Associations between Serum Aflatoxin-B1 and Anemia in Pregnant Women: Evidence from Guangxi Zhuang Birth Cohort in China
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. AFB1 Exposure Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Included Participants
3.2. Serum AFB1-ALB Adduct Levels, Hb, MCV, MCH, and MCHC Concentrations and Anemia Prevalence
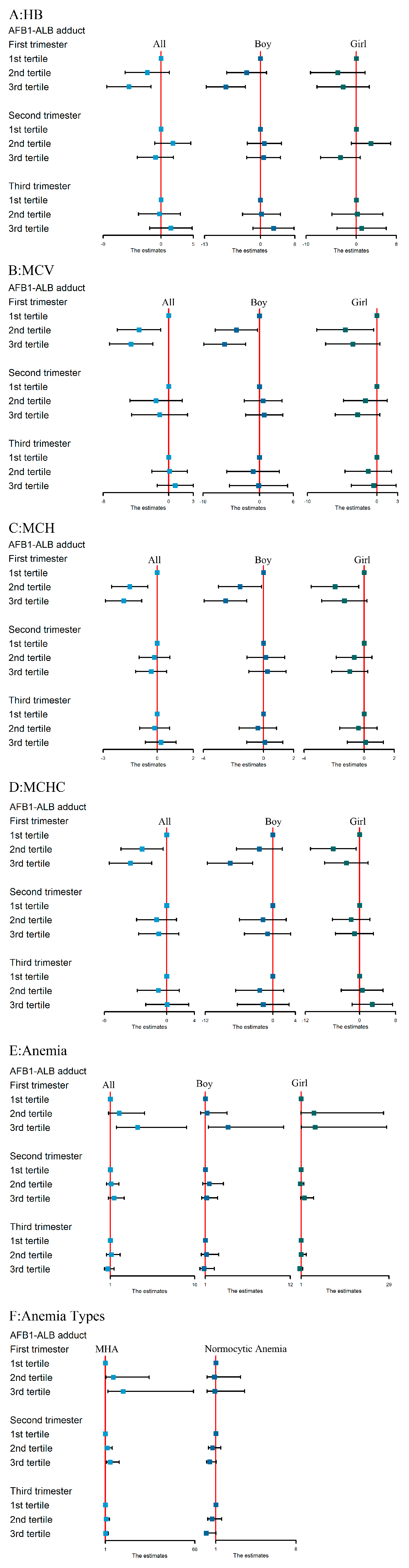
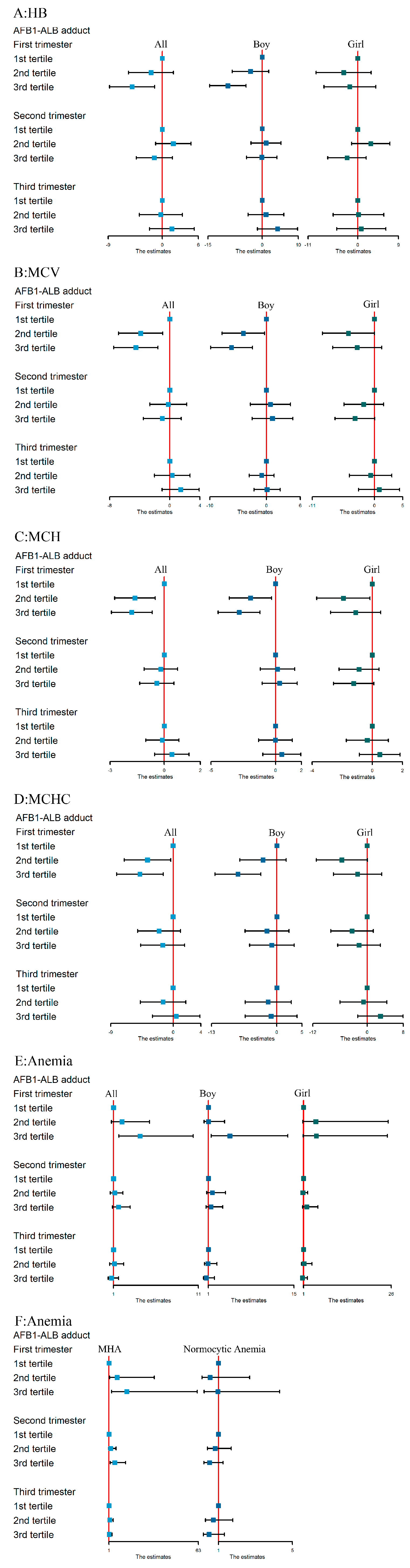
3.3. Associations between AFB1 Exposure and Hb, MCV, MCH, and MCHC Levels
3.4. The Association between AFB1 Exposure and Maternal Anemia Risk
3.5. The Association between AFB1 Exposure and Risk of Different Anemia Types
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- ARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 193–202. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Liang, J.; Yang, D.; Yang, X.; Cao, P.; Wang, X.; Ma, N.; Zhang, L. Spatial analysis of dietary exposure of aflatoxins in peanuts and peanut oil in different areas of China. Food Res. Int. 2021, 140, 109899. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Andrews-Trevino, J.Y.; Webb, P.; Shively, G.; Rogers, B.L.; Baral, K.; Davis, D.; Paudel, K.; Pokharel, A.; Shrestha, R.; Wang, J.S.; et al. Relatively Low Maternal Aflatoxin Exposure Is Associated with Small-for-Gestational-Age but Not with Other Birth Outcomes in a Prospective Birth Cohort Study of Nepalese Infants. J. Nutr. 2019, 149, 1818–1825. [Google Scholar] [CrossRef]
- Lauer, J.M.; Duggan, C.P.; Ausman, L.M.; Griffiths, J.K.; Webb, P.; Wang, J.S.; Xue, K.S.; Agaba, E.; Nshakira, N.; Ghosh, S. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern. Child Nutr. 2019, 15, e12701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groopman, J.D.; Egner, P.A.; Schulze, K.J.; Wu, L.S.; Merrill, R.; Mehra, S.; Shamim, A.A.; Ali, H.; Shaikh, S.; Gernand, A.; et al. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B₁-lysine albumin biomarkers. Food Chem. Toxicol. 2014, 74, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Kyei, N.N.A.; Boakye, D.; Gabrysch, S. Maternal mycotoxin exposure and adverse pregnancy outcomes: A systematic review. Mycotoxin Res 2020, 36, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin Exposure During Pregnancy, Maternal Anemia, and Adverse Birth Outcomes. Am. J. Trop. Med. Hyg. 2017, 96, 770–776. [Google Scholar] [CrossRef] [Green Version]
- WHO. The Global Prevalence of Anemia in 2011; WHO: Geneva, Switzerland, 2015; Volume 126, pp. 5409–5418. [Google Scholar]
- Mitsuda, N.; J-P, N.A.; Eitoku, M.; Maeda, N.; Fujieda, M.; Suganuma, N. Association between maternal hemoglobin concentration and placental weight to birthweight ratio: The Japan Environment and Children’s Study (JECS). Placenta 2020, 101, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Abe, S.K.; Rahman, M.S.; Kanda, M.; Narita, S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.J.; Raval, P.J. Cytotoxicity of aflatoxin on red blood corpuscles. Bull. Environ. Contam. Toxicol. 1991, 47, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Salem, M.H.; Kamel, K.I.; Hassan, G.A.; El-Nouty, F.D. Influence of ascorbic acid supplementation on the haematological and clinical biochemistry parameters of male rabbits exposed to aflatoxin B1. J. Environ. Sci. Health B 2003, 38, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, F.M.; Jolly, P.E.; Ehiri, J.E.; Jiang, Y.; Ellis, W.O.; Stiles, J.K.; Yatich, N.J.; Funkhouser, E.; Person, S.D.; Wilson, C.; et al. Association between anemia and aflatoxin B1 biomarker levels among pregnant women in Kumasi, Ghana. Am. J. Trop. Med. Hyg. 2010, 83, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Liu, S.; Liu, T.; Yang, C.; Wu, Y.; Jennifer Tan, H.J.; Wei, B.; Ma, X.; Feng, B.; Jiang, Q.; et al. Association of prenatal exposure to bisphenols and birth size in Zhuang ethnic newborns. Chemosphere 2020, 252, 126422. [Google Scholar] [CrossRef]
- Wei, B.; Shao, Y.; Liang, J.; Tang, P.; Mo, M.; Liu, B.; Huang, H.; Tan, H.J.J.; Huang, D.; Liu, S.; et al. Maternal overweight but not paternal overweight before pregnancy is associated with shorter newborn telomere length: Evidence from Guangxi Zhuang birth cohort in China. BMC Pregnancy Childbirth 2021, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Flannery, B.; Isitt, C.; Ali, M.; Pestka, J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr. Res. Rev. 2012, 25, 162–179. [Google Scholar] [CrossRef] [Green Version]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001–2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [Green Version]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef] [Green Version]
- McCoy, L.F.; Scholl, P.F.; Sutcliffe, A.E.; Kieszak, S.M.; Powers, C.D.; Rogers, H.S.; Gong, Y.Y.; Groopman, J.D.; Wild, C.P.; Schleicher, R.L. Human aflatoxin albumin adducts quantitatively compared by ELISA, HPLC with fluorescence detection, and HPLC with isotope dilution mass spectrometry. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1653–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.D.; Zhu, B.B.; Gao, H.; Huang, K.; Xu, Y.Y.; Yan, S.Q.; Zhou, S.S.; Cai, X.X.; Zhang, Q.F.; Qi, J.; et al. Repeated measures of prenatal phthalate exposure and maternal hemoglobin concentration trends: The Ma’anshan birth cohort (MABC) study. Environ. Pollut. 2018, 242, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Moore, S.E.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 2003, 111, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Pan, D.; Zhang, T.; Su, M.; Sun, G.; Wei, J.; Guo, Z.; Wang, K.; Song, G.; Yan, Q. Corn Flour Intake, Aflatoxin B(1) Exposure, and Risk of Esophageal Precancerous Lesions in a High-Risk Area of Huai’an, China: A Case-Control Study. Toxins 2020, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Gizachew, D.; Chang, C.H.; Szonyi, B.; De La Torre, S.; Ting, W.E. Aflatoxin B1 (AFB1) production by Aspergillus flavus and Aspergillus parasiticus on ground Nyjer seeds: The effect of water activity and temperature. Int. J. Food Microbiol. 2019, 296, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.J.; Raval, P.J. Alterations in erythrocytes during induced chronic aflatoxicosis in rabbits. Bull. Environ. Contam. Toxicol. 1992, 49, 861–865. [Google Scholar] [CrossRef]
- Partanen, H.A.; El-Nezami, H.S.; Leppänen, J.M.; Myllynen, P.K.; Woodhouse, H.J.; Vähäkangas, K.H. Aflatoxin B1 transfer and metabolism in human placenta. Toxicol. Sci. 2010, 113, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; Kroker-Lobos, M.F.; Lazo, M.; Rivera-Andrade, A.; Egner, P.A.; Wedemeyer, H.; Torres, O.; Freedman, N.D.; McGlynn, K.A.; Guallar, E.; et al. Aflatoxin and viral hepatitis exposures in Guatemala: Molecular biomarkers reveal a unique profile of risk factors in a region of high liver cancer incidence. PLoS ONE 2017, 12, e0189255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, C.S.; Hernández, E.; Escobar, K.; Villagrán, C.I.; Kroker-Lobos, M.F.; Rivera-Andrade, A.; Smith, J.W.; Egner, P.A.; Lazo, M.; Freedman, N.D.; et al. Aflatoxin B(1) exposure and liver cirrhosis in Guatemala: A case-control study. BMJ Open Gastroenterol. 2020, 7, e000380. [Google Scholar] [CrossRef]
- Wang, M. Iron Deficiency and Other Types of Anemia in Infants and Children. Am. Fam. Physician 2016, 93, 270–278. [Google Scholar] [PubMed]
- Chung, W.M.; Lin, J.K.; Wu, K.C.; Hsiung, K.P. In vitro interconversion of aflatoxin B1 and aflatoxicol by rat erythrocytes. Biochem. Pharmacol. 1985, 34, 2566–2569. [Google Scholar] [CrossRef]
- Harvey, R.B.; Clark, D.E.; Huff, W.E.; Kubena, L.F.; Corrier, D.E.; Phillips, T.D. Suppression of serum iron-binding capacity and bone marrow cellularity in pigs fed aflatoxin. Bull. Environ. Contam. Toxicol. 1988, 40, 576–583. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [Green Version]
- Dohnal, V.; Wu, Q.; Kuča, K. Metabolism of aflatoxins: Key enzymes and interindividual as well as interspecies differences. Arch. Toxicol. 2014, 88, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Mary, V.S.; Theumer, M.G.; Arias, S.L.; Rubinstein, H.R. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 2012, 302, 299–307. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin Exposure and Associated Human Health Effects, a Review of Epidemiological Studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Githang’a, D.; Wangia, R.N.; Mureithi, M.W.; Wandiga, S.O.; Mutegi, C.; Ogutu, B.; Agweyu, A.; Wang, J.S.; Anzala, O. The effects of aflatoxin exposure on Hepatitis B-vaccine induced immunity in Kenyan children. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 117–130. [Google Scholar] [CrossRef]
- Deng, Z.J.; Zhao, J.F.; Huang, F.; Sun, G.L.; Gao, W.; Lu, L.; Xiao, Q. Protective Effect of Procyanidin B2 on Acute Liver Injury Induced by Aflatoxin B (1) in Rats. Biomed. Environ. Sci. 2020, 33, 238–247. [Google Scholar]
- Kumara, S.S.; Gayathri, D.; Hariprasad, P.; Venkateswaran, G.; Swamy, C.T. In vivo AFB(1) detoxification by Lactobacillus fermentum LC5/a with chlorophyll and immunopotentiating activity in albino mice. Toxicon 2020, 187, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Tang, L.; Guo, X.; Wang, F.; Massey, M.E.; Su, J.; Guo, T.L.; Williams, J.H.; Phillips, T.D.; Wang, J.S. Aflatoxin B1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 rats. J. Appl. Toxicol. 2014, 34, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and Anemia: A Tight Relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Nicolas, G.; Bennoun, M.; Porteu, A.; Mativet, S.; Beaumont, C.; Grandchamp, B.; Sirito, M.; Sawadogo, M.; Kahn, A.; Vaulont, S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 2002, 99, 4596–4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passarelli, S.; Bromage, S.; Darling, A.M.; Wang, J.S.; Aboud, S.; Mugusi, F.; Griffiths, J.K.; Fawzi, W. Aflatoxin exposure in utero and birth and growth outcomes in Tanzania. Matern. Child Nutr. 2020, 16, e12917. [Google Scholar] [CrossRef] [Green Version]
- Wild, C.P.; Miller, J.D.; Groopman, J.D. Mycotoxin Control in Low- and Middle-Income Countries; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Hell, K.; Fandohan, P.; Bandyopadhyay, R.; Kiewnick, S.; Cotty, P.J. Pre- and postharvest management of aflatoxin in maize: An African perspective. Mycotoxins Detect. Methods Manag. Public Health Agric. Trade 2008, 19, 219–229. [Google Scholar]

| Characteristic | Mean ± SD or n (%) |
|---|---|
| Mothers | |
| Pre-pregnancy BMI (kg/m2) | 20.84 ± 2.53 |
| Ethnic | |
| Zhuang | 578 (93.8) |
| Han | 38 (6.2) |
| Gravidity | |
| Primigravida | 126 (20.5) |
| Multigravida | 490 (79.5) |
| Parity | |
| 0 | 227 (36.9) |
| ≥1 | 389 (63.1) |
| Pre-pregnancy folic acid supplement | |
| No | 537 (87.2) |
| Yes | 79 (12.8) |
| Alcohol consumption pre-pregnancy | |
| No | 582 (94.5) |
| Yes | 34 (5.5) |
| Passive smoking in early pregnancy | |
| No | 330 (53.6) |
| Yes | 286 (46.4) |
| Regular physical activity | |
| No | 361 (58.6) |
| Yes | 255 (41.4) |
| Maternal age (years) | 28.80 ± 5.66 |
| Infants | |
| Infant gender | |
| Boy | 330 (53.6) |
| Girl | 286 (46.4) |
| Gestational age at birth (weeks) | 38.67 ± 1.60 |
| Birth weight (g) | 3089.89 ± 428.39 |
| Birth length (cm) | 49.94 ± 1.92 |
| LBW | 34 (5.5) |
| SGA | 94 (15.3) |
| LGA | 43 (7.0) |
| Variables | First Trimester (n = 303) | Second Trimester (n = 329) | Third Trimester (n = 300) |
|---|---|---|---|
| Hb (g/L) | 118.86 ± 12.36 | 113.37 ± 10.58 | 115.48 ± 11.97 |
| MCV (fl) | 85.48 ± 9.45 | 88.12 ± 8.66 | 87.29 ± 7.80 |
| MCH (pg) | 28.11 ± 3.64 | 28.69 ± 3.21 | 27.98 ± 3.06 |
| MCHC (g/L) | 328.51 ± 10.96 | 324.17 ± 11.03 | 319.95 ± 11.44 |
| Anemia (n%) | 58 (19.1) | 113 (34.3) | 86 (28.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, L.; Liu, S.; Ye, Y.; Qiu, X.; Huang, D.; Pan, D.; Chen, J.; Qian, Z.; McMillin, S.E.; Vaughn, M.G.; et al. Associations between Serum Aflatoxin-B1 and Anemia in Pregnant Women: Evidence from Guangxi Zhuang Birth Cohort in China. Toxins 2021, 13, 806. https://doi.org/10.3390/toxins13110806
Lei L, Liu S, Ye Y, Qiu X, Huang D, Pan D, Chen J, Qian Z, McMillin SE, Vaughn MG, et al. Associations between Serum Aflatoxin-B1 and Anemia in Pregnant Women: Evidence from Guangxi Zhuang Birth Cohort in China. Toxins. 2021; 13(11):806. https://doi.org/10.3390/toxins13110806
Chicago/Turabian StyleLei, Lei, Shun Liu, Ye Ye, Xiaoqiang Qiu, Dongping Huang, Dongxiang Pan, Jiehua Chen, Zhengmin Qian, Stephen Edward McMillin, Michael G. Vaughn, and et al. 2021. "Associations between Serum Aflatoxin-B1 and Anemia in Pregnant Women: Evidence from Guangxi Zhuang Birth Cohort in China" Toxins 13, no. 11: 806. https://doi.org/10.3390/toxins13110806
APA StyleLei, L., Liu, S., Ye, Y., Qiu, X., Huang, D., Pan, D., Chen, J., Qian, Z., McMillin, S. E., Vaughn, M. G., Luo, X., Wu, K., Xiao, S., Li, J., Liu, M., Yang, Y., Lai, M., Dong, G., & Zeng, X. (2021). Associations between Serum Aflatoxin-B1 and Anemia in Pregnant Women: Evidence from Guangxi Zhuang Birth Cohort in China. Toxins, 13(11), 806. https://doi.org/10.3390/toxins13110806







