Dynamic Ochratoxin A Production by Strains of Aspergillus niger Intended Used in Food Industry of China
Abstract
:1. Introduction
2. Results
2.1. OTA Temporal Producing Profile for Strains of A.niger Used in This Study
2.2. Substrates Effect on OTA Production Profile by A.niger Intended Used in Food Industry
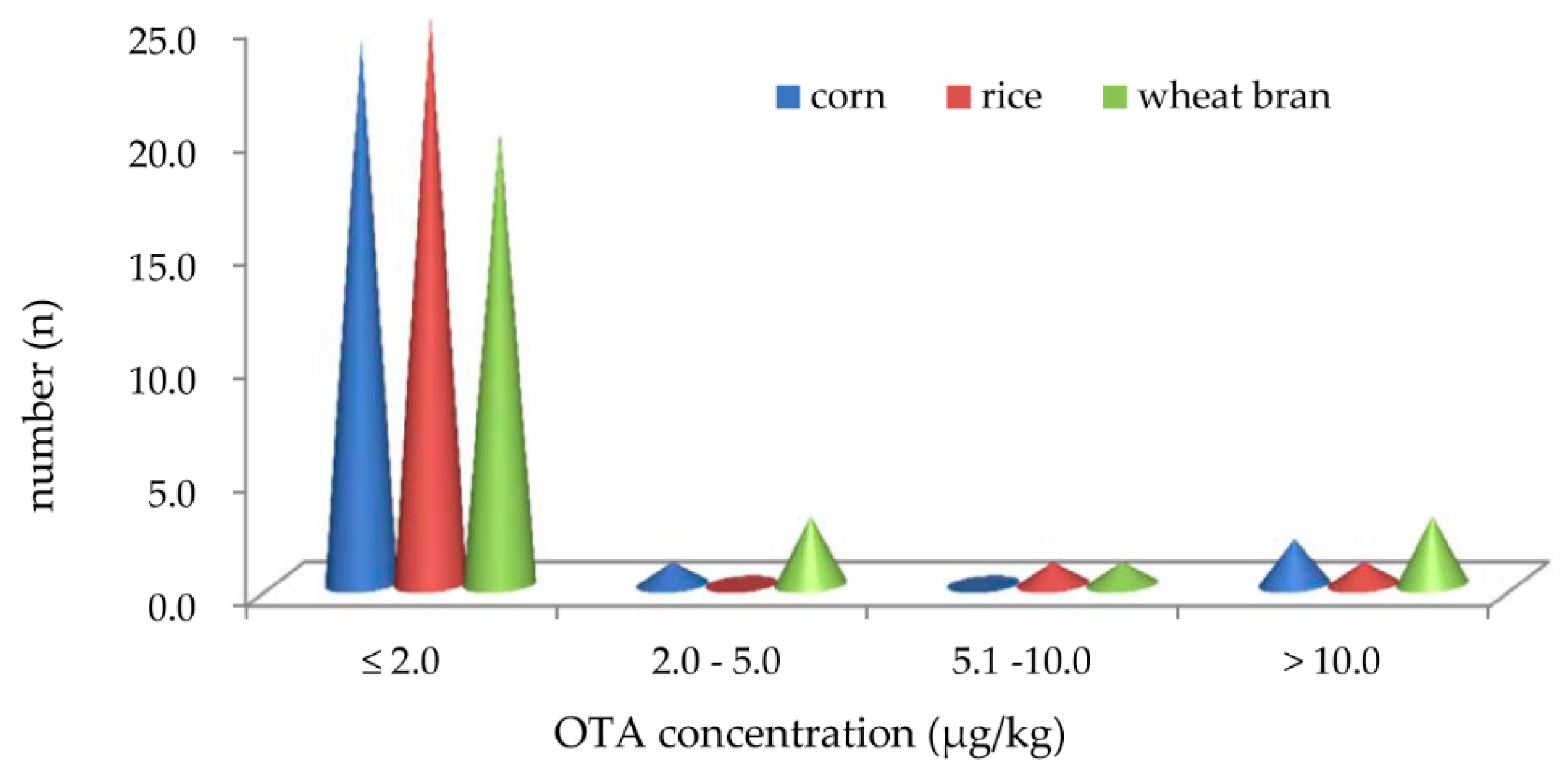
2.3. Average Concentration Distribution of OTA Produced by 27 Strains of A.niger Intended Used in Chinese Food Industry
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Strains of Fungi
4.3. OTA Production
4.4. OTA Extraction
4.5. HPLC Conditions
4.6. HPLC Method Validation
4.7. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Merwe, K.J.; Steyn, P.S.; Fourie, L.; Scott, D.B.; Theron, J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature 1965, 205, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rigó, K.; Téren, J.; Mesterházy, Á. Recent advances in ochratoxin research I. Production, detection and occurrence of ochratoxins. Cereal Res. Commun. 2001, 29, 85–92. [Google Scholar]
- Smith, J.E.; Moss, M.O. Mycotoxins, formation, analysis and significance. Food Microbiol. 1985, 2, 291. [Google Scholar]
- International Agency for Research on Cancer (IARC). Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monogr. Eval. Carcinog. Risks Hum. WHO/IARC Lyons 1993, 56, 1–599. [Google Scholar]
- Pel, H.J.; de Winde, J.H.; Archer, D.B.; Dyer, P.S.; Hofmann, G.; Schaap, P.J.; Turner, G.; de Vries, R.P.; Albang, R.; Albermann, K.; et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger. Nat. Biotechnol. 2007, 25, 221–231. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Accensi, F.; Abarca, M.L.; Cabañes, F.J. Occurrence of Aspergillus species in mixed feeds and component raw materials and their ability to produce ochratoxin A. Food Microbiol. 2004, 21, 623–627. [Google Scholar] [CrossRef]
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Ochratoxin A production by strains of Aspergillus niger var.niger. Appl. Environ. Microbiol. 1994, 60, 2650–2652. [Google Scholar]
- Marino, A.; Fiorentino, C.; Spataro, F.; Nostro, A. Effect of temperature on production of Ochratoxin A by Aspergillus niger in orange juice. J. Toxins 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Astoreca, A.L.; Barberis, C.L.; Magnoli, C.E.; Dalcero, A. Growth and ochratoxin A production by Aspergillus niger group strains in coffee beans in relation to environmental factors. World Mycotoxin J. 2010, 3, 59–65. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Larsen, T.O.; Thrane, U.; Meijer, M.; Varga, J.; Samson, R.A.; Nielsen, K.F. Fumonisin and ochratoxin production in industrial Aspergillus niger strains. PLoS ONE 2011, 6, e23496. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Proctor, R.H.; Morelli, M.; Haidukowski, M.; Gallo, A.; Logrieco, A.F.; Moretti, A. Variation in fumonisin and ochratoxin production associated with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front. Microbiol. 2016, 7, 1412. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Lars, L.H.M.; Larsen, T.O.; Kumar, R.; Arnau, J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl. Environ. Microbiol. 2018, 102, 9481–9515. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Jiang, H.R.; Xu, J.; Zhang, J.; Li, F.Q. Dynamic fumonisin B2 production by Aspergillus niger intended used in food industry in China. Toxins 2017, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.M.; Comeria, R.M.; Larumbe, G.; Ritieni, A.; Vaamonde, G.; Fernández, P.V. Toxigenic fungi isolated from dried vine fruits in Argentina. Int. J. Food Microbiol. 2005, 104, 43–49. [Google Scholar] [CrossRef]
- Magnoli, C.; Violante, M.; Combina, M.; Palacio, G.; Dalcero, A. Mycoflora and ochratoxin-producing strains of Aspergillus section Nigri in wine grapes in Argentina. Lett. Appl. Microbiol. 2010, 37, 179–184. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Butchko, R.A.; Haidukowski, M.; Stea, G.; Logrieco, A.; Moretti, A. Variation in the fumonisin biosynthetic gene cluster in fumonisin-producing and nonproducing black Aspergilli. Fungal Genet. Biol. 2014, 73, 39–52. [Google Scholar] [CrossRef]
- Ferracin, L.M.; Fier, C.B.; Vieira, M.L.C.; Viorello, C.B.M.; Varani Ade, M.; Rossi, M.M.; Santos, M.M.; Taniwaki, M.H.; Lamanaka, B.T.; Fungaro, M.H.P. Strain-specific polyketide synthase genes of Aspergillus niger. Int. J. Food Microbiol. 2012, 155, 137–145. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.Y.; Chen, H.Y.; Li, M.; Zhu, X.J.; Gao, Q.; Wang, D.P.; Zhang, Y. A polyketide synthase encoded by the gene An15g07920 is involved in the biosynthesis of ochratoxin A in Aspergillus niger. J. Agric. Food Chem. 2016, 64, 9680–9688. [Google Scholar] [CrossRef]
- Castellá, G.; Alborch, L.; Bragulat, M.R.; Cabañes, F.J. Real time quantitative expression study of a polyketide synthase gene related to ochratoxin a biosynthesis in Aspergillus niger. Food Control 2015, 53, 147–150. [Google Scholar] [CrossRef]
- von Hertwig, A.M.; Ana, A.S.S.; Sartori, D.; da Silva, J.J.; Nascimento, M.S.; Iamanaka, B.T.; Fungaro, M.H.P.; Taniwaki, M.H. Real-time PCR-based method for rapid detection of Aspergillus niger and Aspergillus welwitschiae isolated from coffee. J. Microbiol. Methods 2018, 148, 87–92. [Google Scholar] [CrossRef]
- Jessica, G.S.; Marta, G.D.; María, T.G.J.; Covadonga, V.; Belén, P. Description of an orthologous cluster of ochratoxin A biosynthetic genes in Aspergillus and Penicillium species. A comparative analysis. Int. J. Food Microbiol. 2018, 268, 35–43. [Google Scholar]
- Standardization Administration of the People’s Republic of China. Determination of Ochratoxin A in Food-High Performance Liquid Chromatographic Method with Immunoaffinity Column Clean-Up. GB 5009.96-2016; SAC: Beijing, China, 2017; Available online: http://down.foodmate.net/standard/sort/3/50478.html (accessed on 23 June 2017).
- European Union Decision 2002/657/EC. Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Common. 2001, L221, 8–36. [Google Scholar]
| Stain No 1 | Corn (μg/kg) | Rice (μg/kg) | Wheat Bran (μg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 28 | Day 7 | Day 14 | Day 21 | Day 28 | Day 7 | Day 14 | Day 21 | Day 28 | |
| SN-01 | 128.0 | 117.4 | 181.5 | 90.3 | nd | nd | nd | nd | nd | nd | 0.1 | nd |
| SN-02 | 1.3 | 0.3 | nd | nd | 0.8 | 0.4 | 0.2 | 0.2 | 2.0 | nd | 0.4 | 3.4 |
| SN-03 | nd 2 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| SN-04 | 3.5 | 0.4 | 0.1 | nd | 0.1 | nd | nd | nd | 35.0 | 0.5 | 0.3 | 0.6 |
| SN-05 | 4.7 | 21.8 | 52.0 | 46.2 | 88.0 | 29.0 | 22.3 | 14.8 | nd | nd | nd | nd |
| SN-06 | nd | nd | 0.2 | nd | 25.0 | 26.0 | 9.8 | 8.0 | nd | nd | nd | nd |
| SN-07 | nd | nd | nd | nd | nd | nd | 0.2 | nd | 5.0 | nd | 0.5 | nd |
| SN-08 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.3 | nd |
| SN-09 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| SN-10 | 2.0 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| SN-11 | 0.2 | 4.1 | nd | nd | nd | 0.7 | 0.9 | 0.2 | 75.9 | 116.2 | 175.4 | 95.3 |
| SN-12 | nd | nd | nd | nd | nd | 0.5 | nd | nd | nd | nd | nd | nd |
| SN-13 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 40.1 | 0.5 | 10.4 |
| OA-01 | nd | nd | nd | 0.7 | nd | nd | 0.2 | nd | nd | nd | 13.2 | nd |
| OA-02 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.9 | 1.5 | nd |
| OA-03 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| OA-04 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| OA-05 | nd | nd | nd | 0.5 | nd | nd | 0.8 | 0.2 | nd | nd | nd | nd |
| OA-06 | 0.4 | 0.4 | 0.3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| TA-01 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| TA-02 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| TA-03 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| TA-04 | nd | nd | nd | nd | nd | nd | nd | nd | 1.9 | 1.9 | 1.0 | 11.7 |
| TA-05 | nd | nd | nd | nd | nd | 0.2 | nd | nd | nd | nd | nd | nd |
| TA-06 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.4 | nd |
| TA-07 | nd | nd | 0.6 | nd | nd | 427.8 | 176.5 | 190.2 | 3.0 | 0.3 | 0.5 | 5.1 |
| GA-01 | nd | nd | nd | nd | nd | nd | 0.2 | 15.9 | nd | nd | nd | nd |
| ACCC 30557 | nd | nd | nd | nd | nd | nd | nd | nd | 0.6 | nd | nd | nd |
| ATCC 16404 | 0.2 | nd | 0.3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| SI-01 | nd | nd | 0.3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Substrate | Time Intervals (Day) | OTA Production for Different Functions of A.niger | |||||
|---|---|---|---|---|---|---|---|
| Saccharifying Enzyme Producer (n = 13) | Organic Acid Producer (n = 6) | Tannase Producer (n = 7) | |||||
| No. of Positive (%) | Mean (Range) (μg/kg) | No. of Positive (%) | Mean (Range) (μg/kg) | No. of Positive (%) | Mean (Range) (μg/kg) | ||
| Corn | 7 | 6 (46) | 23.3 (0.2–128.0) | 1 (17) | 0.4 | 0 | 0 |
| 14 | 5 (38) | 28.8 (0.3–117.4) | 1 (17) | 0.4 | 0 | 0 | |
| 21 | 4 (31) | 58.5 (0.1–181.5) | 1(17) | 0.3 | 1 (14) | 0.6 | |
| 28 | 2 (15) | 68.3 (46.2–90.3) | 2 (33) | 0.6 (0.5–0.7) | 0 | 0 | |
| Rice | 7 | 4 (31) | 28.5 (0.1–88.0) | 0 | 0 | 0 | 0 |
| 14 | 5 (38) | 11.3 (0.4–29.0) | 0 | 0 | 2 (29) | 214.0 (0.2–427.8) | |
| 21 | 5 (38) | 6.7 (0.2–22.3) | 2 (33) | 0.5 (0.2–0.8) | 1 (14) | 176.5 | |
| 28 | 4 (31) | 5.8 (0.2–14.8) | 1 (17) | 0.2 | 1 (14) | 190.2 | |
| Wheat bran | 7 | 4 (31) | 29.5 (2.0–75.9) | 0 | 0 | 2 (29) | 2.5 (1.9–3.0) |
| 14 | 3 (23) | 52.3 (0.5–116.2) | 1 (17) | 0.9 | 2 (29) | 1.1 (0.3–1.9) | |
| 21 | 7 (54) | 25.4 (0.1–175.4) | 2 (33) | 7.4 (1.5–13.2) | 3 (43) | 0.6 (0.4–1.0) | |
| 28 | 4 (31) | 27.4 (0.6–95.3) | 0 | 0 | 2 (29) | 8.4 (5.1–11.7) | |
| Potential Application | Strain No. | Isolated Substrate | Optimum Culture Temperature (°C) | Obtained Center/Place 2 |
|---|---|---|---|---|
| Saccharifying enzyme production (n = 13) | SN-01 | nr 1 | 25–28 | ACCC |
| SN-02 | Mold culture used for wine fermentation | 25–28 | ACCC | |
| SN-03 | nr | 25–28 | ACCC | |
| SN-04 | nr | 25–28 | ACCC | |
| SN-05 | nr | 30–32 | ACCC | |
| SN-06 | nr | 30–32 | ACCC | |
| SN-07 | nr | 25–28 | CGMCC | |
| SN-08 | nr | 25–28 | CGMCC | |
| SN-09 | nr | 25–28 | CGMCC | |
| SN-10 | nr | 25–28 | CGMCC | |
| SN-11 | nr | 25–28 | CGMCC | |
| SN-12 | nr | 25–28 | CGMCC | |
| SN-13 | nr | 25–28 | CGMCC | |
| Organic acid production (n = 6) | OA-01 | nr | 25–28 | CGMCC |
| OA-02 | nr | 25–28 | CGMCC | |
| OA-03 | nr | 29–31 | ACCC | |
| OA-04 | nr | 25–28 | CGMCC | |
| OA-05 | nr | 25–28 | CGMCC | |
| OA-06 | nr | 25–28 | ACCC | |
| Tannase production (n = 7) | TA-01 | nr | 25–28 | CGMCC |
| TA-02 | Eucalyptus leaves | 25–28 | CGMCC | |
| TA-03 | Rotten soil | 25–28 | CGMCC | |
| TA-04 | nr | 25–28 | CGMCC | |
| TA-05 | Rotten wood | 25–28 | CGMCC | |
| TA-06 | Rotten wood | 25–28 | CGMCC | |
| TA-07 | Rotten wood | 25–28 | CGMCC | |
| β-galactosidase production (n = 1) | GA-01 | Moldy bagasse | 25–28 | CGMCC |
| Research and Teaching | ACCC 30557 | Cloth | 24 | ACCC |
| Used as the reference strain for medium control, method validation, etc. | ATCC 16404 | nr | 25–28 | ATCC |
| No practical application | SI-01 | Corn | 25–28 | Our lab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Jiang, H.; Li, F. Dynamic Ochratoxin A Production by Strains of Aspergillus niger Intended Used in Food Industry of China. Toxins 2019, 11, 122. https://doi.org/10.3390/toxins11020122
Han X, Jiang H, Li F. Dynamic Ochratoxin A Production by Strains of Aspergillus niger Intended Used in Food Industry of China. Toxins. 2019; 11(2):122. https://doi.org/10.3390/toxins11020122
Chicago/Turabian StyleHan, Xiaomin, Hongru Jiang, and Fengqin Li. 2019. "Dynamic Ochratoxin A Production by Strains of Aspergillus niger Intended Used in Food Industry of China" Toxins 11, no. 2: 122. https://doi.org/10.3390/toxins11020122
APA StyleHan, X., Jiang, H., & Li, F. (2019). Dynamic Ochratoxin A Production by Strains of Aspergillus niger Intended Used in Food Industry of China. Toxins, 11(2), 122. https://doi.org/10.3390/toxins11020122





