Ostreopsis cf. ovata Bloom in Currais, Brazil: Phylogeny, Toxin Profile and Contamination of Mussels and Marine Plastic Litter
Abstract
1. Introduction
2. Results
2.1. Bloom Detection
2.2. Species Identification
2.3. Colonization of Plastic Litter by Microalgae
2.4. Toxin Production and Accumulation in Marine Organisms
3. Discussion
3.1. Taxonomy and Phylogeny of Ostreopsis Species: Difficulties in Identifying the Toxic Bloom-Forming O. cf. ovata
3.2. Bloom Formation, Toxin Production and Contamination of Marine Organisms
3.3. The Plastic Litter Problem
4. Conclusions
5. Materials and Methods
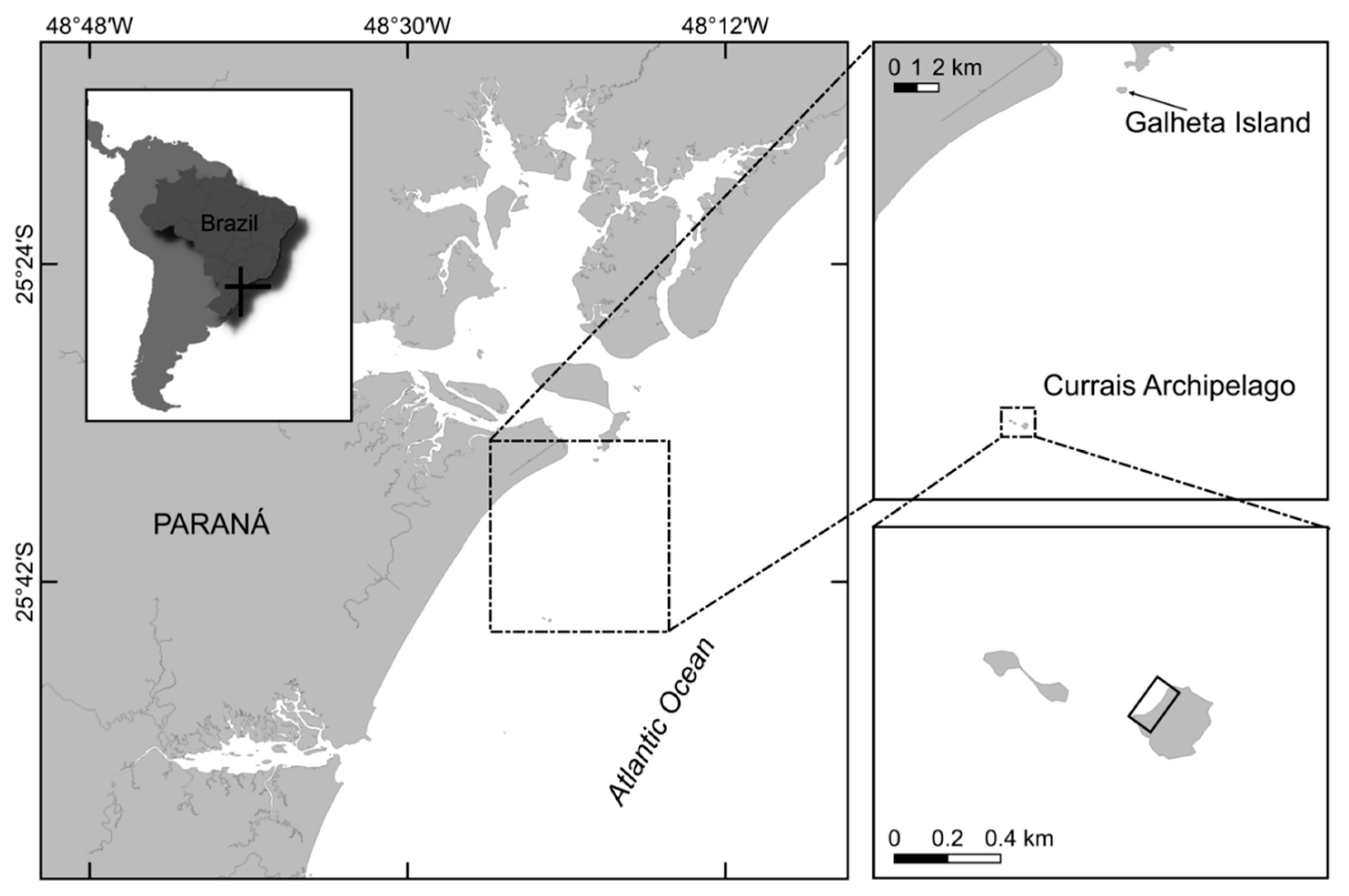
5.1. Sampling
5.2. Cultures
5.3. Morphological Observations
5.4. DNA Amplification, Sequencing and Molecular Phylogeny
5.5. Sampling and Processing of Marine Fauna
5.6. Toxin Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Rhodes, L. World-wide occurrence of the toxic dinoflagellate genus Ostreopsis Schmidt. Toxicon 2011, 57, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Chomérat, N.; Bilien, G.; Derrien, A.; Henry, K.; Ung, A.; Viallon, J.; Darius, H.T.; Mahana iti Gatti, C.; Roué, M.; Hervé, F.; et al. Ostreopsis lenticularis Y. Fukuyo (Dinophyceae, Gonyaulacales) from French Polynesia (South Pacific Ocean): A revisit of its morphology, molecular phylogeny and toxicity. Harmful Algae 2019, 84, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Faimali, M.; Giussani, V.; Piazza, V.; Garaventa, F.; Corrà, C.; Asnaghi, V.; Privitera, D.; Gallus, L.; Cattaneo-Vietti, R.; Mangialajo, L.; et al. Toxic effects of harmful benthic dinoflagellate Ostreopsis ovata on invertebrate and vertebrate marine organisms. Mar. Environ. Res. 2012, 76, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Shears, N.T.; Ross, P.M. Blooms of benthic dinoflagellates of the genus Ostreopsis: An increasing and ecologically important phenomenon on temperate reefs in New Zealand and worldwide. Harmful Algae 2009, 8, 916–925. [Google Scholar] [CrossRef]
- Ferreira, C.E.L. Sea urchins killed by toxic algae. JMBA Glob. Mar. Environ. 2006, 3, 22–23. [Google Scholar]
- Lessios, H.A. The Great Diadema antillarum Die-Off: 30 Years Later. Ann. Rev. Mar. Sci. 2016, 8, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Brissard, C.; Herrenknecht, C.; Séchet, V.; Hervé, F.; Pisapia, F.; Harcouet, J.; Lémée, R.; Chomérat, N.; Hess, P.; Amzil, Z. Complex toxin profile of French Mediterranean Ostreopsis cf. ovata strains, seafood accumulation and ovatoxins prepurification. Mar. Drugs 2014, 12, 2851–2876. [Google Scholar] [CrossRef]
- García-Altares, M.; Tartaglione, L.; Dell’Aversano, C.; Carnicer, O.; De La Iglesia, P.; Forino, M.; Diogène, J.; Ciminiello, P. The novel ovatoxin-g and isobaric palytoxin (so far referred to as putative palytoxin) from Ostreopsis cf. ovata (NW Mediterranean Sea): Structural insights by LC-high resolution MSn. Anal. Bioanal. Chem. 2015, 407, 1191–1204. [Google Scholar]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Yasumoto, T.; Battocchi, C.; Giacobbe, M.; Amorim, A.; et al. Investigation of toxin profile of Mediterranean and Atlantic strains of Ostreopsis cf. siamensis (Dinophyceae) by liquid chromatography-high resolution mass spectrometry. Harmful Algae 2013, 23, 19–27. [Google Scholar] [CrossRef]
- Accoroni, S.; Glibert, P.M.; Pichierri, S.; Romagnoli, T.; Marini, M.; Totti, C. A conceptual model of annual Ostreopsis cf. ovata blooms in the northern Adriatic Sea based on the synergic effects of hydrodynamics, temperature, and the N:P ratio of water column nutrients. Harmful Algae 2015, 45, 14–25. [Google Scholar] [CrossRef]
- Selina, M.S.; Morozova, T.V.; Vyshkvartsev, D.I.; Orlova, T.Y. Seasonal dynamics and spatial distribution of epiphytic dinoflagellates in Peter the Great Bay (Sea of Japan) with special emphasis on Ostreopsis species. Harmful Algae 2014, 32, 1–10. [Google Scholar] [CrossRef]
- Funari, E.; Manganelli, M.; Testai, E. Ostreospis cf. ovata blooms in coastal water: Italian guidelines to assess and manage the risk associated to bathing waters and recreational activities. Harmful Algae 2015, 50, 45–56. [Google Scholar] [CrossRef]
- Proença, L.A.O.; Boemer, G.L.; Dias, J.P.; Hatherly, M.M.; Mendes, I.L.; Mendes, L.A.M.; Mendes, M.C.Q.; Rossi, W.C.; Tamanaha, M.S.; Tenenbaum, D.R.; et al. Can the cases of airborne intoxication of beach users in south coast of Bahia (16°24′S–39°02′W) be related to microalgae? In Proceedings of the GEOHAB Open Science Meeting on HABs in Benthic Systems, Honolulu, HI, USA, 21–23 June 2010; p. 31. [Google Scholar]
- Granéli, E.; Vidyarathna, N.K.; Funari, E.; Cumaranatunga, P.R.T.; Scenati, R. Can increases in temperature stimulate blooms of the toxic benthic dinoflagellate Ostreopsis ovata? Harmful Algae 2011, 10, 165–172. [Google Scholar] [CrossRef]
- Larsson, M.E.; Laczka, O.F.; Suthers, I.M.; Ajani, P.A.; Doblin, M.A. Hitchhiking in the East Australian Current: rafting as a dispersal mechanism for harmful epibenthic dinoflagellates. Mar. Ecol. Prog. Ser. 2018, 596, 49–60. [Google Scholar] [CrossRef]
- Casabianca, S.; Capellacci, S.; Giacobbe, M.G.; Dell’Aversano, C.; Tartaglione, L.; Varriale, F.; Narizzano, R.; Risso, F.; Moretto, P.; Dagnino, A.; et al. Plastic-associated harmful microalgal assemblages in marine environment. Environ. Pollut. 2019, 244, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Masó, M.; Garcés, E.; Pagès, F.; Camp, J. Drifting plastic debris as a potential vector for dispersing Harmful Algal Bloom (HAB) species. Sci. Mar. 2003, 67, 107–111. [Google Scholar] [CrossRef]
- Kershaw, P.J. Marine Plastic Debris and Microplastics—Global Lessons and Research to Inspire Action and Guide Policy Change; United Nations Environment Programme: Nairobi, Kenya, 2016. [Google Scholar]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Baztan, J.; Bergmann, M.; Booth, A.; Broglio, E.; Carrasco, A.; Chouinard, O.; Clüsener-Godt, M.; Cordier, M.; Cozar, A.; Devrieses, L.; et al. Breaking Down the Plastic Age. Fate Impact Microplast. Mar. Ecosyst. 2016, 3, 177–181. [Google Scholar]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in aquatic environments: Implications for Canadian ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Budziak, A.; Van Fanneker, J.A.; Galgani, F.; Hanke, G.; Maes, T.; Matiddi, M.; Nilsson, P.; Oosterbaan, L.; Priestland, E.; et al. Harm Caused by Marine Litter; European Commission: Luxembourg, 2016; ISBN 9789279645358. [Google Scholar]
- Brandão, M.L.; Braga, K.M.; Luque, J.L. Marine debris ingestion by Magellanic penguins, Spheniscus magellanicus (Aves: Sphenisciformes), from the Brazilian coastal zone. Mar. Pollut. Bull. 2011, 62, 2246–2249. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Rodrigues, F.L.; Medeiros, L.; Ortega, I.; Rodrigues, L.; Monteiro, D.S.; Kessler, F.; Proietti, M.C. Ingestion of plastic marine litter by sea turtles in southern Brazil: abundance, characteristics and potential selectivity. Mar. Pollut. Bull. 2019, 140, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Ivar do Sul, J.A.; Fillmann, G. Is marine debris ingestion still a problem for the coastal marine biota of southern Brazil? Mar. Pollut. Bull. 2010, 60, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Murray, S.A.; Chomérat, N.; Horiguchi, T. Marine Benthic Dinoflagellates—Unveiling Their Worldwide Biodiversity; Senckenberg-Reihe: Frankfurt, Germany, 2014; ISBN 978-3-510-61402-8. [Google Scholar]
- Brandini, F.P. Produção Primária nos Oceanos (in Portuguese). In Introdução às Ciências do Mar; Castello, J.P., Krug, L.C., Eds.; Editora Textos: Pelotas, Brazil, 2015; pp. 280–312. [Google Scholar]
- Guebert-Bartholo, F.M.; Barletta, M.; Costa, M.F.; Monteiro-Filho, E.L.A. Using gut contents to assess foraging patterns of juvenile green turtles Chelonia mydas in the Paranaguá Estuary, Brazil. Endanger. Species Res. 2011, 13, 131–143. [Google Scholar] [CrossRef]
- Accoroni, S.; Romagnoli, T.; Penna, A.; Capellacci, S.; Ciminiello, P.; Dell’Aversano, C.; Tartaglione, L.; Abboud-Abi Saab, M.; Giussani, V.; Asnaghi, V.; et al. Ostreopsis fattorussoi sp. nov. (Dinophyceae), a new benthic toxic Ostreopsis species from the eastern Mediterranean Sea. J. Phycol. 2016, 52, 1064–1084. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Hoppenrath, M.; Dorantes-Aranda, J.J.; Harwood, D.T.; Murray, S.A. Molecular and phylogenetic characterization of Ostreopsis (Dinophyceae) and the description of a new species, Ostreopsis rhodesae sp. nov., from a subtropical Australian lagoon. Harmful Algae 2016, 60, 116–130. [Google Scholar] [CrossRef] [PubMed]
- David, H.; Laza-Martínez, A.; Miguel, I.; Orive, E. Ostreopsis cf. siamensis and Ostreopsis cf. ovata from the Atlantic Iberian Peninsula: Morphological and phylogenetic characterization. Harmful Algae 2013, 30, 44–55. [Google Scholar] [CrossRef]
- Penna, A.; Vila, M.; Fraga, S.; Giacobbe, M.G.; Francesco, A.; Riobó, P.; Vernesi, C. Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the western Mediterranean Sea based on morphology, toxicity and internal transcribed spacer 5.8s rDNA sequences. J. Phycol. 2005, 41, 212–225. [Google Scholar] [CrossRef]
- Fukuyo, Y. Taxonomical Study on Benthic Dinoflagellates collected in Coral Reefs. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 967–978. [Google Scholar] [CrossRef]
- Scalco, E.; Brunet, C.; Marino, F.; Rossi, R.; Soprano, V.; Zingone, A.; Montresor, M. Growth and toxicity responses of Mediterranean Ostreopsis cf. ovata to seasonal irradiance and temperature conditions. Harmful Algae 2012, 17, 25–34. [Google Scholar] [CrossRef]
- Accoroni, S.; Totti, C. The toxic benthic dinoflagellates of the genus Ostreopsis in temperate areas: A review. Adv. Oceanogr. Limnol. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, S.; Li, Y.; Cen, J.; Wang, H.; Li, Q.; Nie, X. Morphology and molecular phylogeny of Ostreopsis cf. ovata and O. lenticularis (Dinophyceae) from Hainan Island, South China Sea. Phycol. Res. 2018, 66, 3–14. [Google Scholar] [CrossRef]
- Sato, S.; Nishimura, T.; Uehara, K.; Sakanari, H.; Tawong, W.; Hariganeya, N.; Smith, K.; Rhodes, L.; Yasumoto, T.; Taira, Y.; et al. Phylogeography of Ostreopsis along west Pacific coast, with special reference to a novel clade from Japan. PLoS ONE 2011, 6, e27983. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and Palytoxin Accumulation in Seafood in Relation to Ostreopsis cf. ovata Blooms on the French Mediterranean Coast. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [CrossRef]
- Nascimento, S.M.; França, J.V.; Gonçalves, J.E.A.; Ferreira, C.E.L. Ostreopsis cf. ovata (Dinophyta) bloom in an equatorial island of the Atlantic Ocean. Mar. Pollut. Bull. 2012, 64, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.M.; Monteiro, P.O.; Ferreira, C.E.L.; González-Rodríguez, E. Ostreopsis ovata blooms on Rio de Janeiro coast. Harmful Algae News 2008, 37, 2–5. [Google Scholar]
- Accoroni, S.; Romagnoli, T.; Colombo, F.; Pennesi, C.; di Camillo, C.G.; Marini, M.; Battocchi, C.; Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; et al. Ostreopsis cf. ovata bloom in the northern Adriatic Sea during summer 2009: Ecology, molecular characterization and toxin profile. Mar. Pollut. Bull. 2011, 62, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Mangialajo, L.; Ganzin, N.; Accoroni, S.; Asnaghi, V.; Blanfuné, A.; Cabrini, M.; Cattaneo-Vietti, R.; Chavanon, F.; Chiantore, M.; Cohu, S.; et al. Trends in Ostreopsis proliferation along the Northern Mediterranean coasts. Toxicon 2011, 57, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Grillo, C.; Melchiorre, N. Putative Palytoxin and its new analogue, Ovatoxin-a, in Ostreopsis ovata collected along the Ligurian Coasts during the 2006 toxic outbreak. J. Am. Soc. Mass Spectrom. 2008, 19, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Illoul, H.; Hernández, F.R.; Vila, M.; Adjas, N.; Younes, A.A.; Bournissa, M.; Koroghli, A.; Marouf, N.; Rabia, S.; Ameur, F.L.K. The Genus Ostreopsis along the Algerian Coastal Waters (SW Mediterranean Sea) Associated with a Human Respiratory Intoxication Episode. Cryptogam. Algol. 2012, 33, 209–216. [Google Scholar] [CrossRef]
- Totti, C.; Accoroni, S.; Cerino, F.; Cucchiari, E.; Romagnoli, T. Ostreopsis ovata bloom along the Conero Riviera (northern Adriatic Sea): Relationships with environmental conditions and substrata. Harmful Algae 2010, 9, 233–239. [Google Scholar] [CrossRef]
- Tubaro, A.; Durando, P.; Del Favero, G.; Ansaldi, F.; Icardi, G.; Deeds, J.R.; Sosa, S. Case definitions for human poisonings postulated to palytoxins exposure. Toxicon 2011, 57, 478–495. [Google Scholar] [CrossRef]
- Ramos, V.; Vasconcelos, V. Palytoxin and analogs: Biological and ecological effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Contins, M.; Nascimento, S.M. Effects of the toxic benthic dinoflagellate Ostreopsis cf. ovata on fertilization and early development of the sea urchin Lytechinus variegatus. Mar. Environ. Res. 2018, 135, 11–17. [Google Scholar] [CrossRef]
- Privitera, D.; Giussani, V.; Isola, G.; Faimali, M.; Piazza, V.; Garaventa, F.; Asnaghi, V.; Cantamessa, E.; Cattaneo-Vietti, R.; Chiantore, M. Toxic effects of Ostreopsis ovata on larvae and juveniles of Paracentrotus lividus. Harmful Algae 2012, 18, 16–23. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine Biotoxins: Occurrence, Toxicity, Regulatory Limits and Reference Methods. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Forino, M.; Tartaglione, L. Liquid chromatography-high-resolution mass spectrometry for palytoxins in mussels. Anal. Bioanal. Chem. 2015, 407, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Pezzolesi, L.; Guerrini, F.; Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Pistocchi, R. Influence of temperature and salinity on Ostreopsis cf. ovata growth and evaluation of toxin content through HR LC-MS and biological assays. Water Res. 2012, 46, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, L.; Mazzeo, A.; Dell’Aversano, C.; Forino, M.; Giussani, V.; Capellacci, S.; Penna, A.; Asnaghi, V.; Faimali, M.; Chiantore, M.; et al. Chemical, molecular, and eco-toxicological investigation of Ostreopsis sp. from Cyprus Island: Structural insights into four new ovatoxins by LC-HRMS/MS. Anal. Bioanal. Chem. 2016, 408, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.D.Q.; Nunes, J.M.C.; Menezes, M.; Fraga, S.; Rodríguez, F.; Vázquez, J.A.; Blanco, J.; Franco, J.M.; Riobó, P. Toxin production, growth kinetics and molecular characterization of Ostreopsis cf. ovata isolated from Todos os Santos Bay, tropical southwestern Atlantic. Toxicon 2017, 138, 18–30. [Google Scholar] [CrossRef]
- Nascimento, S.M.; Corrêa, E.V.; Menezes, M.; Varela, D.; Paredes, J.; Morris, S. Growth and toxin profile of Ostreopsis cf. ovata (Dinophyta) from Rio de Janeiro, Brazil. Harmful Algae 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Biré, R.; Trotereau, S.; Lemée, R.; Delpont, C.; Chabot, B.; Aumond, Y.; Krys, S. Occurrence of palytoxins in marine organisms from different trophic levels of the French Mediterranean coast harvested in 2009. Harmful Algae 2013, 28, 10–22. [Google Scholar] [CrossRef]
- Biré, R.; Trotereau, S.; Lemée, R.; Oregioni, D.; Delpont, C.; Krys, S.; Guérin, T. Hunt for palytoxins in a wide variety of marine organisms harvested in 2010 on the French Mediterranean coast. Mar. Drugs 2015, 13, 5425–5446. [Google Scholar] [CrossRef]
- Aligizaki, K.; Katikou, P.; Nikolaidis, G.; Panou, A. First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece). Toxicon 2008, 51, 418–427. [Google Scholar] [CrossRef]
- Eich, A.; Mildenberger, T.; Laforsch, C.; Weber, M. Biofilm and diatom succession on polyethylene (PE) and biodegradable plastic bags in two marine habitats: Early signs of degradation in the pelagic and benthic zone? PLoS ONE 2015, 10, e0137201. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Osborn, A.M.; Duhaime, M.B. Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 2016, 11, e0159289. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181203. [Google Scholar] [CrossRef] [PubMed]
- Bugoni, L.; Krauseand, M.; Petry, L. Marine debris and human impacts on sea turtles in southern Brazil. Mar. Pollut. Bull. 2001, 42, 1330–1334. [Google Scholar] [CrossRef]
- Poli, C.; Mesquita, D.O.; Saska, C.; Mascarenhas, R. Plastic ingestion by sea turtles in Paraíba State, Northeast Brazil. Iheringia Série Zool. 2015, 105, 265–270. [Google Scholar] [CrossRef]
- Antiquera, M.S.; Onofre, E.V.; Tiepolo, L.M. Desafios para conservação da Tartaruga Verde (Chelonia mydas) no litoral paranaense (in Portuguese). Realização 2018, 5, 14–20. [Google Scholar]
- Pozdnyakov, I.; Skarlato, S. Dinoflagellate amphiesma at different stages of the life cycle. Protistology 2012, 7, 108–115. [Google Scholar]
- Moreira-González, A.R.; Fernandes, L.F.; Uchida, H.; Uesugi, A.; Suzuki, T.; Chomérat, N.; Bilien, G.; Mafra, L.L. Variations in morphology, growth, and toxicity among strains of the Prorocentrum lima species complex isolated from Cuba and Brazil. J. Appl. Phycol. 2019, 31, 519–532. [Google Scholar] [CrossRef]
- Nézan, E.; Tillmann, U.; Bilien, G.; Boulben, S.; Chèze, K.; Zentz, F.; Salas, R.; Chomérat, N. Taxonomic revision of the dinoflagellate Amphidoma caudata: Transfer to the genus Azadinium (Dinophyceae) and proposal of two varieties, based on morphological and molecular phylogenetic analyses. J. Phycol. 2012, 48, 925–939. [Google Scholar] [CrossRef]
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]








| Sample | DV | Wide (W) | DV/W Ratio | AP |
|---|---|---|---|---|
| Cultures (all) | 40.8 (23.7–60.1, n = 237) | 31 (15.4–48.9, n = 203) | 1.33 (1.04–1.68, n = 238) | 28.1 (18.6–44.5, n = 26) |
| LM062 | 50.3 (34.1–58, n = 62) | 40.0 (29–48.9, n = 57) | 1.27 (1.06–1.5, n = 62) | 37.9 (35.3–44.5, n = 4) |
| LM086 | 35.5 (27.8–46.3, n = 63) | 28.8 (22.8–44.7, n = 53) | 1.25 (1.04–1.44, n = 63) | 27.3 (23.5–34.2, n = 9) |
| LM129 | 33.7 (23.7–49.5, n = 66) | 24.1 (15.4–39.3, n = 62) | 1.40 (1.05–1.65, n = 66) | 22.7 (19.7–25.5, n = 4) |
| LM130 | 45.3 (27.4–60.1, n = 46) | 32.1 (20.7–40.9, n = 31) | 1.45 (1.23–1.68, n = 47) | 27 (18.6–39.4, n = 9) |
| Field | 49.9 (29.9–65.9, n = 81) | 32.6 (17.1–45.9, n = 67) | 1.53 (1.31–1.79, n = 88) | 23.4 (16.7–30.7, n = 19) |
| All specimens | 43.1 (23.7–65.9, n = 318) | 31.4 (15.4–48.9, n = 270) | 1.38 (1.04–1.79, n = 326) | 26.1 (16.7–44.5, n = 45) |
| Animal | Site | n | Mean Total (Min–Max) | %OVT-a (Min–Max) | %OVT-b (Min–Max) | %OVT-c (Min–Max) | %OVT-d (Min–Max) | %OVT-e (Min–Max) |
|---|---|---|---|---|---|---|---|---|
| Sea urchin | Currais | 4 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Sea cucumber | Currais | 1 | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Coral | Currais | 1 | 29.5 | 67.8% | 32.2% | <LOD | <LOD | <LOD |
| Mussel | Currais | 5 | 22.4 (<LOQ–32.9) | 68.4% (65.9–70.4) | 31.6% (29.6–34.1) | <LOD | <LOD | <LOD |
| Mussel | Currais (transp.) | 5 | 98.0 (52.6–131) | 68.5% (60.1–77.1) | 26.7% (22.9–32.1) | 6.18% (2.62–9.75) | <LOD | 3.80% (2.85–4.48) |
| Origin | OVTX-a | OVTX-b | OVTX-c | OVTX-d/e | Others 3 | Total (pg cell−1) | Reference |
|---|---|---|---|---|---|---|---|
| Italy | 47–56% | 34–37% | 4–8% | 15–18% | 0.5–3% | 12.0–20.0 | [59] |
| Italy | ~50–70% 1 | ~20–25% 1 | ~0–5% 1 | ~5–25% 1 | ~0–5% 1 | 6.0–15.8 | [41] |
| France | 51–61% | 14–16% | 4–6% | 6–18% | 5–17% | 22.5–300 | [7] |
| Spain | 52–59% | 20–29% | 3–6% | 12–16% | 0.9–1.6% | 50.0–250 | [8] |
| Greece | 76.2% | N/A 2 | N/A 2 | 20.4% | 3.4% | 44.0 | [60] |
| Brazil (NE) | 56–61% | 31–37% | 0.3–0.7% | 3–7% | N/A | 21.0–43.4 | [61] |
| Brazil (SE) | 19–45% | 27–51% | 2–18% | 3–4–0% | N/A | 60.0–468 | [62] |
| Brazil (S) | 57–59% | 30–35% | 1.5–5% | 5.5–8% | N/A | 11.3–35.5 | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tibiriçá, C.E.J.A.; Leite, I.P.; Batista, T.V.V.; Fernandes, L.F.; Chomérat, N.; Herve, F.; Hess, P.; Mafra, L.L. Ostreopsis cf. ovata Bloom in Currais, Brazil: Phylogeny, Toxin Profile and Contamination of Mussels and Marine Plastic Litter. Toxins 2019, 11, 446. https://doi.org/10.3390/toxins11080446
Tibiriçá CEJA, Leite IP, Batista TVV, Fernandes LF, Chomérat N, Herve F, Hess P, Mafra LL. Ostreopsis cf. ovata Bloom in Currais, Brazil: Phylogeny, Toxin Profile and Contamination of Mussels and Marine Plastic Litter. Toxins. 2019; 11(8):446. https://doi.org/10.3390/toxins11080446
Chicago/Turabian StyleTibiriçá, Carlos Eduardo J. A., Isabel P. Leite, Talita V. V. Batista, Luciano F. Fernandes, Nicolas Chomérat, Fabienne Herve, Philipp Hess, and Luiz L. Mafra. 2019. "Ostreopsis cf. ovata Bloom in Currais, Brazil: Phylogeny, Toxin Profile and Contamination of Mussels and Marine Plastic Litter" Toxins 11, no. 8: 446. https://doi.org/10.3390/toxins11080446
APA StyleTibiriçá, C. E. J. A., Leite, I. P., Batista, T. V. V., Fernandes, L. F., Chomérat, N., Herve, F., Hess, P., & Mafra, L. L. (2019). Ostreopsis cf. ovata Bloom in Currais, Brazil: Phylogeny, Toxin Profile and Contamination of Mussels and Marine Plastic Litter. Toxins, 11(8), 446. https://doi.org/10.3390/toxins11080446






