The Effect of a Single Bout of Chinese Archery on Core Executive Functions in Preadolescent Children in Shanghai
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
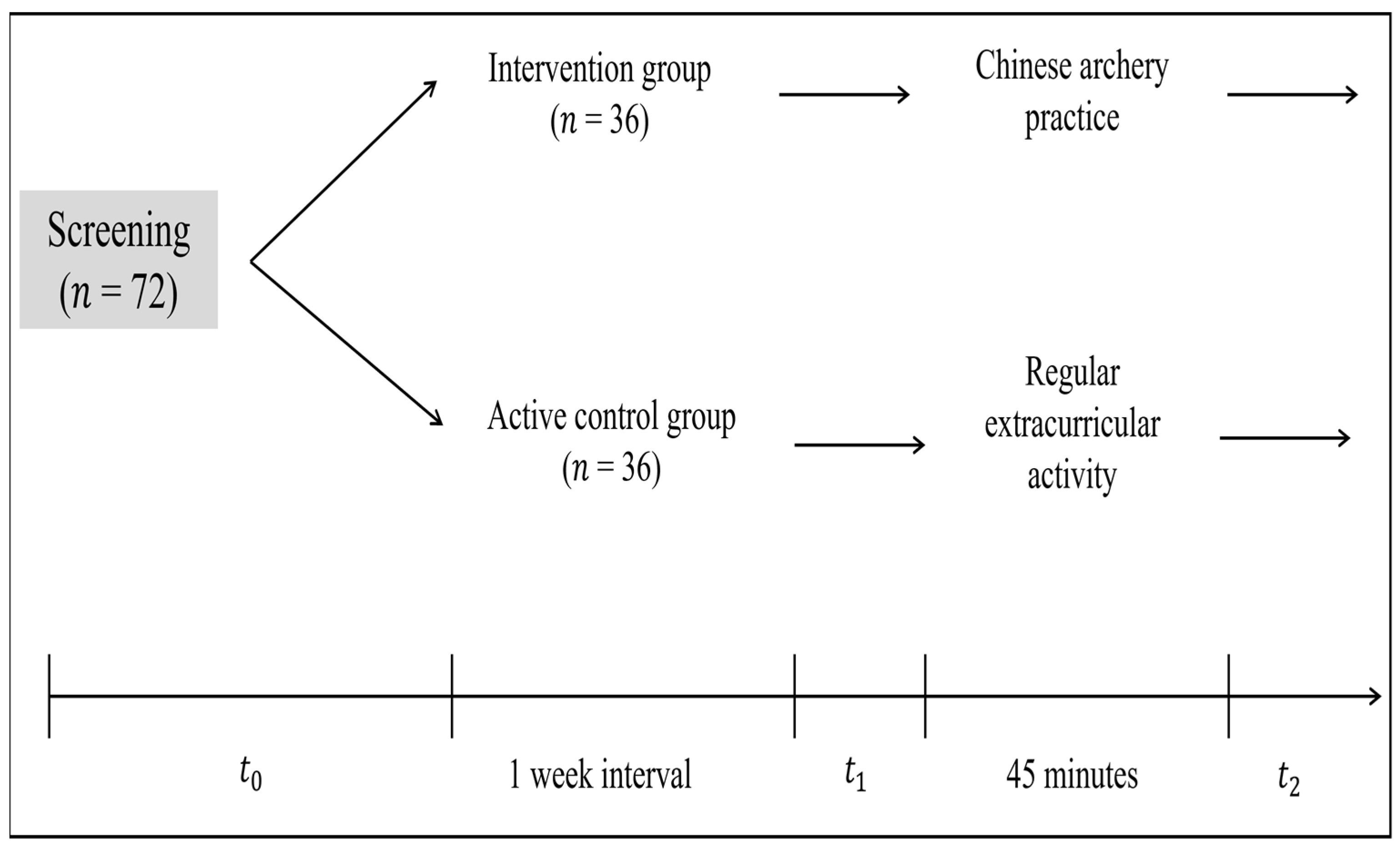
2.2. Experimental Design
2.3. Measurements
2.3.1. Inhibition Control
2.3.2. Working Memory
2.3.3. Cognitive Flexibility
2.4. Experimental Procedure
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Core Executive Functions Performance
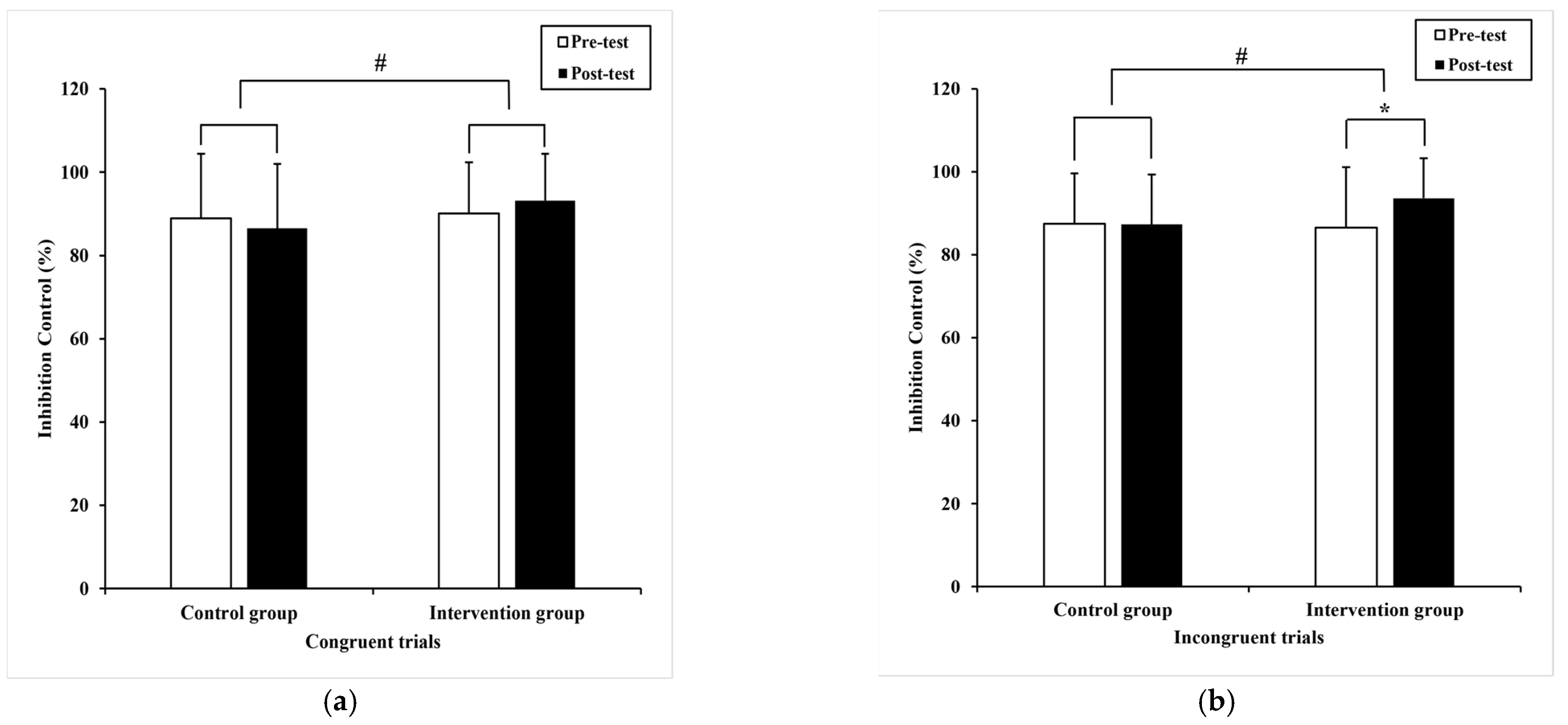
3.2.1. Inhibition Control
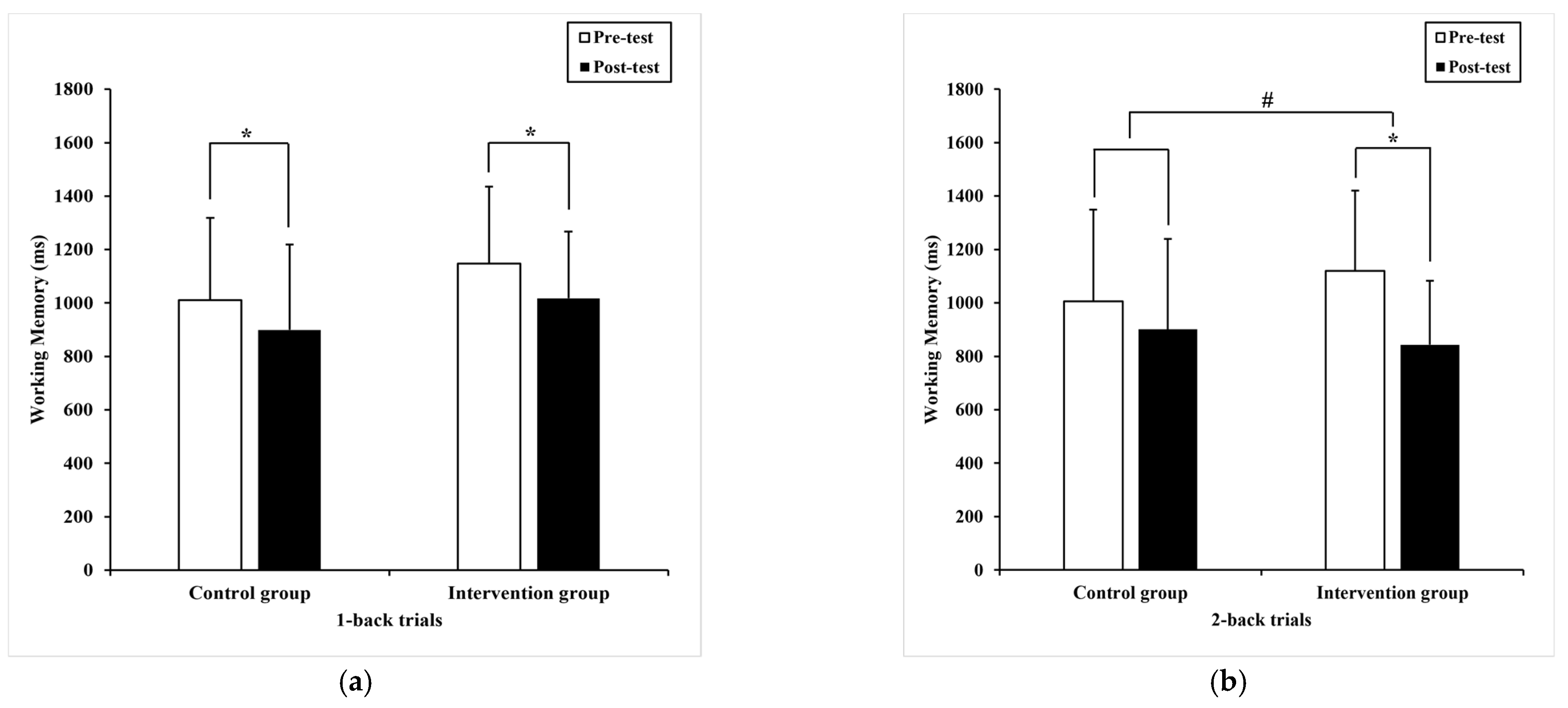
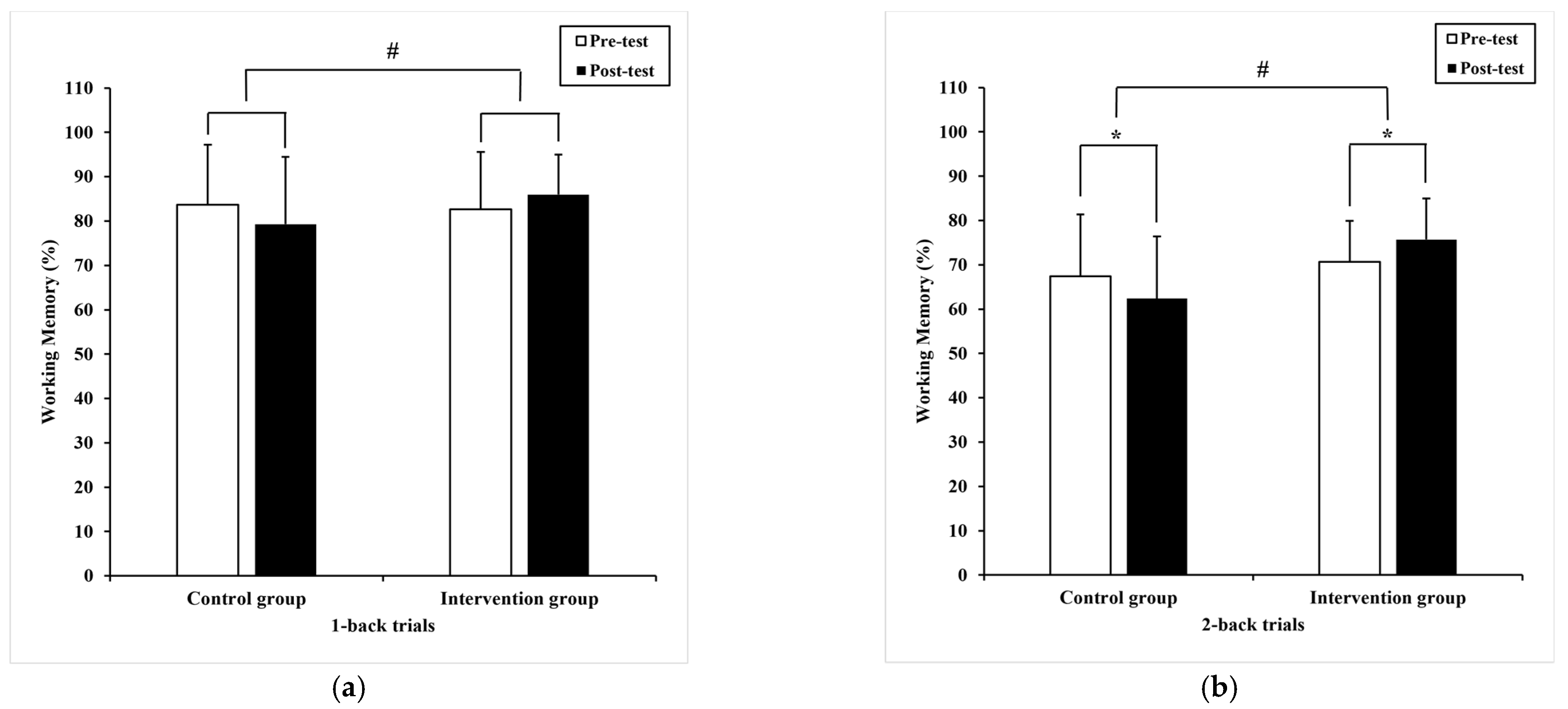
3.2.2. Working Memory
3.2.3. Cognitive Flexibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Diamond, A.; Ling, D.S. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev. Cogn. Neurosci. 2016, 18, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Pontifex, M.B.; Raine, L.B.; Castelli, D.M.; Hall, E.E.; Kramer, A. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience 2009, 159, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Pesce, C.; Ben-Soussan, T.D. “Cogito ergo sum” or “ambulo ergo sum”? New perspectives in developmental exercise and cognition research. In Exercise-Cognition Interaction: Neuroscience Perspectives; Elsevier Academic Press: San Diego, CA, USA, 2016; pp. 251–282. [Google Scholar]
- Singh, A.S.; Saliasi, E.; van den Berg, V.; Uijtdewilligen, L.; de Groot, R.H.M.; Jolles, J.; Andersen, L.B.; Bailey, R.; Chang, Y.K.; Diamond, A.; et al. Effects of physical activity interventions on cognitive and academic performance in children and adolescents: A novel combination of a systematic review and recommendations from an expert panel. Br. J. Sport. Med. 2019, 53, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Etnier, J.L.; Salazar, W.; Landers, D.M.; Petruzzello, S.J.; Han, M.; Nowell, P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. J. Sport Exerc. Psychol. 1997, 19, 249–277. [Google Scholar] [CrossRef]
- Best, J.R.; Miller, P.H. A developmental perspective on executive function. Child Dev. 2010, 81, 1641–1660. [Google Scholar] [CrossRef]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Brown, T.E.; Landgraf, J.M. Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: Evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad. Med. 2010, 122, 42–51. [Google Scholar] [CrossRef]
- Morrison, F.J.; Ponitz, C.C.; McClelland, M.M. Self-regulation and academic achievement in the transition to school. In Child Development at the Intersection of Emotion and Cognition; Calkins, S., Bell, M., Eds.; American Psychological Association: Washington, DC, USA, 2010; pp. 203–224. [Google Scholar]
- Bailey, C.E. Cognitive accuracy and intelligent executive function in the brain and in business. Ann. N. Y. Acad. Sci. 2007, 1118, 122–141. [Google Scholar] [CrossRef]
- Riggs, N.R.; Spruijt-Metz, D.; Chou, C.-P.; Pentz, M.A. Relationships between executive cognitive function and lifetime substance use and obesity-related behaviors in fourth grade youth. Child Neuropsychol. 2012, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; McGowan, A.L.; Chandler, M.C.; Gwizdala, K.L.; Parks, A.C.; Fenn, K.; Kamijo, K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019, 40, 1–22. [Google Scholar] [CrossRef]
- Chen, A.-G.; Yan, J.; Yin, H.-C.; Pan, C.-Y.; Chang, Y.-K. Effects of acute aerobic exercise on multiple aspects of executive function in preadolescent children. Psychol. Sport Exerc. 2014, 15, 627–636. [Google Scholar] [CrossRef]
- Soga, K.; Shishido, T.; Nagatomi, R. Executive function during and after acute moderate aerobic exercise in adolescents. Psychol. Sport Exerc. 2015, 16, 7–17. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Q.; Li, Z.; Cunha, P.M.; Zhang, Y.; Kong, Z.; Lin, W.; Chen, S.; Cai, Y. Effects of Acute and Chronic Exercises on Executive Function in Children and Adolescents: A Systemic Review and Meta-Analysis. Front. Psychol. 2020, 11, 554915. [Google Scholar] [CrossRef] [PubMed]
- Verburgh, L.; Konigs, M.; Scherder, E.J.; Oosterlaan, J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: A meta-analysis. Br. J. Sport. Med. 2014, 48, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.S.; Chueh, T.Y.; Huang, C.J.; Kao, S.C.; Hillman, C.H.; Chang, Y.K.; Hung, T.M. Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J. Sport. Sci. 2021, 39, 10–22. [Google Scholar] [CrossRef]
- Schwarck, S.; Schmicker, M.; Dordevic, M.; Rehfeld, K.; Muller, N.; Muller, P. Inter-Individual Differences in Cognitive Response to a Single Bout of Physical Exercise-A Randomized Controlled Cross-Over Study. J. Clin. Med. 2019, 8, 1101. [Google Scholar] [CrossRef]
- Piepmeier, A.T.; Etnier, J.L. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J. Sport Health Sci. 2015, 4, 14–23. [Google Scholar] [CrossRef]
- Hindin, S.B.; Zelinski, E.M. Extended practice and aerobic exercise interventions benefit untrained cognitive outcomes in older adults: A meta-analysis. J. Am. Geriatr. Soc. 2012, 60, 136–141. [Google Scholar] [CrossRef]
- Diamond, A.; Kirkham, N. Not quite as grown-up as we like to think: Parallels between cognition in childhood and adulthood. Psychol. Sci. 2005, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Chu, C.H.; Wang, C.C.; Wang, Y.C.; Song, T.F.; Tsai, C.L.; Etnier, J.L. Dose-response relation between exercise duration and cognition. Med. Sci. Sport. Exerc. 2015, 47, 159–165. [Google Scholar] [CrossRef]
- Lambrick, D.; Stoner, L.; Grigg, R.; Faulkner, J. Effects of continuous and intermittent exercise on executive function in children aged 8–10 years. Psychophysiology 2016, 53, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Masaki, H.; Gerber, M.; Ludyga, S. Acute and long-term effects of resistance training on executive function. J. Cogn. Enhanc. 2018, 2, 200–207. [Google Scholar] [CrossRef]
- Tian, S.; Mou, H.; Qiu, F. Sustained Effects of High-Intensity Interval Exercise and Moderate-Intensity Continuous Exercise on Inhibitory Control. Int. J. Environ. Res. Public Health 2021, 18, 2687. [Google Scholar] [CrossRef]
- Golden, D.; Liang, L.-Y.; Getchell, N. The Effects of Xbox Kinect Active Video Gaming on Executive Function, Inhibition, in Children with and without Autism Spectrum Disorder: A Pilot Study. J. Behav. Brain Sci. 2022, 12, 287–301. [Google Scholar] [CrossRef]
- Wu, C.H.; Karageorghis, C.I.; Wang, C.C.; Chu, C.H.; Kao, S.C.; Hung, T.M.; Chang, Y.K. Effects of acute aerobic and resistance exercise on executive function: An ERP study. J. Sci. Med. Sport 2019, 22, 1367–1372. [Google Scholar] [CrossRef]
- Sudo, M.; Komiyama, T.; Aoyagi, R.; Nagamatsu, T.; Higaki, Y.; Ando, S. Executive function after exhaustive exercise. Eur. J. Appl. Physiol. 2017, 117, 2029–2038. [Google Scholar] [CrossRef]
- Mehren, A.; Diaz Luque, C.; Brandes, M.; Lam, A.P.; Thiel, C.M.; Philipsen, A.; Ozyurt, J. Intensity-Dependent Effects of Acute Exercise on Executive Function. Neural Plast. 2019, 2019, 8608317. [Google Scholar] [CrossRef]
- Vazou, S.; Pesce, C.; Lakes, K.; Smiley-Oyen, A. More than one road leads to Rome: A narrative review and meta-analysis of physical activity intervention effects on cognition in youth. Int. J. Sport Exerc. Psychol. 2019, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Ling, D.S. Aerobic-Exercise and resistance-training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev. Cogn. Neurosci. 2019, 37, 100572. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; McAuley, E.; Erickson, K.I.; Liu-Ambrose, T.; Kramer, A.F. On mindful and mindless physical activity and executive function: A response to Diamond and Ling (2016). Dev. Cogn. Neurosci. 2019, 37, 100529. [Google Scholar] [CrossRef] [PubMed]
- Flook, L.; Smalley, S.L.; Kitil, M.J.; Galla, B.M.; Kaiser-Greenland, S.; Locke, J.; Ishijima, E.; Kasari, C. Effects of Mindful Awareness Practices on Executive Functions in Elementary School Children. J. Appl. Sch. Psychol. 2010, 26, 70–95. [Google Scholar] [CrossRef]
- Lambourne, K.; Tomporowski, P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Res. 2010, 1341, 12–24. [Google Scholar] [CrossRef]
- Ishihara, T.; Sugasawa, S.; Matsuda, Y.; Mizuno, M. Improved executive functions in 6–12-year-old children following cognitively engaging tennis lessons. J. Sport. Sci. 2017, 35, 2014–2020. [Google Scholar] [CrossRef]
- Alesi, M.; Bianco, A.; Luppina, G.; Palma, A.; Pepi, A. Improving children’s coordinative skills and executive functions: The effects of a football exercise program. Percept. Mot. Ski. 2016, 122, 27–46. [Google Scholar] [CrossRef]
- Egger, F.; Conzelmann, A.; Schmidt, M. The effect of acute cognitively engaging physical activity breaks on children’s executive functions: Too much of a good thing? Psychol. Sport Exerc. 2018, 36, 178–186. [Google Scholar] [CrossRef]
- Luu, K.; Hall, P.A. Examining the Acute Effects of Hatha Yoga and Mindfulness Meditation on Executive Function and Mood. Mindfulness 2016, 8, 873–880. [Google Scholar] [CrossRef]
- Jacob, R.; Parkinson, J. The potential for school-based interventions that target executive function to improve academic achievement: A review. Rev. Educ. Res. 2015, 85, 512–552. [Google Scholar] [CrossRef]
- Diamond, A. Activities and programs that improve children’s executive functions. Curr. Dir. Psychol. Sci. 2012, 21, 335–341. [Google Scholar] [CrossRef]
- Formenti, D.; Cavaggioni, L.; Duca, M.; Trecroci, A.; Rapelli, M.; Alberti, G.; Komar, J.; Iodice, P. Acute effect of exercise on cognitive performance in middle-aged adults: Aerobic versus balance. J. Phys. Act. Health 2020, 17, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Gothe, N.; Pontifex, M.B.; Hillman, C.; McAuley, E. The acute effects of yoga on executive function. J. Phys. Act. Health 2013, 10, 488–495. [Google Scholar] [CrossRef]
- Bo, Z.; Songping, Y. Cultural Comparison between Chinese and Western Sports Competition-Based on the Chinese Ritual Archery and Ancient Olympic Games. J. Shanghai Univ. Sport 2018, 42, 87–92, 98. [Google Scholar]
- Selby, S. Chinese Archery; Hong Kong University Press: Hong Kong, China, 2000; Volume 1. [Google Scholar]
- Baifa, Z.; Xinglong, Z.; Dongmei, L. Muscle coordination during archery shooting: A comparison of archers with different skill levels. Eur. J. Sport Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Clemente, F.; Couceiro, M.; Rocha, R.; Mendes, R. Study of the heart rate and accuracy performance of archers. J. Phys. Educ. Sport 2011, 11, 434. [Google Scholar]
- Lu, Q.; Li, P.; Wu, Q.; Liu, X.; Wu, Y. Efficiency and Enhancement in Attention Networks of Elite Shooting and Archery Athletes. Front. Psychol. 2021, 12, 638822. [Google Scholar] [CrossRef]
- Wang, D.; Hu, T.; Luo, R.; Shen, Q.; Wang, Y.; Li, X.; Qiao, J.; Zhu, L.; Cui, L.; Yin, H. Effect of Cognitive Reappraisal on Archery Performance of Elite Athletes: The Mediating Effects of Sport-Confidence and Attention. Front. Psychol. 2022, 13, 860817. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Zelazo, P.D. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nat. Protoc. 2006, 1, 297–301. [Google Scholar] [CrossRef]
- Hillman, C.H.; Snook, E.M.; Jerome, G.J. Acute cardiovascular exercise and executive control function. Int. J. Psychophysiol. 2003, 48, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Puhse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Puhse, U.; Lucchi, S.; Marti, J.; Gerber, M. Immediate and sustained effects of intermittent exercise on inhibitory control and task-related heart rate variability in adolescents. J. Sci. Med. Sport 2019, 22, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.B.; Bandelow, S.; Nute, M.L.; Dring, K.J.; Stannard, R.L.; Morris, J.G.; Nevill, M.E. Sprint-based exercise and cognitive function in adolescents. Prev. Med. Rep. 2016, 4, 155–161. [Google Scholar] [CrossRef]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Ai, J.Y.; Chen, F.T.; Hsieh, S.S.; Kao, S.C.; Chen, A.G.; Hung, T.M.; Chang, Y.K. The Effect of Acute High-Intensity Interval Training on Executive Function: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3593. [Google Scholar] [CrossRef]
- Moreau, D.; Chou, E. The Acute Effect of High-Intensity Exercise on Executive Function: A Meta-Analysis. Perspect. Psychol. Sci. 2019, 14, 734–764. [Google Scholar] [CrossRef]
- Graham, J.D.; Bremer, E.; Fenesi, B.; Cairney, J. Examining the Acute Effects of Classroom-Based Physical Activity Breaks on Executive Functioning in 11- to 14-Year-Old Children: Single and Additive Moderation Effects of Physical Fitness. Front. Pediatr. 2021, 9, 688251. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Sato, D.; Yamashiro, K.; Tsubaki, A.; Takehara, N.; Uetake, Y.; Nakano, S.; Maruyama, A. Inter-individual differences in working memory improvement after acute mild and moderate aerobic exercise. PLoS ONE 2018, 13, e0210053. [Google Scholar] [CrossRef]
- Pommier, P.S. Effects Of Exercise Intensity And Type On Executive Function In Elementary-Aged Children. Ph.D. Thesis, Illinois State University, Normal, IL, USA, 2021. [Google Scholar]
- Benzing, V.; Chang, Y.K.; Schmidt, M. Acute Physical Activity Enhances Executive Functions in Children with ADHD. Sci. Rep. 2018, 8, 12382. [Google Scholar] [CrossRef]
- Ishihara, T.; Sugasawa, S.; Matsuda, Y.; Mizuno, M. The beneficial effects of game-based exercise using age-appropriate tennis lessons on the executive functions of 6-12-year-old children. Neurosci. Lett. 2017, 642, 97–101. [Google Scholar] [CrossRef]
- Song, W.; Feng, L.; Wang, J.; Ma, F.; Chen, J.; Qu, S.; Luo, D. Play Smart, Be Smart? Effect of Cognitively Engaging Physical Activity Interventions on Executive Function among Children 4~12 Years Old: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 762. [Google Scholar] [CrossRef]
- Best, J.R. Effects of physical activity on children’s executive function: Contributions of experimental research on aerobic exercise. Dev. Rev. 2010, 30, 331–351. [Google Scholar] [CrossRef]
- Contreras-Osorio, F.; Campos-Jara, C.; Martinez-Salazar, C.; Chirosa-Rios, L.; Martinez-Garcia, D. Effects of Sport-Based Interventions on Children’s Executive Function: A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 755. [Google Scholar] [CrossRef]
- Menglu, S.; Ruiwen, L.; Suyong, Y.; Dong, Z. Effects of Tai Chi on the Executive Function and Physical Fitness of Female Methamphetamine Dependents: A Randomized Controlled Trial. Front. Psychiatry 2021, 12, 653229. [Google Scholar] [CrossRef]
- Wayne, P.M.; Walsh, J.N.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of Tai Chi on cognitive performance in older adults: Systematic review and meta-Analysis. J. Am. Geriatr. Soc. 2014, 62, 25–39. [Google Scholar] [CrossRef]
- Taylor-Piliae, R.E.; Newell, K.A.; Cherin, R.; Lee, M.J.; King, A.C.; Haskell, W.L. Effects of Tai Chi and Western exercise on physical and cognitive functioning in healthy community-dwelling older adults. J. Aging Phys. Act. 2010, 18, 261–279. [Google Scholar] [CrossRef]
- Fatemi, H.; Chan, A.S.; Sze, S.L.; Siu, N.Y.; Lau, E.M.; Cheung, M.-c. A Chinese Mind-Body Exercise Improves Self-Control of Children with Autism: A Randomized Controlled Trial. PLoS ONE 2013, 8, e68184. [Google Scholar]
- Lakes, K.D.; Bryars, T.; Sirisinahal, S.; Salim, N.; Arastoo, S.; Emmerson, N.; Kang, D.; Shim, L.; Wong, D.; Kang, C.J. The Healthy for Life Taekwondo Pilot Study: A Preliminary Evaluation of Effects on Executive Function and BMI, Feasibility, and Acceptability. Ment. Health Phys. Act. 2013, 6, 181–188. [Google Scholar] [CrossRef]
- Novick, J.M.; Bunting, M.F.; Dougherty, M.R.; Engle, R.W. Cognitive and Working Memory Training: Perspectives from Psychology, Neuroscience, and Human Development; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Arnsten, A.F.; Goldman-Rakic, P.S. Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry 1998, 55, 362–368. [Google Scholar] [CrossRef]
- Cerqueira, J.J.; Mailliet, F.; Almeida, O.F.; Jay, T.M.; Sousa, N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007, 27, 2781–2787. [Google Scholar] [CrossRef]
- Gothe, N.P.; Keswani, R.K.; McAuley, E. Yoga practice improves executive function by attenuating stress levels. Biol. Psychol. 2016, 121, 109–116. [Google Scholar] [CrossRef]
- Purohit, S.P.; Pradhan, B. Effect of yoga program on executive functions of adolescents dwelling in an orphan home: A randomized controlled study. J. Tradit. Complement. Med. 2017, 7, 99–105. [Google Scholar] [CrossRef]
- Suwabe, K.; Hyodo, K.; Fukuie, T.; Ochi, G.; Inagaki, K.; Sakairi, Y.; Soya, H. Positive Mood while Exercising Influences Beneficial Effects of Exercise with Music on Prefrontal Executive Function: A Functional NIRS Study. Neuroscience 2021, 454, 61–71. [Google Scholar] [CrossRef]
- Bigelow, H.; Gottlieb, M.D.; Ogrodnik, M.; Graham, J.D.; Fenesi, B. The Differential Impact of Acute Exercise and Mindfulness Meditation on Executive Functioning and Psycho-Emotional Well-Being in Children and Youth With ADHD. Front. Psychol. 2021, 12, 660845. [Google Scholar] [CrossRef]
- Henning, S.E. Chinese Martial Arts Training Manuals: A Historical Survey. China Rev. Int. 2008, 15, 247–249. [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. Review of the evidence on, and fundamental questions about, efforts to improve executive functions, including working memory. In Cognitive and Working Memory Training: Perspectives from Psychology, Neuroscience, and Human Development; Oxford University Press: Oxford, UK, 2020; pp. 143–431. [Google Scholar]


| Variables | Control Group | Intervention Group | t/x2 | p |
|---|---|---|---|---|
| M (SD) | M (SD) | |||
| n (male/female) | 36 (15/21) | 36 (23/13) | 3.57 | 0.06 |
| Age (month) | 115.94 (3.46) | 115.44 (3.23) | 0.63 | 0.53 |
| Height (cm) | 143.76 (6.28) | 147.07 (6.56) | −2.19 | 0.00 * |
| Weight (kg) | 41.90 (11.56) | 42.01 (9.59) | −0.04 | 0.97 |
| BMI (kg/m2) | 20.07 (4.47) | 19.21 (3.07) | 0.95 | 0.35 |
| Aerobic fitness (ml/min/kg) | 46.08 (2.74) | 45.20 (2.65) | 1.39 | 0.17 |
| Control Group (n = 36) | Intervention Group (n = 36) | F | p | η2p | |||
|---|---|---|---|---|---|---|---|
| Pre-Test M (SD) | Post-Test M (SD) | Pre-Test M (SD) | Post-Test M (SD) | ||||
| Reaction time (ms) | |||||||
| Inhibition Control | |||||||
| Congruent trials | 542.61 (91.90) | 504.31 * (63.28) 507.71 a (6.59) | 558.95 (99.40) | 472.05 * (47.18) 468.66 a (6.59) | 17.48 | 0.00 # | 0.20 |
| Incongruent trials | 493.96 (92.28) | 529.08 (95.40) 529.81 a (15.24) | 498.98 (115.94) | 465.33 (95.99) 464.60 a (15.24) | 9.14 | 0.00 # | 0.12 |
| Working Memory | |||||||
| 1-back trials | 1010.47 * (308.65) | 899.33 (319.07) 935.00 a (40.96) | 1147.54 (287.8) | 1017.16 * (249.75) 981.48 a (40.96) | 0.62 | 0.34 | 0.01 |
| 2-back trials | 1005.43 (342.97) | 901.02 (338.57) 932.13 a (39.91) | 1120.25 (300.12) | 843.33 * (240.06) 812.22 a (39.91) | 4.44 | 0.04 # | 0.01 |
| Cognitive Flexibility | |||||||
| color trials | 874.12 (194.15) | 833.60 (170.65) 802.56 a (18.12) | 768.77 (165.50) | 707.87 * (123.19) 738.05 a (17.86) | 6.18 | 0.02 # | 0.08 |
| shape trials | 784.37 (263.64) | 755.96 (153.89) 742.30 a (22.46) | 694.36 (176.09) | 640.59 * (138.60) 654.22 a (22.14) | 7.64 | 0.01 # | 0.10 |
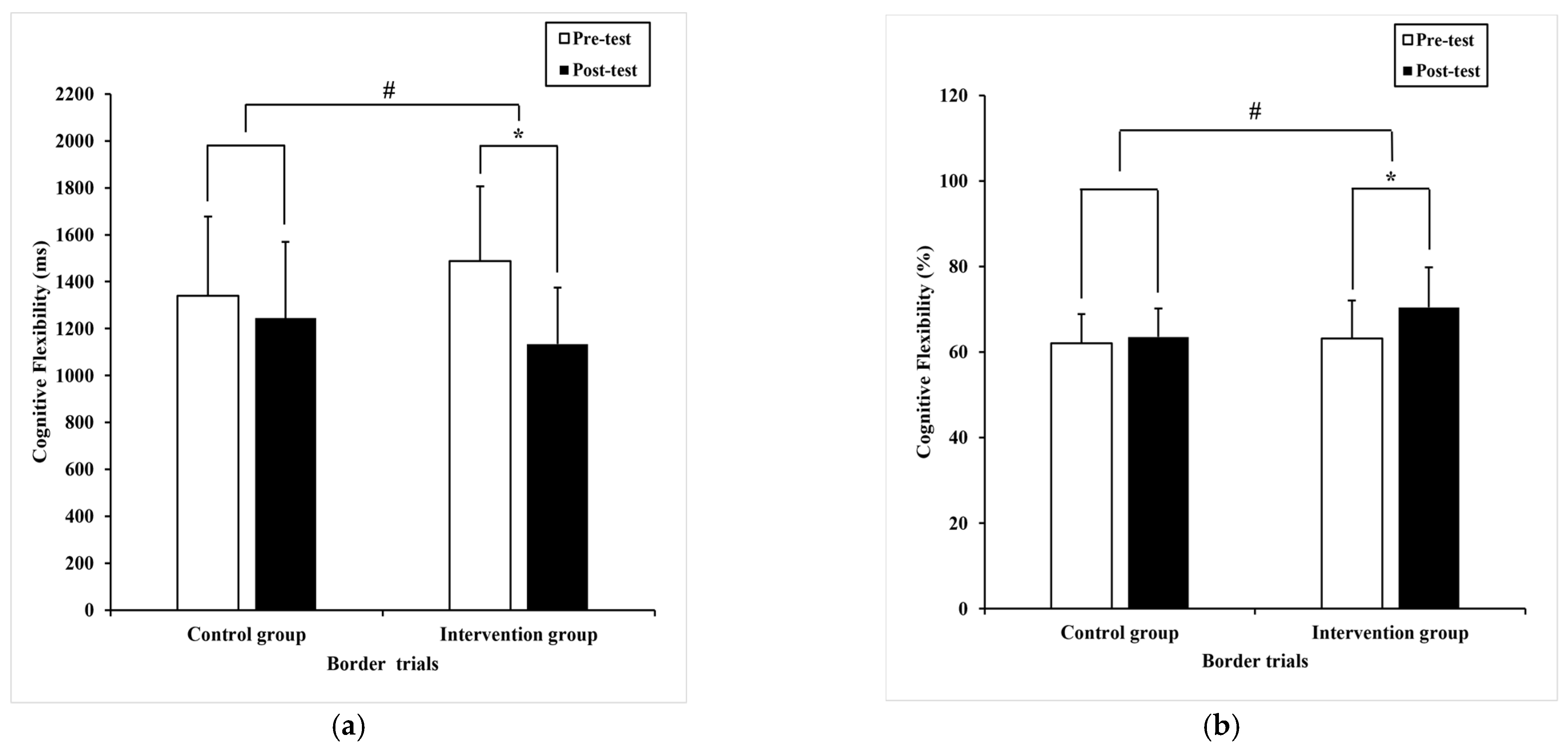
| Border trials | 1339.90 (336.90) | 1245.0 (324.87) 1269.39 a (45.00) | 1487.79 (319.05) | 1133.81 * (241.83) 1109.43 a (45.00) | 6.16 | 0.02 # | 0.08 |
| Accuracy (%) | |||||||
| Inhibition Control | |||||||
| Congruent trials | 88.9 (15.5) | 86.52 (15.47) 86.46 a (2.26) | 90.06 (12.32) | 93.19 (11.23) 93.26 a (2.26) | 4.53 | 0.04 # | 0.06 |
| Incongruent trials | 87.5 (12.1) | 87.29 (12.07) 87.34 a (1.82) | 86.56 (14.54) | 93.61 * (9.62) 93.57 a (1.82) | 5.85 | 0.02 # | 0.08 |
| Working Memory | |||||||
| 1-back trials | 83.7 (13.5) | 79.30 (15.18) 79.02 a (1.70) | 82.68 (12.87) | 85.98(8.97) 86.27 a (1.70) | 9.07 | 0.00 # | 0.12 |
| 2-back trials | 67.4 (14.0) | 62.41 * (14.03) 62.91 a (1.94) | 70.65 (9.24) | 75.71 * (9.28) 75.21 a (1.94) | 19.89 | 0.00 # | 0.22 |
| Cognitive flexibility | |||||||
| Color trials | 90.2 (20.0) | 95.5 (6.62) 95.49 a (1.29) | 91.35 (18.71) | 93.61 (8.69) 93.59 a (1.29) | 1.07 | 0.30 | 0.02 |
| Shape trials | 93.4 (8.5) | 90.4 (18.4) 90.4 a (3.0) | 92.29 (16.78) | 91.95 (17.85) 91.9 a (3.0) | 0.131 | 0.72 | 0.00 |
| Border trials | 62.1 (6.8) | 63.53 (6.66) 63.77 a (1.24) 59.6 a (1.0) | 63.19 (8.91) | 70.43 * (9.41) 70.19 a (1.24) 65.5 a (1.0)65.6 * (6.5) 65.5 a (1.0) | 13.40 | 0.00 # | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Taneepanichskul, S.; Zhang, B.; Xenos, P. The Effect of a Single Bout of Chinese Archery on Core Executive Functions in Preadolescent Children in Shanghai. Int. J. Environ. Res. Public Health 2023, 20, 1415. https://doi.org/10.3390/ijerph20021415
Liu J, Taneepanichskul S, Zhang B, Xenos P. The Effect of a Single Bout of Chinese Archery on Core Executive Functions in Preadolescent Children in Shanghai. International Journal of Environmental Research and Public Health. 2023; 20(2):1415. https://doi.org/10.3390/ijerph20021415
Chicago/Turabian StyleLiu, Jianjun, Surasak Taneepanichskul, Bo Zhang, and Peter Xenos. 2023. "The Effect of a Single Bout of Chinese Archery on Core Executive Functions in Preadolescent Children in Shanghai" International Journal of Environmental Research and Public Health 20, no. 2: 1415. https://doi.org/10.3390/ijerph20021415
APA StyleLiu, J., Taneepanichskul, S., Zhang, B., & Xenos, P. (2023). The Effect of a Single Bout of Chinese Archery on Core Executive Functions in Preadolescent Children in Shanghai. International Journal of Environmental Research and Public Health, 20(2), 1415. https://doi.org/10.3390/ijerph20021415






