respiratory Burst Measurement
4.8. Measurement of Microcystin and Microcystin Conjugates by Liquid Chromatography Mass Spectrometry Analyses
4.9. Determination of Whole Immunoglobulin Concentration and Specific Antibodies against Carp Spring Virus by ELISA
4.10. Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OIE. Aquatic Animals Commission Spring Viraenia of Carps. In Manual of Diagnostic Tests for Aquatic Animals; OIE: Paris, France, 2016; ISBN 9789290448877. [Google Scholar]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- US EPA. Harmful algal Blooms. Available online: http://www2.epa.gov/nutrientpollution/harmful-algal-blooms (accessed on 19 April 2020).
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial poisoning in livestock, wild mammals and birds—An overview. Adv. Exp. Med. Biol. 2008, 619, 613–637. [Google Scholar]
- Hilborn, E.; Beasley, V. One Health and Cyanobacteria in Freshwater Systems: Animal Illnesses and Deaths Are Sentinel Events for Human Health Risks. Toxins 2015, 7, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Adamovsky, O.; Moosova, Z.; Pekarova, M.; Basu, A.; Babica, P.; Svihalkova Sindlerova, L.; Kubala, L.; Blaha, L. Immunomodulatory Potency of Microcystin, an Important Water-Polluting Cyanobacterial Toxin. Environ. Sci. Technol. 2015, 49, 12457–12464. [Google Scholar] [CrossRef]
- Moosova, Z.; Hrouzek, P.; Kapuscik, A.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of selected cyanobacterial peptides in vitro. Toxicon 2018, 149, 20–25. [Google Scholar] [CrossRef]
- Moosova, Z.; Pekarova, M.; Sindlerova, L.S.; Vasicek, O.; Kubala, L.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 2019, 226, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Palikova, M.; Ondrackova, P.; Mares, J.; Adamovsky, O.; Pikula, J.; Kohoutek, J.; Navratil, S.; Blaha, L.; Kopp, R. In vivo effects of microcystins and complex cyanobacterial biomass on rats (Rattus norvegicus var. alba): Changes in immunological and haematological parameters. Toxicon 2013, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palikova, M.; Navratil, S.; Papezikova, I.; Ambroz, P.; Vesely, T.; Pokorova, D.; Mares, J.; Adamovsky, O.; Navratil, L.; Kopp, R. Combined exposure of carps (Cyprinus carpio L.) to cyanobacterial biomass and white spot disease. Neuro. Endocrinol. Lett. 2012, 33 (Suppl. 3), 77–83. [Google Scholar] [PubMed]
- Clarke, J.D.; Dzierlenga, A.; Arman, T.; Toth, E.; Li, H.; Lynch, K.D.; Tian, D.-D.; Goedken, M.; Paine, M.F.; Cherrington, N. Nonalcoholic fatty liver disease alters microcystin-LR toxicokinetics and acute toxicity. Toxicon 2019, 162, 1–8. [Google Scholar] [CrossRef]
- Lad, A.; Su, R.; Breidenbach, J.; Stemmer, P.; Carruthers, N.; Sanchez, N.; Khalaf, F.; Zhang, S.; Kleinhenz, A.; Dube, P.; et al. Chronic Low Dose Oral Exposure to Microcystin-LR Exacerbates Hepatic Injury in a Murine Model of Non-Alcoholic Fatty Liver Disease. Toxins 2019, 11, 486. [Google Scholar] [CrossRef] [Green Version]
- Su, R.C.; Blomquist, T.M.; Kleinhenz, A.L.; Khalaf, F.K.; Dube, P.; Lad, A.; Breidenbach, J.D.; Mohammed, C.J.; Zhang, S.; Baum, C.E.; et al. Exposure to the harmful algal bloom (HAB) toxin microcystin-lr (MC-LR) prolongs and increases severity of dextran sulfate sodium (DSS)-induced colitis. Toxins 2019, 11, 317. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Kimono, D.; Albadrani, M.; Seth, R.K.; Busbee, P.; Alghetaa, H.; Porter, D.E.; Scott, G.I.; Brooks, B.; Nagarkatti, M.; et al. Environmental microcystin targets the microbiome and increases the risk of intestinal inflammatory pathology via NOX2 in underlying murine model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 8742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosová, Z.; Šindlerová, L.; Ambrůzová, B.; Ambrožová, G.; Vašíček, O.; Velki, M.; Babica, P.; Kubala, L. Lipopolysaccharides from Microcystis Cyanobacteria-Dominated Water Bloom and from Laboratory Cultures Trigger Human Immune Innate Response. Toxins 2019, 11, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, H.Y.; Lone, Y.; Koiri, R.K.; Mishra, P.K.; Srivastava, R.K. Microcystin-leucine arginine (MC-LR) induces bone loss and impairs bone micro-architecture by modulating host immunity in mice: Implications for bone health. Environ. Pollut. 2018, 238, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Rymuszka, A.; Sieroslawska, A.; Bownik, A.; Skowronski, T.; Sierosławska, A.; Bownik, A.; Skowroński, T. Microcystin-Lr Modulates Selected Immune Parameters and Induces Necrosis/Apoptosis of Carp Leucocytes. Environ. Toxicol. Chem. 2010, 29, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Sieroslawska, A.; Rymuszka, A.; Bownik, A.; Skowroiiski, T. The influence of microcystin-LR on fish phagocytic cells. Hum. Exp. Toxicol. 2007, 26, 603–607. [Google Scholar] [CrossRef]
- Sandoval, H.; Kodali, S.; Wang, J. Regulation of B cell fate, survival, and function by mitochondria and autophagy. Mitochondrion 2018, 41, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Caro-Maldonado, A.; Wang, R.; Nichols, A.G.; Kuraoka, M.; Milasta, S.; Sun, L.D.; Gavin, A.L.; Abel, E.D.; Kelsoe, G.; Green, D.R.; et al. Metabolic Reprogramming Is Required for Antibody Production That Is Suppressed in Anergic but Exaggerated in Chronically BAFF-Exposed B Cells. J. Immunol. 2014, 192, 3626–3636. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline Phosphatase: An Overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Skocovska, B.; Hilscherova, K.; Babica, P.; Adamovsky, O.; Bandouchova, H.; Horakova, J.; Knotkova, Z.; Marsalek, B.; Paskova, V.; Pikula, J. Effects of cyanobacterial biomass on the Japanese quail. Toxicon 2007, 49, 793–803. [Google Scholar] [CrossRef]
- Pikula, J.; Bandouchova, H.; Hilscherova, K.; Paskova, V.; Sedlackova, J.; Adamovsky, O.; Knotkova, Z.; Lany, P.; Machat, J.; Marsalek, B.; et al. Combined exposure to cyanobacterial biomass, lead and the Newcastle virus enhances avian toxicity. Sci. Total Environ. 2010, 408, 4984–4992. [Google Scholar] [CrossRef]
- Rabergh, C.M.I.; Bylund, G.; Eriksson, J.E. Histopathological Effects of Microcystin-Lr, a Cyclic Peptide Toxin from the Cyanobacterium (Blue-Green-Alga) Microcystis-Aeruginosa, on Common Carp (Cyprinus carpio L). Aquat. Toxicol. 1991, 20, 131–145. [Google Scholar] [CrossRef]
- Adamovsky, O.; Palikova, M.; Ondrackova, P.; Zikova, A.; Kopp, R.; Mares, J.; Pikula, J.; Paskerova, H.; Kohoutek, J.; Blaha, L.; et al. Biochemical and histopathological responses of Wistar rats to oral intake of microcystins and cyanobacterial biomass. Neuro Endocrinol. Lett. 2013, 34, 11–20. [Google Scholar] [PubMed]
- Nemes, K.; Åberg, F.; Gylling, H.; Isoniemi, H. Cholesterol metabolism in cholestatic liver disease and liver transplantation: From molecular mechanisms to clinical implications. World J. Hepatol. 2016, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Ahne, W.; Bjorklund, H.V.; Essbauer, S.; Fijan, N.; Kurath, G.; Winton, J.R. Spring viremia of carp (SVC). Dis. Aquat. Organ. 2002, 52, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.; Carlisle, J.C.; Linville, R.; Washburn, B. Micorcystins: A Brief Overview of their Toxicity and Effects, with Special Reference to fish, Wildlife and Livestock. Calif. Environ. Prot. Agency 2009, 2009, 5. [Google Scholar]
- Adamovský, O.; Kopp, R.; Hilscherová, K.; Babica, P.; Palíková, M.; Pašková, V.; Navrátil, S.; Maršálek, B.; Bláha, L. Microcystin kinetics (bioaccumulation and elimination) and biochemical responses in common carp (Cyprinus carpio) and silver carp (Hypophthalmichthys molitrix) exposed to toxic cyanobacterial blooms. Environ. Toxicol. Chem. 2007, 26, 2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, K.; Zimmermann, L.; Hagenbuch, B.; Dietrich, D. Zebrafish Oatp-mediated transport of microcystin congeners. Arch. Toxicol. 2016, 90, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Hoeger, S.J.; Stemmer, K.; Feurstein, D.J.; Knobeloch, D.; Nussler, A.; Dietrich, D.R. The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: A comparison of primary human hepatocytes and OATP-transfected HEK293 cells. Toxicol. Appl. Pharmacol. 2010, 245, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta-Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Xie, P.; Zhang, D.; Li, G.; Wu, L.; Zhang, W.; Guo, X.; Li, S. Quantitatively evaluating detoxification of the hepatotoxic microcystins through the glutathione and cysteine pathway in the cyanobacteria-eating bighead carp. Aquat. Toxicol. 2012, 116–117, 61–68. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Xie, P.; He, J.; Guo, X.; Tuo, X.; Zhang, W.; Wu, L. Rapid conversion and reversible conjugation of glutathione detoxification of microcystins in bighead carp (Aristichthys nobilis). Aquat. Toxicol. 2014, 147, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Amado, L.L.; Monserrat, J.M. Oxidative stress generation by microcystins in aquatic animals: Why and how. Environ. Int. 2010, 36, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Funato, Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J. Biochem. 2012, 151, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeij, W.P.; Backendorf, C. Reactive Oxygen Species (ROS) Protection via Cysteine Oxidation in the Epidermal Cornified Cell Envelope. In Epidermal Cells; Springer: New York, NY, USA, 2013; pp. 157–169. [Google Scholar]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive Oxygen Species in the Immune System. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef]
- Bláha, L.; Bláhová, L.; Kohoutek, J.; Adamovský, O.; Babica, P.; Marsálek, B. Temporal and spatial variability of cyanobacterial toxins microcystins in three interconnected freshwater reservoirs. J. Serbian Chem. Soc. 2010, 75, 1303–1312. [Google Scholar] [CrossRef]
- Embregts, C.W.E.; Rigaudeau, D.; Veselý, T.; Pokorová, D.; Lorenzen, N.; Petit, J.; Houel, A.; Dauber, M.; Schütze, H.; Boudinot, P.; et al. Intramuscular DNA Vaccination of Juvenile Carp against Spring Viremia of Carp Virus Induces Full Protection and Establishes a Virus-Specific B and T Cell Response. Front. Immunol. 2017, 8, 1340. [Google Scholar] [CrossRef] [Green Version]
- Forlenza, M.; de Carvalho Dias, J.D.; Veselý, T.; Pokorová, D.; Savelkoul, H.F.; Wiegertjes, G.F. Transcription of signal-3 cytokines, IL-12 and IFNαβ, coincides with the timing of CD8αβ up-regulation during viral infection of common carp (Cyprinus carpio L.). Mol. Immunol. 2008, 45, 1531–1547. [Google Scholar] [CrossRef]
- Koutná, M.; Vesely, T.; Psikal, I.; Hulová, J. Identification of spring viraemia of carp virus (SVCV) by combined RT-PCR and nested PCR. Dis. Aquat. Organ. 2003, 55, 229–235. [Google Scholar] [CrossRef]
- Svobodová, Z.; Pravda, D.; Modrá, H. Methods of haematological examination of fish. Edice Metod. 2012, 122, 38. (In Czech) [Google Scholar]
- IFCC Expert Panel on Enzymes. Provisional Recommendations on IFCC Methods for the Measurement of Catalytic Con-centrations of Enzymes. CIin. Chim. Acta 1976, 70, F19–F42. [Google Scholar] [CrossRef]
- Hajzer, S.; Jagelkova, J. Proposition of standard method for catalytic concentration of lactate dehydrogenase in plasma diagnosis. Biochem. Clin. Bohemoslov. 1988, 17, 371–379. [Google Scholar]
- Tiez, N. Progress in the development of a recommended method for alkaline phosphatase activity measurements. Clin. Chem. 1980, 26, 1023. [Google Scholar]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef]
- Doumas, B.T.; Ard Watson, W.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142. [Google Scholar] [CrossRef]
- Michaylova, V.; Ilkova, P. Photometric determination of micro amounts of calcium with arsenazo III. Anal. Chim. Acta 1971, 53, 194–198. [Google Scholar] [CrossRef]
- Kratochvila, J.; Garcic, A. Assignment of organic phosphorus in biological matter. Biochem. Clin. Bohemoslov. 1977, 6, 65. [Google Scholar]
- Higgins, T. Novel chromogen for serum iron determinations. Clin. Chem. 1981, 27, 1619–1620. [Google Scholar] [CrossRef] [PubMed]
- Roch-Ramel, F. An enzymic and fluorophotometric method for estimating urea concentrations in nanoliter specimens. Anal. Biochem. 1967, 21, 372–381. [Google Scholar] [CrossRef]
- Roschlau, P.; Bernt, E.; Gruber, W. Enzymatic determination of total cholesterol in serum. J. Clin. Chem. Clin. Biochem. 1974, 12, 403. [Google Scholar]
- Jaffe, M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Z Physiol.Chem. 1886, 10, 391–400. [Google Scholar]
- Kubala, L.; Lojek, A.; Číž, M.; Vondráček, J.; Dušková, M.; Slavíková, H. Determination of phagocyte activity in whole blood of carp (Cyprinus carpio) by luminol-enhanced chemiluminescence. Vet. Med. (Praha) 1996, 41, 323–327. [Google Scholar]
- Buchtíková, S.; Šimková, A.; Rohlenová, K.; Flajšhans, M.; Lojek, A.; Lilius, E.-M.M.; Hyršl, P. The seasonal changes in innate immunity of the common carp (Cyprinus carpio). Aquaculture 2011, 318, 169–175. [Google Scholar] [CrossRef]
- Kohoutek, J.; Procházková, T.; Adamovský, O.; Palíková, M.; Hilscherová, K. Stable-isotope dilution LC-MS/MS method for quantitative determination of microcystin conjugates with cysteine and glutathione in biotic matrices. Anal. Bioanal. Chem. 2019, 411, 5267–5275. [Google Scholar] [CrossRef]
- Kohoutek, J.; Adamovský, O.; Oravec, M.; Šimek, Z.; Palíková, M.; Kopp, R.; Bláha, L. LC-MS analyses of microcystins in fish tissues overestimate toxin levels—Critical comparison with LC-MS/MS. Anal. Bioanal. Chem. 2010, 398, 1231–1237. [Google Scholar] [CrossRef]
- Vesely, T.; Reschova, S.; Pokorova, D.; Hulova, J.; Nevorankova, Z. Production of monoclonal antibodies against immunoglobulin heavy chain in common carp (Cyprinus carpio L.). Vet. Med. (Praha) 2006, 51, 296. [Google Scholar] [CrossRef] [Green Version]
- Boorsma, D.M.; Streefkerk, J.G. Periodate or glutaraldyehyde for preparing peroxidase conjugates? J. Immunol. Methods 1979, 30, 245–255. [Google Scholar] [CrossRef]

| Indices | Group | T0 | T7 | T14 | T21 | T28 | T42 |
|---|---|---|---|---|---|---|---|
| ALP (µkat/l) | C | 0.37 ± 0.22 0.67 ± 0.55 | 0.69 ± 0.33 | 0.36 ± 0.46ab | 0.69 ± 0.32 | 0.95 ± 1.26 | 0.56 ± 0.50 |
| B | 0.46 ± 0.40 | 0.16 ± 0.16a | 0.59 ± 0.27 | 0.25 ± 0.13 | 0.46 ± 0.42 | ||
| SVC | 0.78 ± 0.34 | 0.93 ± 0.75 b | 0.56 ± 0.24 | 0.50 ± 0.12 | 0.32 ± 0.27 | ||
| B+SVC | 0.62 ± 0.34 | 0.54 ± 0.23 ab | 0.74 ± 0.37 | 0.72 ± 0.42 | 0.42 ± 0.22 | ||
| ALT (µkat/l) | C | 0.38 ± 0.18 0.53 ± 0.32 | 0.24 ± 0.08 | 0.24 ± 0.21 ab | 0.22 ± 0.07 ab | 0.16 ± 0.05 a | 0.18 ± 0.09 |
| B | 0.35 ± 0.20 | 0.15 ± 0.07 A | 0.18 ± 0.06 A | 0.29 ± 0.12 ab | 0.24 ± 0.09 | ||
| SVC | 0.27 ± 0.07 | 0.47 ± 0.05 ab | 0.43 ± 0.11 bc | 0.33 ± 0.13 b | 0.19 ± 0.08 | ||
| B+SVC | 0.34 ± 0.10 | 0.62 ± 0.24 B | 0.51 ± 0.19 C | 0.22 ± 0.07 ab | 0.25 ± 0.11 | ||
| LDH (µkat/l) | C | 25.51 ± 21.29 30.40 ± 15.25 | 7.43 ± 3.79 ab | 3.49 ± 0.58 | 9.48 ± 7.10 | 4.96 ± 3.26 | 6.36 ± 7.08 |
| B | 11.72 ± 19.62 a | 4.55 ± 4.46 | 10.39 ± 11.20 | 11.08 ± 9.32 | 7.63 ± 5.13 | ||
| SVC | 14.09 ± 4.58 b | 14.38 ± 11.96 | 14.48 ± 7.52 | 7.00 ± 7.44 | 4.53 ± 3.21 | ||
| B+SVC | 11.82 ± 6.22 ab | 16.97 ± 11.36 | 6.41 ± 4.05 | 6.23 ± 4.88 | 7.63 ± 7.30 | ||
| CHOL (mmol/l) | C | 5.21 ± 1.09 5.06 ± 0.93 | 5.51 ± 1.10 | 5.34 ± 0.34 ab | 4.66 ± 0.49 a | 4.62 ± 0.45 A | 4.88 ± 0.52 A |
| B | 5.63 ± 0.75 | 4.77 ± 0.82 ab | 5.43 ± 0.85 ab | 6.16 ± 0.67 B | 5.55 ± 0.73 ab | ||
| SVC | 4.93 ± 0.62 | 4.87 ± 0.30 a | 5.46 ± 0.59 ab | 6.58 ± 0.77 b | 5.31 ± 0.56 ab | ||
| B+SVC | 5.02 ± 0.87 | 5.74 ± 0.40 b | 6.62 ± 0.81 b | 4.84 ± 0.71 a | 6.22 ± 0.80 B | ||
| CREA (µmol/l) | C | 31.41 ± 13.22 33.61 ± 10.03 | 34.75 ± 7.72 | 21.58 ± 6.36 a | 26.52 ± 2.55 | 32.69 ± 3.96 a | 25.20 ± 4.21 a |
| B | 37.00 ± 13.57 | 31.97 ± 6.97 ab | 24.97 ± 2.83 | 30.11 ± 3.95 ab | 31.27 ± 3.94 ab | ||
| SVC | 40.41 ± 11.35 | 33.97 ± 4.15 b | 29.72 ± 3.63 | 29.39 ± 4.45 ab | 26.51 ± 4.03 ab | ||
| B+SVC | 37.75 ± 20.08 | 31.58 ± 7.69 ab | 25.75 ± 3.54 | 25.54 ± 3.52 b | 32.92 ± 5.80 b | ||
| TP (g/l) | C | 33.31 ± 2.46 33.01 ± 3.22 | 33.21 ± 2.71 | 30.70 ± 3.27 ab | 28.81 ± 1.89 | 32.33 ± 6.38 | 26.32 ± 1.76 A |
| B | 33.45 ± 3.25 | 31.37 ± 2.06 a | 29.70 ± 2.89 | 32.18 ± 3.11 | 29.30 ± 2.59 ab | ||
| SVC | 31.30 ± 1.52 | 36.06 ± 2.20 b | 35.73 ± 2.97 | 35.52 ± 3.22 | 31.43 ± 1.58 B | ||
| B+SVC | 35.58 ± 7.58 | 35.46 ± 3.96 ab | 36.77 ± 1.91 | 30.78 ± 3.64 | 32.72 ± 3.17 B | ||
| UREA (mmol/l) | C | 1.06 ± 0.32 1.72 ± 0.55 | 1.78 ± 0.57 ab | 1.49 ± 0.75 ab | 1.79 ± 0.66 ab | 2.43 ± 0.73 | 1.80 ± 0.59 |
| B | 1.71 ± 0.54 a | 1.24 ± 0.17 a | 1.24 ± 0.60 A | 1.60 ± 0.64 | 1.92 ± 0.37 | ||
| SVC | 2.58 ± 0.50 b | 3.80 ± 1.09 b | 2.46 ± 0.45 b | 1.62 ± 0.46 | 1.98 ± 0.71 | ||
| B+SVC | 2.03 ± 0.49 ab | 1.89 ± 1.05 ab | 2.64 ± 0.63 B | 2.22 ± 0.73 | 2.17 ± 0.97 | ||
| Ca (mmol/l) | C | 2.38 ± 0.13 2.37 ± 0.19 | 2.64 ± 0.23 | 2.28 ± 0.24 | 2.36 ± 0.15 a | 2.61 ± 0.18 | 2.30 ± 0.09 a |
| B | 2.68 ± 0.35 | 2.56 ± 0.53 | 2.38 ± 0.13 ab | 2.51 ± 0.15 | 2.43 ± 0.25 ab | ||
| SVC | 2.50 ± 0.22 | 2.55 ± 0.19 | 2.81 ± 0.46 b | 2.53 ± 0.24 | 2.48 ± 0.15 ab | ||
| B+SVC | 2.49 ± 0.11 | 2.52 ± 0.45 | 2.74 ± 0.28 b | 2.36 ± 0.17 | 2.67 ± 0.49 b | ||
| Mg (mmol/l) | C | 1.13 ± 0.14 1.03 ± 0.11 | 1.08 ± 0.12 | 1.01 ± 0.15 | 1.09 ± 0.10 a | 1.18 ± 0.20 | 1.09 ± 0.10 a |
| B | 1.12 ± 0.06 | 0.82 ± 0.34 | 1.12 ± 0.11 ab | 1.18 ± 0.10 | 1.16 ± 0.10 ab | ||
| SVC | 1.22 ± 0.09 | 1.14 ± 0.11 | 1.24 ± 0.14 b | 1.23 ± 0.11 | 1.30 ± 0.16 ab | ||
| B+SVC | 1.18 ± 0.18 | 1.09 ± 0.11 | 1.22 ± 0.07 b | 1.09 ± 0.10 | 1.26 ± 0.13 b | ||
| P (mmol/l) | C | 1.36 ± 0.39 1.50 ± 0.23 | 1.39 ± 0.24 A | 1.43 ± 0.22 | 1.54 ± 0.38 | 1.26 ± 0.19 | 1.15 ± 0.27 |
| B | 1.47 ± 0.46 A | 1.35 ± 0.27 | 1.41 ± 0.18 | 1.25 ± 0.17 | 1.20 ± 0.26 | ||
| SVC | 1.75 ± 0.18 B | 1.30 ± 0.19 | 1.76 ± 0.24 | 1.28 ± 0.12 | 1.47 ± 0.42 | ||
| B+SVC | 1.03 ± 0.38 ab | 1.22 ± 0.38 | 1.51 ± 0.37 | 1.18 ± 0.24 | 1.38 ± 0.36 |
| Ion mode | ESI |
|---|---|
| Polarity | positive |
| Capillary voltage (V) | 5500 |
| Drying gas temperature (°C) | 350 |
| Drying gas (GAS1, psi) | 40 |
| Nebulizer (GAS2, psi) | 30 |
| Curtain gas (psi) | 15 |
| Analyte | Ion | Parent (m/z) | Fragment (m/z) | Rt (min) | MQL (ng/g FW) | Recovery (%) | S.D. ( ± %) |
|---|---|---|---|---|---|---|---|
| MC-RR-Cys | [M+2H]2+ | 580.3 | 135.1 | 10.4 | 3.3 | 88.5 | 13.0 |
| 102.9 | |||||||
| MC-RR-GSH | [M+2H]2+ | 673.2 | 608.9 | 10.2 | 4.2 | 91.5 | 12.0 |
| 102.9 | |||||||
| MC-LR-Cys | [M+2H]2+ | 558.8 | 135.1 | 11.1 | 3.4 | 107.0 | 8.8 |
| 102.9 | |||||||
| MC-LR-GSH | [M+2H]2+ | 651.9 | 135.1 | 10.8 | 5.0 | 100.0 | 10.0 |
| 102.9 | |||||||
| MC-RR | [M+2H]+ | 519.8 | 135.2 | 8.4 | 3.6 | 25.0 | 9.0 |
| 127.1 | |||||||
| MC-LR | [M+H]+ | 1045.5 | 135.2 | 10.2 | 16.2 | 25.0 | 10.0 |
| 127.1 | |||||||
| MC-YR | [M+H]+ | 995.5 | 135.2 | 14.0 | 16.2 | 25.0 | 7.0 |
| 127.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palikova, M.; Kopp, R.; Kohoutek, J.; Blaha, L.; Mares, J.; Ondrackova, P.; Papezikova, I.; Minarova, H.; Pojezdal, L.; Adamovsky, O. Cyanobacteria Microcystis aeruginosa Contributes to the Severity of Fish Diseases: A Study on Spring Viraemia of Carp. Toxins 2021, 13, 601. https://doi.org/10.3390/toxins13090601
Palikova M, Kopp R, Kohoutek J, Blaha L, Mares J, Ondrackova P, Papezikova I, Minarova H, Pojezdal L, Adamovsky O. Cyanobacteria Microcystis aeruginosa Contributes to the Severity of Fish Diseases: A Study on Spring Viraemia of Carp. Toxins. 2021; 13(9):601. https://doi.org/10.3390/toxins13090601
Chicago/Turabian StylePalikova, Miroslava, Radovan Kopp, Jiri Kohoutek, Ludek Blaha, Jan Mares, Petra Ondrackova, Ivana Papezikova, Hana Minarova, Lubomir Pojezdal, and Ondrej Adamovsky. 2021. "Cyanobacteria Microcystis aeruginosa Contributes to the Severity of Fish Diseases: A Study on Spring Viraemia of Carp" Toxins 13, no. 9: 601. https://doi.org/10.3390/toxins13090601
APA StylePalikova, M., Kopp, R., Kohoutek, J., Blaha, L., Mares, J., Ondrackova, P., Papezikova, I., Minarova, H., Pojezdal, L., & Adamovsky, O. (2021). Cyanobacteria Microcystis aeruginosa Contributes to the Severity of Fish Diseases: A Study on Spring Viraemia of Carp. Toxins, 13(9), 601. https://doi.org/10.3390/toxins13090601







