Toxic Potential of Traditionally Consumed Mushroom Species—A Controversial Continuum with Many Unanswered Questions
Abstract
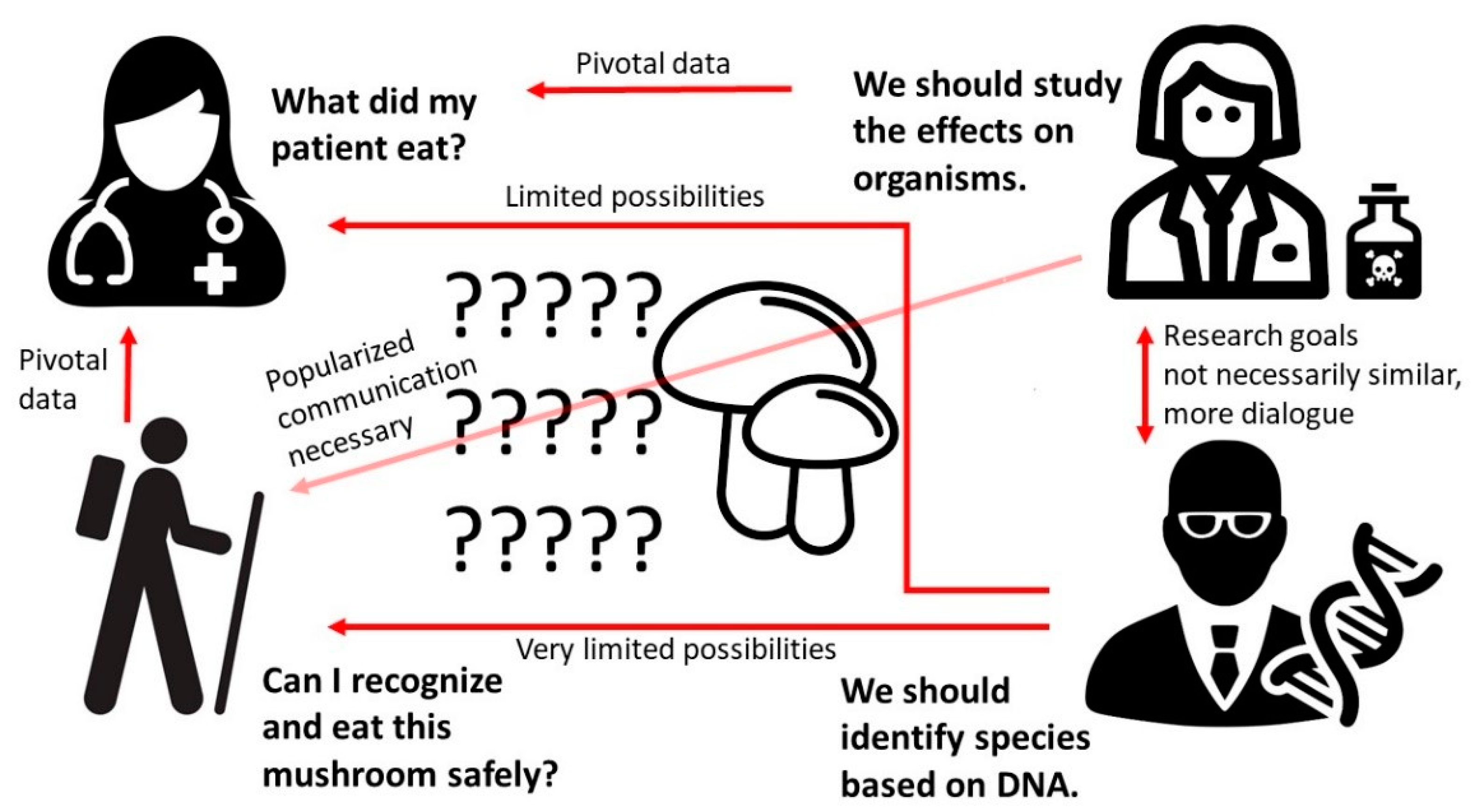
1. Introduction
- What is the current state of knowledge regarding species considered edible but that have been reported to cause toxic effects?
- What types of poisonings do these species potentially cause?
- Are there confounding issues, such as species identification and differences in toxicity of related species?
- Are there other factors, such as contamination, that complicate the use of species that were previously considered edible?
- Should scientists, especially the medical and mycological communities, revise the recommendations and warnings considering these species?
2. From Delicacy to Danger—The Case of Tricholoma equestre
3. Are Edible Mushrooms Really Edible?
4. Causative Agents of Toxicity in Edible Mushrooms
5. How to Tell Poisonous Species apart from the Safe Ones?
6. Conclusions
- The demarcation between poisonous and edible mushroom species is not simple to define, with several edible wild and cultivated species having potential health benefits but also containing toxic compounds.
- Species considered to be edible can cause allergic reactions (including anaphylaxis), rhabdomyolysis, cardiac toxicity, and mutagenic effects, among others. Mostly, these effects have hitherto been observed in small populations, case studies, laboratory rodents, or in assays on cell cultures.
- Species identification is a major problem, not only for layperson mushroom collectors but also for scientific studies and in the emergency room. Previous methodology (spore identification) is in some cases inadequate and requires special skills not readily available in hospitals. Thus, genetic testing may be necessary to distinguish between related species that might have very different levels of toxicity.
- Determining toxicity is further complicated by other causes of food poisoning that may coincide with mushroom ingestion, for instance, microbiological contamination and accumulation of environmental toxins caused by repeated consumption.
- As the identification of related species is exceedingly complex even in research, it is clear that it is virtually impossible for layperson collectors. Thus, recommendations should be based upon visual recognition, and thus, exclude species that cannot be distinguished from toxic ones by conventional methods. It should be acknowledged that in addition to being an issue of high-quality science and precision cladistics on one hand and of mushroom consumption on the other, edibility also remains a medical issue that has to take into account uncertainty and assess risks based on inadequate data.
Author Contributions
Funding
Conflicts of Interest
References
- Cervellin, G.; Comelli, I.; Rastelli, G.; Sanchis-Gomar, F.; Negri, F.; De Luca, C.; Lippi, G. Epidemiology and clinics of mushroom poisoning in Northern Italy: A 21-year retrospective analysis. Hum. Exp. Toxicol. 2018, 37, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.A.; Klukowska-Rötzler, J.; Schenk-Jaeger, K.M.; Kupferschmidt, H.; Exadaktylos, A.K.; Lehmann, B.; Liakoni, E. Mushroom poisoning—A 17 year retrospective study at a level I university emergency department in Switzerland. Int. J. Environ. Res. Public Health 2018, 15, 2855. [Google Scholar] [CrossRef]
- Chan, C.K.; Lam, H.C.; Chiu, S.W.; Tse, M.L.; Lau, F.L. Mushroom poisoning in Hong Kong: A ten-year review. Hong Kong Med. J. 2016, 22, 124–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gawlikowski, T.; Romek, M.; Satora, L. Edible mushroom-related poisoning: A study on circumstances of mushroom collection, transport, and storage. Hum. Exp. Toxicol. 2015, 34, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, H.; Rauber-Lüthy, C. Vergiftungen in der Schweiz. Zur Beratungstätigkeit 2016 von Tox Info Suisse. Schweiz. Ärztezeitung 2017, 98, 1406–1410. [Google Scholar]
- Phillips, R. Der Kosmos-Pilzatlas. Über 900 Einheimische Pilzarten in Farbe [The Kosmos Mushroom Atlas. Over 900 Domestic Mushroom Species in Colour], 2nd ed.; Franckh-Kosmos Verlags GmbH & Co: Stuttgart, Germany, 1990; pp. 24–25, 33–35, 162, 191, 214–215. (In German) [Google Scholar]
- Centers for Disease Control and Prevention. Outbreak of Listeria Infections Linked to Enoki Mushrooms. Available online: https://www.cdc.gov/listeria/outbreaks/enoki-mushrooms-03-20/index.html#:~:text=Epidemiologic%2C%20traceback%2C%20and%20laboratory%20evidence,were%20reported%20from%2017%20states (accessed on 23 September 2020).
- Lange, M. Retkeilijän Sieniopas [Field Guide to Mushrooms]; Otava: Helsinki, Finland, 1964; pp. 86, 212, (In Finnish from the Danish Original). [Google Scholar]
- Bedry, R.; Baudrimont, I.; Deffieux, G.; Creppy, E.E.; Pomies, J.P.; Ragnaud, J.M.; Dupon, M.; Neau, D.; Gabinski, C.; De Witte, S.; et al. Wild-mushroom intoxication as a cause of rhabdomyolysis. N. Engl. J. Med. 2001, 345, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Chodorowski, Z.; Waldman, W.; Sein Anand, J. Acute poisoning with Tricholoma equestre. Przegl. Lek. 2002, 59, 386–387, (In Polish with an English Abstract). [Google Scholar]
- Laubner, G.; Mikulevičienė, G. A series of cases of rhabdomyolysis after ingestion of Tricholoma equestre. Acta Med. Litu. 2016, 23, 193–197. [Google Scholar] [CrossRef]
- North American Mycological Association. Mushroom Poisoning Syndromes. Available online: https://namyco.org/mushroom_poisoning_syndromes.php (accessed on 23 September 2020).
- Nieminen, P.; Mustonen, A.-M.; Kirsi, M. Increased plasma creatine kinase activities triggered by edible wild mushrooms. Food Chem. Toxicol. 2005, 43, 133–138. [Google Scholar] [CrossRef]
- Nieminen, P.; Kärjä, V.; Mustonen, A.-M. Indications of hepatic and cardiac toxicity caused by subchronic Tricholoma flavovirens consumption. Food Chem. Toxicol. 2008, 46, 781–786. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Radović, J.; Sułkowska-Ziaja, K.; Krakowska, A.; Gdula-Argasińska, J.; Opoka, W.; Kundaković, T. Study of biological activity of Tricholoma equestre fruiting bodies and their safety for human. Eur. Food Res. Technol. 2018, 244, 2255–2264. [Google Scholar] [CrossRef]
- Nieminen, P.; Kirsi, M.; Mustonen, A.-M. Suspected myotoxicity of edible wild mushrooms. Exp. Biol. Med. 2006, 231, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Kärjä, V.; Mustonen, A.-M. Myo- and hepatotoxic effects of cultivated mushrooms in mice. Food Chem. Toxicol. 2009, 47, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.-M.; Määttänen, M.; Kärjä, V.; Puukka, K.; Aho, J.; Saarela, S.; Nieminen, P. Myo- and cardiotoxic effects of the wild winter mushroom (Flammulina velutipes) on mice. Exp. Biol. Med. 2018, 243, 639–644. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Ko, W.-C.; Lin, L.-Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). Biomed. Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef]
- Wakimoto, T.; Asakawa, T.; Akahoshi, S.; Suzuki, T.; Nagai, K.; Kawagishi, H.; Kan, T. Proof of the existence of an unstable amino acid: Pleurocybellaziridine in Pleurocybella porrigens. Angew. Chem. Int. Ed. 2011, 50, 1168–1170. [Google Scholar] [CrossRef]
- Beaumier, M.; Rioult, J.-P.; Georges, M.; Brocheriou, I.; Lobbedez, T.; Lanot, A. Mushroom poisoning presenting with acute kidney injury and elevated transaminases. Kidney Int. Rep. 2019, 4, 877–881. [Google Scholar] [CrossRef]
- Salo, P.; Niemelä, T.; Salo, U. Suomen Sieniopas [Guide to Mushrooms in Finland]; WSOY and Kasvimuseo: Helsinki, Finland, 2006; pp. 34–55, 139. (In Finnish) [Google Scholar]
- Finnish Food Authority. Suositeltavat Ruokasienet [Recommended Mushrooms for Consumption]. Available online: https://www.ruokavirasto.fi/henkiloasiakkaat/tietoa-elintarvikkeista/elintarvikeryhmat/ruokasienet/suositeltavat-ruokasienet/ (accessed on 20 August 2020). (In Finnish).
- Parker, H. Alaska’s Mushrooms. A Practical Guide, 3rd ed.; Alaska Northwest Books: Portland, OR, USA, 1999; pp. 22–24. [Google Scholar]
- Faulstich, H.; Cochet-Meilhac, M. Amatoxins in edible mushrooms. FEBS Lett. 1976, 64, 73–75. [Google Scholar] [CrossRef]
- Knuutinen, J.; von Wright, A. The mutagenicity of Lactarius mushrooms. Mutat. Res. 1982, 103, 115–118. [Google Scholar] [CrossRef]
- Grüter, A.; Friederich, U.; Würgler, F.E. The mutagenicity of edible mushrooms in a histidine-independent bacterial test system. Food Chem. Toxicol. 1991, 29, 159–165. [Google Scholar] [CrossRef]
- Toth, B.; Gannett, P.; Visek, W.J.; Patil, K. Carcinogenesis studies with the lyophilized mushroom Agaricus bisporus in mice. In Vivo 1998, 12, 239–244. [Google Scholar] [PubMed]
- Akıllı, N.B.; Dündar, Z.D.; Köylü, R.; Günaydın, Y.K.; Cander, B. Rhabdomyolysis induced by Agaricus bisporus. JAEM 2014, 13, 212–213. [Google Scholar] [CrossRef]
- Morales, P.; Bermúdez, E.; Sanz, B.; Hernández, P.E. A study of the mutagenicity of some commercially canned Spanish mushrooms. Food Chem. Toxicol. 1990, 28, 607–611. [Google Scholar] [CrossRef]
- Al-Deen, I.H.S.; Twaij, H.A.A.; Al-Badr, A.A.; Istarabadi, T.A.W. Toxicologic and histopathologic studies of Pleurotus ostreatus mushroom in mice. J. Ethnopharmacol. 1987, 21, 297–305. [Google Scholar] [CrossRef]
- Saviuc, P.; Danel, V. New syndromes in mushroom poisoning. Toxicol. Rev. 2006, 25, 199–209. [Google Scholar] [CrossRef]
- Cho, J.T.; Han, J.H. A case of mushroom poisoning with Russula subnigricans: Development of rhabdomyolysis, acute kidney injury, cardiogenic shock, and death. J. Korean Med. Sci. 2016, 31, 1164–1167. [Google Scholar] [CrossRef]
- Otsuji, K.; Ohara, K.; Nakamura, M.; Amazumi, R.; Higa, C.; Kakazu, K.; Kondo, Y. [A case of anaphylaxis caused by enokitake (Flammulina velutipes) ingestion]. Arerugi 2015, 64, 63–67, (In Japanese with an English Abstract). [Google Scholar]
- Matsui, S.; Nakazawa, T.; Umegae, Y.; Mori, M. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Intern. Med. 1992, 31, 1204–1206. [Google Scholar] [CrossRef][Green Version]
- Fukushima, M.; Ohashi, T.; Fujiwara, Y.; Sonoyama, K.; Nakano, M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001, 226, 758–765. [Google Scholar] [CrossRef]
- Klimaszyk, P.; Rzymski, P. The yellow knight fights back: Toxicological, epidemiological, and survey studies defend edibility of Tricholoma equestre. Toxins 2018, 10, 468. [Google Scholar] [CrossRef]
- Matsuura, M.; Saikawa, Y.; Inui, K.; Nakae, K.; Igarashi, M.; Hashimoto, K.; Nakata, M. Identification of the toxic trigger in mushroom poisoning. Nat. Chem. Biol. 2009, 5, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, T.; Shang, J.-H.; Zhao, Y.-L.; Wang, F.; Li, Z.-H.; Dong, Z.-J.; Luo, X.-D.; Liu, J.-K. Chemical and toxicological investigations of a previously unknown poisonous European mushroom Tricholoma terreum. Chem. Eur. J. 2014, 20, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-S.; Hossain, M.A.; Park, S.-C. Toxicological profiles of poisonous, edible, and medicinal mushrooms. Mycobiology 2014, 42, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Suortti, T.; von Wright, A.; Koskinen, A. Necatorin, a highly mutagenic compound from Lactarius necator. Phytochemistry 1983, 22, 2873–2874. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Wu, H.-L.; Shi, G.-Y. Toxicity of the cardiotoxic protein, flammutoxin, isolated from the edible mushroom Flammulina velutipes. Toxicon 1975, 13, 323–331. [Google Scholar] [CrossRef]
- Garcia, J.; Oliveira, A.; de Pinho, P.G.; Freitas, V.; Carvalho, A.; Baptista, P.; Pereira, E.; de Lourdes Bastos, M.; Carvalho, F. Determination of amatoxins and phallotoxins in Amanita phalloides mushrooms from northeastern Portugal by HPLC-DAD-MS. Mycologia 2015, 107, 679–687. [Google Scholar] [CrossRef]
- Tofani, L. Rabdomiolisi da farmaci antidislipidemizzanti e da funghi: Nuovi parallelismi e spunti per una corretta condotta alimentare. Difesa Sociale 2003, LXXXII, 47–58, (In Italian with an English Abstract). [Google Scholar]
- Korhonen, M. Onko keltavalmuska myrkyllinen? [Is the yellow tricholoma poisonous?]. Sienilehti 2002, 54, 35. (In Finnish) [Google Scholar]
- Rzymski, P.; Klimaszyk, P. Is the yellow knight mushroom edible or not? A systematic review and critical viewpoints on the toxicity of Tricholoma equestre. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1309–1324. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P. Comment on “Mushroom poisoning: A proposed new clinical classification”. Toxicon 2019, 159, 63–64. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Benjamin, D. Comment on “Study of biological activity of Tricholoma equestre fruiting bodies and their safety for human”. Eur. Food Res. Technol. 2019, 245, 963–965. [Google Scholar] [CrossRef]
- Cocchi, L.; Vescovi, L.; Petrini, L.E.; Petrini, O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006, 98, 277–284. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Shnyreva, A.A.; Sivolapova, A.B.; Shnyreva, A.V. The commercially cultivated edible oyster mushrooms Pleurotus sajor-caju and P. pulmonarius are two separate species, similar in morphology but reproductively isolated. Russ. J. Genet. 2012, 48, 1080–1088. [Google Scholar] [CrossRef]
- Muszyńska, B.; Gdula-Argasińska, J.; Opoka, W. Update the comments on “Study of biological activity of Tricholoma equestre fruiting bodies and their safety for human”. Eur. Food Res. Technol. 2019, 245, 1783–1785. [Google Scholar] [CrossRef]
- White, J.; Weinstein, S.A.; De Haro, L.; Bédry, R.; Schaper, A.; Rumack, B.H.; Zilker, T. Reply to Rzymski and Klimaszyk regarding comment on “Mushroom poisoning: A proposed new clinical classification”. Toxicon 2019, 160, 59. [Google Scholar] [CrossRef]
- Chwaluk, P. Rhabdomyolysis as an unspecyfic symptom of mushroom poisoning—A case report. Przegl. Lek. 2013, 70, 684–686, (In Polish with an English Abstract). [Google Scholar]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Khan, T.M.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieminen, P.; Mustonen, A.-M. Toxic Potential of Traditionally Consumed Mushroom Species—A Controversial Continuum with Many Unanswered Questions. Toxins 2020, 12, 639. https://doi.org/10.3390/toxins12100639
Nieminen P, Mustonen A-M. Toxic Potential of Traditionally Consumed Mushroom Species—A Controversial Continuum with Many Unanswered Questions. Toxins. 2020; 12(10):639. https://doi.org/10.3390/toxins12100639
Chicago/Turabian StyleNieminen, Petteri, and Anne-Mari Mustonen. 2020. "Toxic Potential of Traditionally Consumed Mushroom Species—A Controversial Continuum with Many Unanswered Questions" Toxins 12, no. 10: 639. https://doi.org/10.3390/toxins12100639
APA StyleNieminen, P., & Mustonen, A.-M. (2020). Toxic Potential of Traditionally Consumed Mushroom Species—A Controversial Continuum with Many Unanswered Questions. Toxins, 12(10), 639. https://doi.org/10.3390/toxins12100639



