OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity
Abstract
1. Introduction
2. Results
3. Discussion
Limitations of the study
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. Experimental Procedure
5.3. Neurophysiological Evaluation
5.4. Botulinum Neurotoxin Type A treatment
5.5. Study Design
5.6. Statistical Methods
Author Contributions
Funding
Conflicts of Interest
References
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 1980, 30, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Leathley, M.J.; Gregson, J.M.; Moore, A.P.; Smith, T.L.; Sharma, A.K. Prevalence of spasticity post stroke. Clin. Rehabil. 2002, 16, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Beard, S.; Hunn, A.; Wight, J. Treatments for spasticity and pain in multiple sclerosis: A systematic review. Health Technol. Assess. 2003, 7, 1–111. [Google Scholar] [CrossRef]
- Brainin, M.; Norrving, B.; Sunnerhagen, K.S.; Goldstein, L.B.; Cramer, S.C.; Donnan, G.A.; Duncan, P.W.; Francisco, G.; Good, D.; Graham, G.; et al. Poststroke chronic disease management: Towards improved identification and interventions for poststroke spasticity-related complications. Int. J. Stroke 2011, 6, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, P.; Anderson, P. The psychosocial impact of spasticity related problems for people with multiple sclerosis: A focus group study. J. Health Psychol. 2001, 6, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Albanese, A.; Chancellor, M.B.; Elovic, E.; Segal, K.R.; Simpson, D.M.; Ward, A.B. Evidence-based review and assessment of botulinum neurotoxin for the treatment of adult spasticity in the upper motor neuron syndrome. Toxicon 2013, 67, 115–128. [Google Scholar] [CrossRef]
- Haikh, A.; Phadke, C.P.; Ismail, F.; Boulias, C. Relationship between Botulinum Toxin, Spasticity, and Pain: A Survey of Patient Perception. Can. J. Neurol. Sci. 2016, 43, 311–315. [Google Scholar]
- Bartolo, M.; Chiò, A.; Ferrari, S.; Tassorelli, C.; Tamburin, S.; Avenali, M.; Azicnuda, E.; Calvo, A.; Caraceni, A.T.; Defazio, G.; et al. Italian Consensus Conference on Pain in Neurorehabilitation (ICCPN). Assessing and treating pain in movement disorders, amyotrophic lateral sclerosis, severe acquired brain injury, disorders of consciousness, dementia, oncology and neuroinfectivology. Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2016, 52, 841–854. [Google Scholar]
- Paolucci, S.; Martinuzzi, A.; Scivoletto, G.; Smania, N.; Solaro, C.; Aprile, I.; Armando, M.; Bergamaschi, R.; Berra, E.; Berto, G.; et al. Italian Consensus Conference on Pain in Neurorehabilitation (ICCPN). Assessing and treating pain associated with stroke, multiple sclerosis, cerebral palsy, spinal cord injury and spasticity. Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2016, 52, 827–840. [Google Scholar]
- Kermen, R. Botulinum toxin for chronic pain conditions. Dis. Mon. 2016, 62, 353–357. [Google Scholar] [CrossRef]
- Sandrini, G.; Perrotta, A.; Tassorelli, C.; Torelli, P.; Brighina, F.; Sances, G.; Nappi, G. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: A multicenter, double-blind, randomized, placebo-controlled, parallel group study. J. Headache Pain 2011, 12, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Finkel, A.G. Botulinum toxin and the treatment of headache: A clinical review. Toxicon 2015, 1, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Schütz, S.G.; Simpson, D.M. Botulinum toxin for neuropathic pain and spasticity: An overview. Pain Manag. 2014, 4, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; De Icco, R.; Tassorelli, C.; Smania, N.; Tamburin, S. Botulinum neurotoxin type A for the treatment of pain: Not just in migraine and trigeminal neuralgia. J. Headache Pain 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Matak, I.; Lacković, Z. Botulinum toxin A, brain and pain. Prog. Neurobiol. 2014, 119–120, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Matak, I.; Tékus, V.; Bölcskei, K.; Lacković, Z.; Helyes, Z. Involvement of substance P in the antinociceptive effect of botulinum toxin type A: Evidence from knockout mice. Neuroscience 2017, 1, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Yao, L.; Ni, L.; Wang, L.; Hu, X. Antinociceptive effect of botulinum toxin A involves alterations in AMPA receptor expression and glutamate release in spinal dorsal horn neurons. Neuroscience 2017, 15, 197–207. [Google Scholar] [CrossRef]
- Aoki, K.R. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005, 26, 785–793. [Google Scholar] [CrossRef]
- Price, D.D. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp. Neurol. 1972, 37, 57–68. [Google Scholar] [CrossRef]
- Alvisi, E.; Serrao, M.; Conte, C.; Alfonsi, E.; Tassorelli, C.; Prunetti, P.; Cristina, S.; Perrotta, A.; Pierelli, F.; Sandrini, G. Botulinum toxin A modifies nociceptive withdrawal reflex in subacute stroke patients. Brain Behav. 2018, 8, e01069. [Google Scholar] [CrossRef]
- Sandrini, G.; Serrao, M.; Rossi, P.; Romaniello, A.; Cruccu, G.; Willer, J.C. The lower limb reflex in humans. Prog. Neurobiol. 2005, 77, 353–395. [Google Scholar] [CrossRef]
- Price, D.D.; Hayes, R.L.; Ruda, M.; Dubner, R. Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J. Neurophysiol. 1978, 41, 933–947. [Google Scholar] [CrossRef]
- Li, J.; Simone, D.A.; Larson, A.A. Windup leads to characteristics of central sensitization. Pain 1999, 79, 75–82. [Google Scholar] [CrossRef]
- La Cesa, S.; Tamburin, S.; Tugnoli, V.; Sandrini, G.; Paolucci, S.; Lacerenza, M.; Marchettini, P.; Cruccu, G.; Truini, A. How to diagnose neuropathic pain? The contribution from clinical examination, pain questionnaires and diagnostic tests. Neurol. Sci. 2015, 36, 2169–2675. [Google Scholar] [CrossRef]
- Lundberg, L.E.R.; Jorum, E.; Holm, E.; Torebjrk, H.E. Intra-neural electrical stimulation of cutaneous nociceptive fibres in humans: Effects of different pulse patterns on magnitude of pain. Acta Physiol. Scand. 1992, 146, 41–48. [Google Scholar] [CrossRef]
- Herrero, J.F.; Laird, J.M.; López-García, J.A. Wind-up of spinal cord neurones and pain sensation: Much ado about something? Prog. Neurobiol. 2000, 61, 169–203. [Google Scholar] [CrossRef]
- Eide, P.K. Wind-up and the NMDA receptor complex from a clinical perspective. Eur. J. Pain 2000, 4, 5–15. [Google Scholar] [CrossRef]
- Staud, R.; Vierck, C.J.; Cannon, R.L.; Mauderli, A.P.; Price, D.D. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain 2001, 91, 165–175. [Google Scholar] [CrossRef]
- Staud, R.; Weyl, E.E.; Riley, J.L.; Fillingim, R.B. Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS ONE 2014, 18, e89086. [Google Scholar] [CrossRef]
- Perrotta, A.; Serrao, M.; Sandrini, G.; Burstein, R.; Sances, G.; Rossi, P.; Bartolo, M.; Pierelli, F.; Nappi, G. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 2010, 30, 272–284. [Google Scholar] [CrossRef]
- Rhudy, J.L.; France, C.R. Reliability and validity of a brief method to assess nociceptive flexion reflex (NFR) threshold. J. Pain 2011, 12, 782–791. [Google Scholar] [CrossRef]
- Perrotta, A.; Sandrini, G.; Serrao, M.; Buscone, S.; Tassorelli, C.; Tinazzi, M.; Zangaglia, R.; Pacchetti, C.; Bartolo, M.; Pierelli, F.; et al. Facilitated temporal summation of pain at spinal level in Parkinson’s disease. Mov. Disord. 2011, 15, 442–448. [Google Scholar] [CrossRef]
- Perrotta, A.; Serrao, M.; Ambrosini, A.; Bolla, M.; Coppola, G.; Sandrini, G.; Pierelli, F. Facilitated temporal processing of pain and defective supraspinal control of pain in cluster headache. Pain 2013, 154, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, A.; Bolla, M.; Anastasio, M.G.; Serrao, M.; Sandrini, G.; Pierelli, F. Modulation of temporal summation threshold of the nociceptive withdrawal reflex by transcutaneous spinal direct current stimulation in humans. Clin. Neurophysiol. 2016, 127, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Shippen, C.; Bavikatte, G.; Mackarel, D. The benefit of botulinum toxin A in the management of central post stroke pain: A case report. J. Neurol. Stroke 2017, 6, 00218. [Google Scholar] [CrossRef]
- Wissel, J.; Ganapathy, V.; Ward, A.B.; Borg, J.; Ertzgaard, P. OnabotulinumtoxinA Improves Pain in Patients with Post-Stroke Spasticity: Findings from a Randomized, Double-Blind, Placebo Controlled Trial. J. Pain Symptom. Manag. 2016, 52, 17–26. [Google Scholar] [CrossRef]
- Favre-Guilmard, C.; Auguet, M.; Chabrier, P.E. Different antinociceptive effects of botulinum toxin type A in inflammatory and peripheral polyneuropathic rat models. Eur. J. Pharmacol. 2009, 617, 48–53. [Google Scholar] [CrossRef]
- Oh, H.M.; Chung, M.E. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins 2015, 14, 3127–3154. [Google Scholar] [CrossRef]
- Intiso, D.; Basciani, M.; Santamato, A.; Intiso, M.; Di Rienzo, F. Botulinum Toxin Type A for the Treatment of Neuropathic Pain in Neuro-Rehabilitation. Toxins 2015, 30, 2454–2480. [Google Scholar] [CrossRef]
- Porro, C.A.; Sandrini, G.; Truini, A.; Tugnoli, V.; Alfonsi, E.; Berliocchi, L.; Cacciatori, C.; La Cesa, S.; Magrinelli, F.; Sacerdote, P.; et al. Diagnosing and assessing pain in neurorehabilitation: From translational research to the clinical setting. Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Eur. J. Phys. Rehabil. Med. 2016, 52, 717–729. [Google Scholar]
- Mallik, A.; Weir, A.I. Nerve conduction studies: Essentials and pitfalls in practice. J. Neurol. Neurosurg. Psychiatry 2005, 76, 23–31. [Google Scholar] [CrossRef]
| All Patients | Stroke | MS | SCI | ANOVA (F or χ2) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Number (N) | 25 | 10 | 10 | 5 | |||
| Age (years) | 59.4 ± 12.6 | 66.5 ± 8.1 | 49.9 ± 8.8 | 39.5 ± 9.2 | F (2,21) 15.699 | 0.001 | |
| Sex—Male (N - %) | 14–56% | 6–60% | 3–30% | 5 – 100% | χ2 (2) 3.745 | 0.154 | |
| Height (cm) | 166.8 ± 5.7 | 167.1 ± 5.6 | 165.6 ± 5.6 | 169.0 ± 9.9 | F (2,18) 0.301 | 0.744 | |
| Weight (Kg) | 69.8 ± 11.8 | 73.7 ± 10.6 | 63.1 ± 12.9 | 69.5 ± 7.8 | F (2,18) 1.961 | 0.170 | |
| Disease duration (years) | 11.3 ± 11.9 | 4.7 ± 6.2 | 23.3 ± 8.6 | 18.4 ± 23.4 | F (2,21) 0.266 | 0.001 | |
| Botulinum toxin dosage (units) | 221.9 ± 135.1 | 218.0 ± 164.1 | 210.7 ± 73.2 | 290.0 ± 14.1 | F (2,21) 12.100 | 0.769 | |
| First treatment with botulinum toxin (N - %) | 13–52% | 5–50% | 3–30% | 5–100% | χ2 (2) 0.705 | 0.703 | |
| Affected side (N - %) | R | 13–52% | 8–80% | 4–40% | 0–0% | χ2 (4) 4.419 | 0.352 |
| L | 6–24% | 1–10% | 2–20% | 3–60% | |||
| B | 6–24% | 1–10% | 4–40% | 2–40% | |||
| FIM (20–140) | 81.6 ± 28.5 | 83.3 ± 27.2 | 70.3 ± 32.3 | 105.0 ± 14.1 | F (2,21) 1.125 | 0.344 | |
| BARTHEL INDEX (0–20) | 11.5 ± 5.9 | 13.0 ± 4.8 | 6.8 ± 6.1 | 16.5 ± 2.1 | F (2,20) 3.522 | 0.049 | |
| BDI (0–63) | 8.0 ± 4.6 | 8.6 ± 4.5 | 8.5 ± 5.1 | 3.5 ± 2.1 | F (2,20) 1.117 | 0.347 | |
| MMSE (0–30) | 26.2 ± 3.7 | 25.5 ± 2.8 | 26.3 ± 5.4 | 29.5 ± 0.7 | F (2,19) 1.461 | 0.257 | |
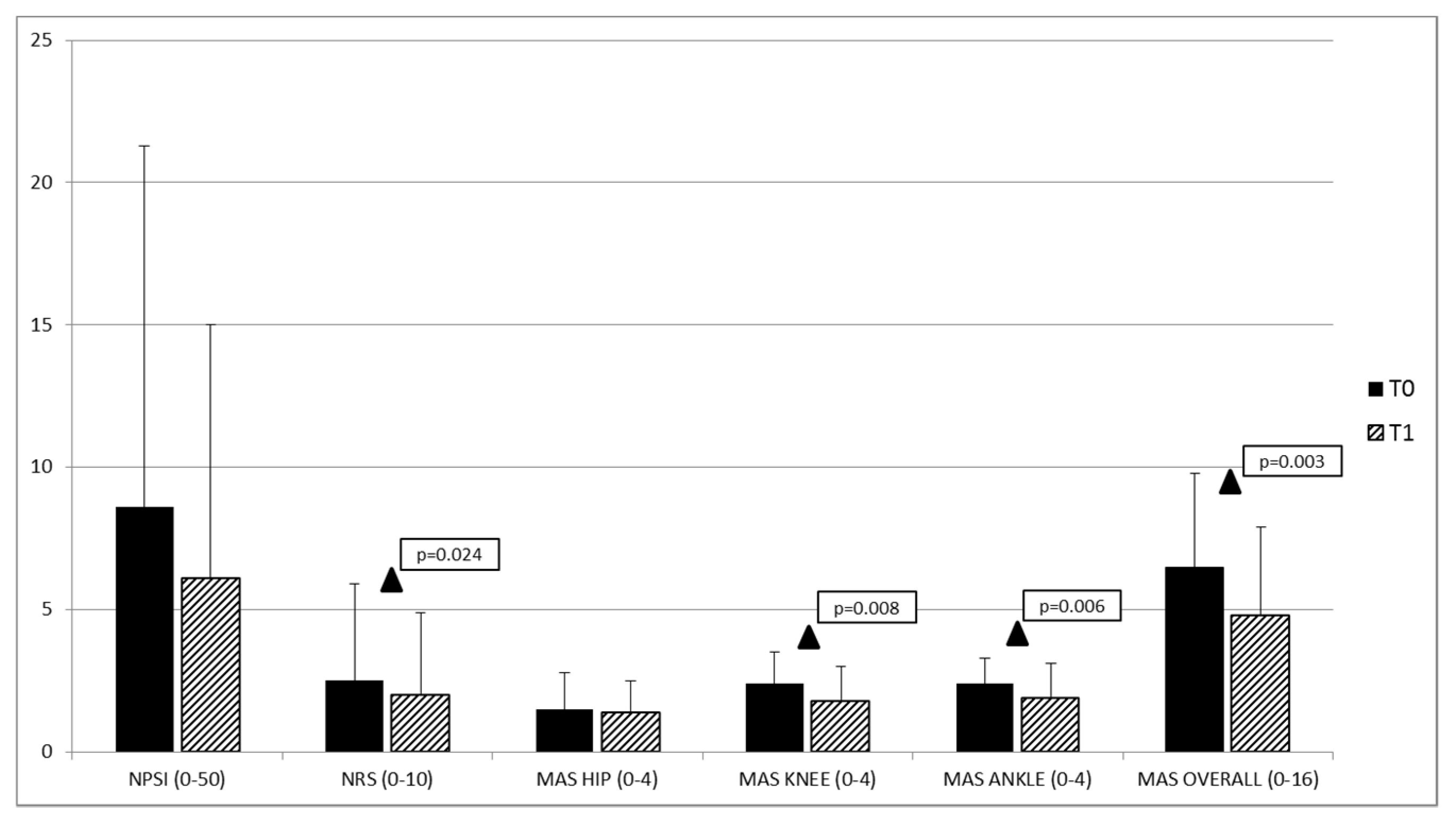
| NPSI (0–50) | 8.6 ± 12.7 | 5.8 ± 11.9 | 13.6 ± 14.8 | 6.5 ± 9.2 | F (2,18) 0.325 | 0.727 | |
| NRS (0–10) | 2.5 ± 3.4 | 1.5 ± 2.7 | 3.7 ± 3.8 | 4.0 ± 5.7 | F (2,18) 0.768 | 0.479 | |
| MAS–injected lower limb | |||||||
| HIP (0–4) | 1.5 ± 1.3 | 1.1 ± 0.9 | 2.3 ± 1.6 | 2.0 ± 0.0 | F (2,20) 2.572 | 0.101 | |
| KNEE (0–4) | 2.4 ± 1.1 | 1.8 ± 1.0 | 3.3 ± 0.5 | 3.0 ± 0.0 | F (2,20) 4.833 | 0.019 | |
| ANKLE (0–4) | 2.4 ± 0.9 | 2.3 ± 1.0 | 2.8 ± 0.9 | 2.0 ± 0.0 | F (2,20) 1.035 | 0.374 | |
| OVERALL (0–16) | 6.5 ± 3.3 | 5.1 ± 2.7 | 9.2 ± 3.7 | 7.0 ± 0.0 | F (2,20) 2.366 | 0.120 | |
| T0 | T1 | Paired t-Test | p-Value | |
|---|---|---|---|---|
| FIM (20–140) | 81.6 ± 28.5 | 82.4 ± 32.1 | (1,19) −0.237 | 0.815 |
| BARTHEL (0–20) | 11.5 ± 5.9 | 14.7 ± 11.6 | (1,19) −1.386 | 0.182 |
| NPSI (0–100) | 8.6 ± 12.7 | 6.1 ± 8.9 | (1,19) 1.873 | 0.077 |
| NRS (0–10) | 2.5 ± 3.4 | 2.0 ± 2.9 | (1,19) 2.463 | 0.024 |
| MAS–injected lower limb | ||||
| HIP (0–4) | 1.5 ± 1.3 | 1.4 ± 1.1 | (1,19) 0.900 | 0.379 |
| KNEE (0–4) | 2.4 ± 1.1 | 1.8 ± 1.2 | (1,19) 2.979 | 0.008 |
| ANKLE (0–4) | 2.4 ± 0.9 | 1.9 ± 1.2 | (1,19) 3.123 | 0.006 |
| OVERALL (0–16) | 6.5 ± 3.3 | 4.8 ± 3.1 | (1,19) 3.478 | 0.003 |
| Stroke | MS | SCI | ANOVA | p-Value | |
|---|---|---|---|---|---|
| ST (mA) | 1.8 ± 1.6 | 1.2 ± 0.9 | 2.0 ± 2.3 | F (2,21) 1.150 | 0.336 |
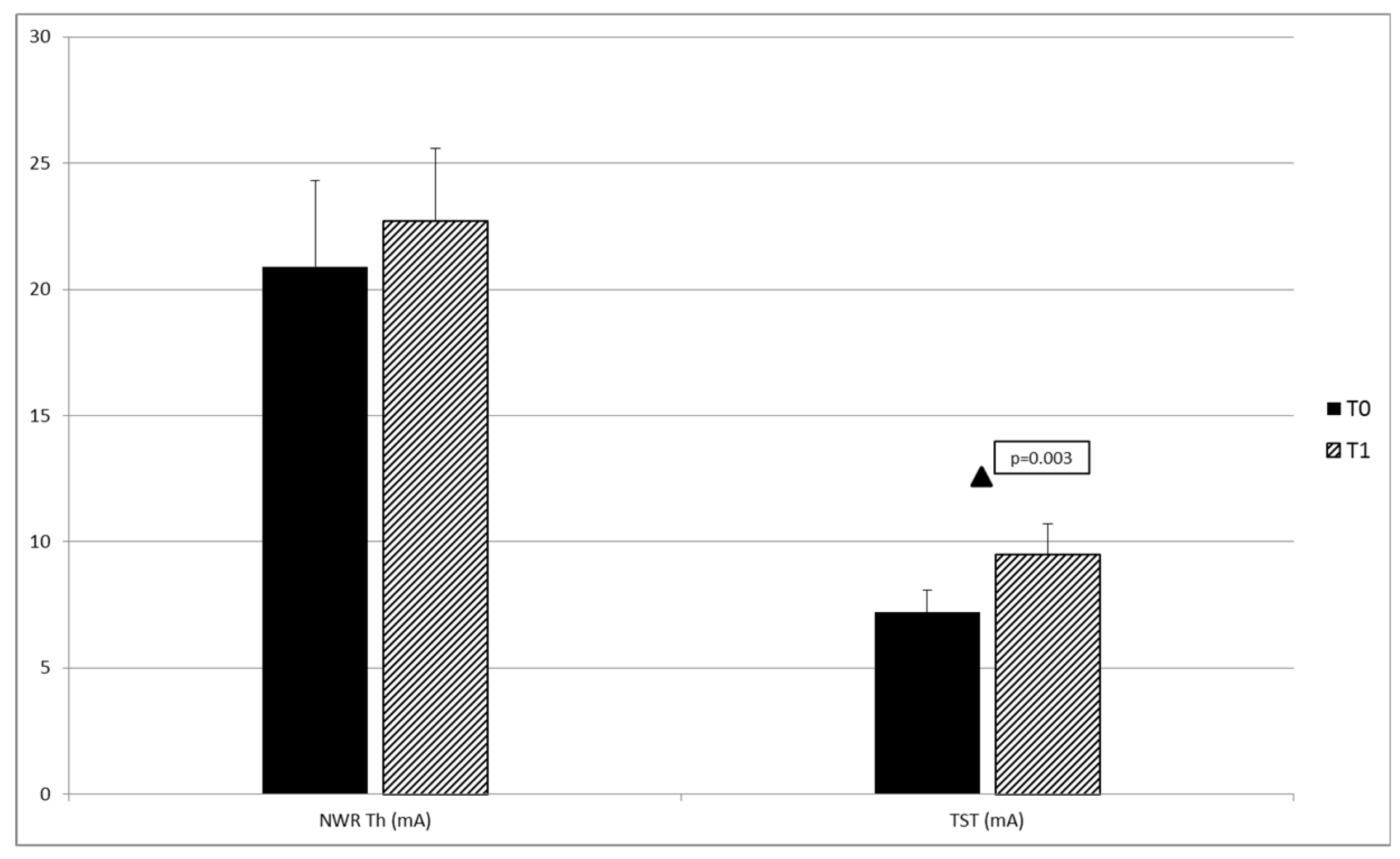
| NWR Th (mA) | 15.9 ± 3.2 | 27.5 ± 16.0 | 18.0 ± 16.9 | F (2,19) 1.199 | 0.323 |
| NRS Th | 5.9 ± 1.6 | 6.0 ± 2.0 | 5.0 ± 0.4 | F (2,19) 0.270 | 0.766 |
| Area (msec*mA) | 3516.4 ± 3272.0 | 1690.7 ± 909.1 | 8797.6 ± 10,783.0 | F (2,19) 3.744 | 0.043 |
| TST (mA) | 6.6 ± 2.3 | 7.2 ± 4.5 | 9.6 ± 8.9 | F (2,20) 0.416 | 0.665 |
| NRS 5°st | 5.3 ± 1. | 4.3 ± 0.8 | 3.0 | F (2,20) 1.495 | 0.248 |
| T0 | T1 | Paired t-Test | p-Value | |
|---|---|---|---|---|
| ST (mA) | 1.6 ± 1.4 | 2.1 ± 1.6 | (1,14) −1.429 | 0.175 |
| NWR Th (mA) | 20.9 ± 12.2 | 22.7 ± 10.4 | (1,14) −0.807 | 0.433 |
| NRS Th | 5.8 ± 1.7 | 6.6 ± 1.9 | (1,14) −2.175 | 0.057 |
| Area (msec*mV) | 3490.3 ± 4312.9 | 2230.6 ± 2125.1 | (1,14) 1.599 | 0.132 |
| TST (mA) | 7.2 ± 4.0 | 9.5 ± 5.1 | (1,14) −2.655 | 0.019 |
| NRS 5°st | 4.7 ± 1.3 | 4.8 ± 1.7 | (1,13) 0.16 | 0.856 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Icco, R.; Perrotta, A.; Berra, E.; Allena, M.; Alfonsi, E.; Tamburin, S.; Serrao, M.; Sandrini, G.; Tassorelli, C. OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity. Toxins 2019, 11, 359. https://doi.org/10.3390/toxins11060359
De Icco R, Perrotta A, Berra E, Allena M, Alfonsi E, Tamburin S, Serrao M, Sandrini G, Tassorelli C. OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity. Toxins. 2019; 11(6):359. https://doi.org/10.3390/toxins11060359
Chicago/Turabian StyleDe Icco, Roberto, Armando Perrotta, Eliana Berra, Marta Allena, Enrico Alfonsi, Stefano Tamburin, Mariano Serrao, Giorgio Sandrini, and Cristina Tassorelli. 2019. "OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity" Toxins 11, no. 6: 359. https://doi.org/10.3390/toxins11060359
APA StyleDe Icco, R., Perrotta, A., Berra, E., Allena, M., Alfonsi, E., Tamburin, S., Serrao, M., Sandrini, G., & Tassorelli, C. (2019). OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity. Toxins, 11(6), 359. https://doi.org/10.3390/toxins11060359







