Abstract
Background: This work aimed to perform a comprehensive investigation of organic Moroccan honeys obtained from plants of euphorbia, arbutus, and carob, based on the determination of physico-chemical profiles and volatile fingerprints. Methods: The selected analytical approach involved different techniques, including physico-chemical procedures for determination of humidity, acidity, diastase activity; solid-phase microextraction (SPME) coupled to GC-MS for aromatic fraction exploration; and ICP-MS for multi-element analysis. Results: The results obtained from the physico-chemical analyses were highly comparable to those of other commercial honeys. In 50% of samples investigated, the diastase number was just above the legal limit fixed by Honey Quality Standards. The analysis of the volatile fraction highlighted the presence of numerous compounds from the terpenoid group along with characteristic molecules such as furfural, isophorone, and derivatives. In most cases, VOCs were distinct markers of origin; in others, it was not possible to assess an exclusive source for bees to produce honey. Conclusion: The results contributed to place the three varieties of honey investigated among the commercial products available in the market. Many variables determined returned positive indications about quality and safety of these special honeys.
1. Introduction
According to the Codex Alimentarius, “honey is the natural sweet substance produced by honey bees from the nectar of plants or from secretions of living parts of plants or excretions of plant sucking insects on the living parts of plants” [1]. Honey is regarded as a natural sweetener, being composed of about 95% sugars and several other components, including proteins (mostly enzymes), minerals, phenolics, and organic acids. However, honey composition greatly depends on a varied list of factors, such as floral source, original raw material (nectar, honeydew, secretion), site of production, season, etc. Beyond its use as a food, honey has a long tradition in ethnomedicine and pharmacognosy [2]. Numerous are the ailments treated with honey or mixtures containing honey: wounds and ulcers, cardiovascular disease, microbial infections, and inflammation [2]. Ultimately, honey has also been demonstrated to inhibit in vitro proliferation of cancer cells [3]. Most part of its health-promoting effects have been attributed to the polyphenolic content and therefore to a significant antioxidant power [4]. Statistics show that the annual production volume of natural honey worldwide amounted to 1.77 million tons in 2020, with China as the leading producer [5]. From an overview of the works on honey published in the last ten years, it appears that the main focuses of research have been (i) composition; (ii) bioactivity (antioxidant and antimicrobial); (iii) definition of markers of origin and authenticity (i.e., phenolics, minerals, floral volatiles, sugars); (iv) determination of quality parameters (i.e., hydroxymethylfurfural, namely HMF, and diastase activity) [6,7,8]. The predominant techniques used for honey investigation include gas and liquid chromatography, spectroscopy, solid-phase microextraction (SPME), and physico-chemical procedures, all with the support of statistical analysis [9,10]. Generally, gas chromatographic analyses have been coupled to SPME as a sampling technique. The latter is a solventless sample preparation methodology that has been widely applied in the last years for the determination of volatiles released by a honey matrix [11,12,13,14]. Advantages of the technique include friendliness, rapidity, eco-sustainability, very low sample handling, analytical sensitivity and selectivity. On the other hand, SPME-GC-MS requires specific expertise both in the use of instrumental apparatus and in the interpretation of data. With respect to HPLC, it has been demonstrated to be a valid tool for the elucidation of phenolic fractions in honey [15,16]. For instance, mono- and multidimensional LC techniques have been shown to be powerful tools for the study of the phenolic profile of Serbian propolis [17], whereas another study investigated the stability of polyphenols in honey by means of LC-MS/MS [18]. Another tool for the analysis of polyphenols and other bioactives in honey is Fourier-Transform Infrared Spectroscopy (FTIR), a versatile, fast, and non-invasive technique that provides structural elucidation of honey constituents [19,20,21]. Nonetheless, the exploitation of advanced technologies for the chemical exploration of honey has been constantly supported by chemometrics, such as principal component analysis, cluster analysis, and linear and partial least squares discriminant analysis [22,23,24,25]. Investigations have been conducted toward the clarification of the chemical and biological properties of peculiar unifloral honeys, such as manuka, citrus, and eucalyptus, as well as honey from stingless bees [26,27,28,29]. The aim of the present work was to carry out a comprehensive investigation on some honeys from the Moroccan market obtained from plants of Euphorbia, strawberry tree, and carob. Besides the conventional physico-chemical parameters (i.e., acidity, humidity, refractive index, diastase), the volatile fingerprints were explored by means of SPME-GC-MS.
2. Materials and Methods
2.1. Samples
Six honey samples were investigated and are described in Table 1. Honeys were produced and sold by local cooperatives in Morocco, with the exception of one sample (#5) produced in France. All samples were declared “organic” by the producer and were kept at room temperature (20 °C) in a cool and dry place until analysis.

Table 1.
Description of the investigated honey samples.
2.2. Physico-Chemical Parameters
Each analysis was carried out in triplicate.
Refractive index, water content (humidity), and Total Soluble Solids (TSS) were determined by means of an Abbe refractometer, measuring the refractive index at 20 °C. Values of humidity were extrapolated from Wedmore’s formula [30]. Honey samples were homogenized by stirring thoroughly (3 min); in the case of crystallized honey, this was heated in a thermostatic bath at 40 °C. TSS is measured in Brix degrees (by switching the reader of the refractometer), and it basically indicates the quantity of sugars present in honey. The procedure for its determination was in accordance with international harmonized methods [31].
The electrical conductivity was measured on a 20% (DW) honey solution at 20 °C in accordance with a previously described methodology by means of a pH/conductivity meter (Eutech PC700, Thermo Fisher Scientific Inc., Waltham, MA, USA) [32]. Honey samples were prepared as reported above for humidity. Successively, 20 g (DW) of honey was weighed and diluted with water until reaching a 100 mL volume. An immersion conductivity cell was immersed in this solution, and the reading was registered. The same equipment was used for pH determination. The pHmeter (resolution 0.01 units) was immersed in a 133 ppm (mg/L) honey solution (reference buffer solutions at pH 4 and pH 9). Free, combined, and total acidity values were measured by the titrimetric method [33]. Free acidity (FA) was obtained by titrating honey (130 ppm solution) with 0.05 N sodium hydroxide solution to pH 8.5. Combined acidity (CA) was measured by adding to the honey solution 10 mL NaOH solution and backtitrating with 0.05 N hydrogen chloride to pH 8.3. Total acidity (TA) is regarded as the sum of free and combined acidities.
The diastatic activity was photometrically quantified using the Phadebas® Honey Diastase Test (Phadebas, Lund, Sweden) [34]. The method is based on the use of an insoluble substrate made of starch bearing a blue dye. The substrate is hydrolyzed by α-amylase, yielding blue particles that promptly solubilize in water. The blue dye is determined spectrophotometrically by setting the absorbance at a 620 nm wavelength. The value of absorbance is proportional to the diastatic activity, which is expressed as diastase number (DN). One DN corresponds to the amount of enzyme that converts 0.01 g of starch to the prescribed endpoint in one hour at 40 °C. For the expression of the results, the following equation was used:
where ΔA620 is the difference between sample absorbance and blank absorbance. If (1) gave values < 8, then the following equation was used:
DN = 28.2·ΔA620 + 2.64
DN = 35.2·ΔA620 − 0.46
2.3. Multi-Element Analysis
With the exception of mercury (Hg), all the elements were determined by means of a Thermo Scientific iCAP-Q ICP-MS system, equipped with an autosampler ASX520 (Cetac Technologies Inc., Omaha, NE, USA). Analyses were run in triplicate. Samples were preliminarily digested in a closed-vessel microwave digestion system (Ethos 1, Milestone, Italy). Stock standard solutions of all the target analytes were purchased from Fluka (Milan, Italy) and Thermo Scientific and used as internal standards for calibration (validation data available in Table S1).
2.3.1. Sample Preparation
An aliquot of 500 mg honey was accurately weighed into acid-washed vessels, added with 1 mL of 0.5 ppm Rhenium solution, and digested with 7 mL of 69% v/v HNO3 and 1 mL H2O2. The instrumental settings were 10 min at 1000 W up to 200 °C, and then held at 20 min. Afterwards, the extracts were filtered through 0.45 μm filters.
2.3.2. ICP-MS Conditions
The RF power was set at 1550 W; plasma gas flow rate was 14 L min−1; auxiliary gas flow rate was 0.8 L min−1; carrier gas flow rate was 1.1 L min−1; helium collision gas flow rate was 4.7 mL min−1; spray chamber temperature was 2.7 °C; sample depth was 5 mm; sample introduction flow rate was 0.93 mL min−1; nebulizer pump was 0.1 rps; extract lens was set at 1 voltage, 1.5 V.
Monitored isotopes were 7Li, 9Be, 11B, 23Na, 24Mg, 27Al, 39K, 48Ti, 51V, 52Cr, 55Mn, 56Fe, 59Co, 60Ni, 63Cu, 66Zn, 75As, 80Se, 88Sr, 98Mo, 107Ag, 114Cd, 121Sb, 138Ba, 205Tl, and 208Pb.
Integration times were 0.5 s/point for As, V, Se, and Fe; 0.01 s/point for Na, Mg, and K, and 0.1 s/point for other elements. All samples were analyzed in batches, with blank samples and known standards.
2.3.3. Analysis of Mercury
For the determination of Hg, a direct analyzer DMA-80 (Milestone s.r.l., Bergamo, Italy) was used according to the US EPA 7473 method [35]. About 0.1 g of each honey sample was put in a specific cuvette and submitted to a temperature increase from 60 °C to 650 °C in about 5–6 min, allowing for sample thermal decomposition, in oxygen or air atmosphere. Hg and other present species were then released and transported by a gas flow. The Hg was selectively trapped on a gold-containing amalgamator, whereas the decomposition fumes were fluxed away to avoid signal distresses. By heating the amalgamator, Hg was released and transferred to the lecture cell for its determination via atomic absorption spectroscopy at a 253.7 nm wavelength. Hg was finally calibrated by means of an equation built with Hg 1000 mg/L certified standard (CZECH Metrology Institute Analytika).
2.4. Volatile Fingerprint
2.4.1. SPME-GC Parameters
The flavor fingerprint of honey samples was determined by headspace solid-phase microextraction (HS-SPME) followed by gas chromatography (GC) coupled to FID and MS detection systems. The SPME fiber consisted of a Divinylbenzene/Carbon WR/Polydimethylsiloxane 80 μm coating (Agilent Technologies, Santa Clara, CA, USA). Following method optimization, 1.0 g of honey was put into a 10 mL headspace crimped vial, added with 3 mL water, and stirred. After an equilibration period for 10 min at 50 °C, the fiber was exposed to sample headspace for 20 min at 50 °C; during fiber exposure samples were stirred at a speed of 300 rpm. Then, the fiber was thermally desorbed into the GC injection port and set at a temperature of 250 °C for 5 min. GC-FID analyses were performed on a GC-2010 (Shimadzu, Milan, Italy) equipped with a Zebron-5 ms capillary column, 30 m × 0.25 mm ID × 0.25 μm df (Phenomenex, Torrance, CA, USA). The oven program temperature was from 50 °C (1 min) to 250 °C (held 1 min) at 4 °C·min−1, to 300 °C (held 10 min) at 10 °C·min−1. The injection port was equipped with a narrow inlet liner (0.75 mm ID, Agilent Technologies). Sample injection took place in splitless mode, with a 5 min sampling time, and then using split ratio 20:1. Carrier gas (He, 210.0 KPa, pressure control mode) was used at a linear velocity of 30 cm·s−1. An FID detector (300 °C) was used, and gas flows were 40 mL·min−1 for hydrogen and 400 mL·min−1 for air. Data handling was performed by means of GCsolution 2.32 software.
2.4.2. Mass Spectrometry
For mass spectrometric analysis, a GCMS-TQ8030 (Shimadzu, Kyoto, Japan) was used. The instrument was equipped with the same Zebron-5 ms capillary column and operated at the same experimental conditions as reported above. The MS set-up was as follows: ion source, 200 °C; interface temperature, 250 °C; electron multiplier voltage, 1.0 kV; mass range, 40–400 amu. For qualitative analysis, mass spectral databases were: FFNSC2 (Wiley), Adams 4th edition (Allured), and NIST11, each provided with Retention Index parameters, as an aid to identification. Experimental Retention Indices were measured by injecting an HS-SPME extract from a laboratory-made solution of n-paraffins ranging from n-hexane to n-hexadecane (concentration range: 5.0–50.0 ppm). Specifically, to avoid the SPME fiber oversaturation caused by lower boiling point paraffins, the solution was prepared by adding to a 25 mL volumetric flask, 0.125 mg/each of C6, C7, C8, and C9; 0.25 mg/each of C10, C11, and C12; 12.5 mg/each of C13, C14, and C15; and finally adding C16 as the main solvent until reaching volume.
3. Results and Discussion
3.1. Physico-Chemical Parameters
3.1.1. Humidity
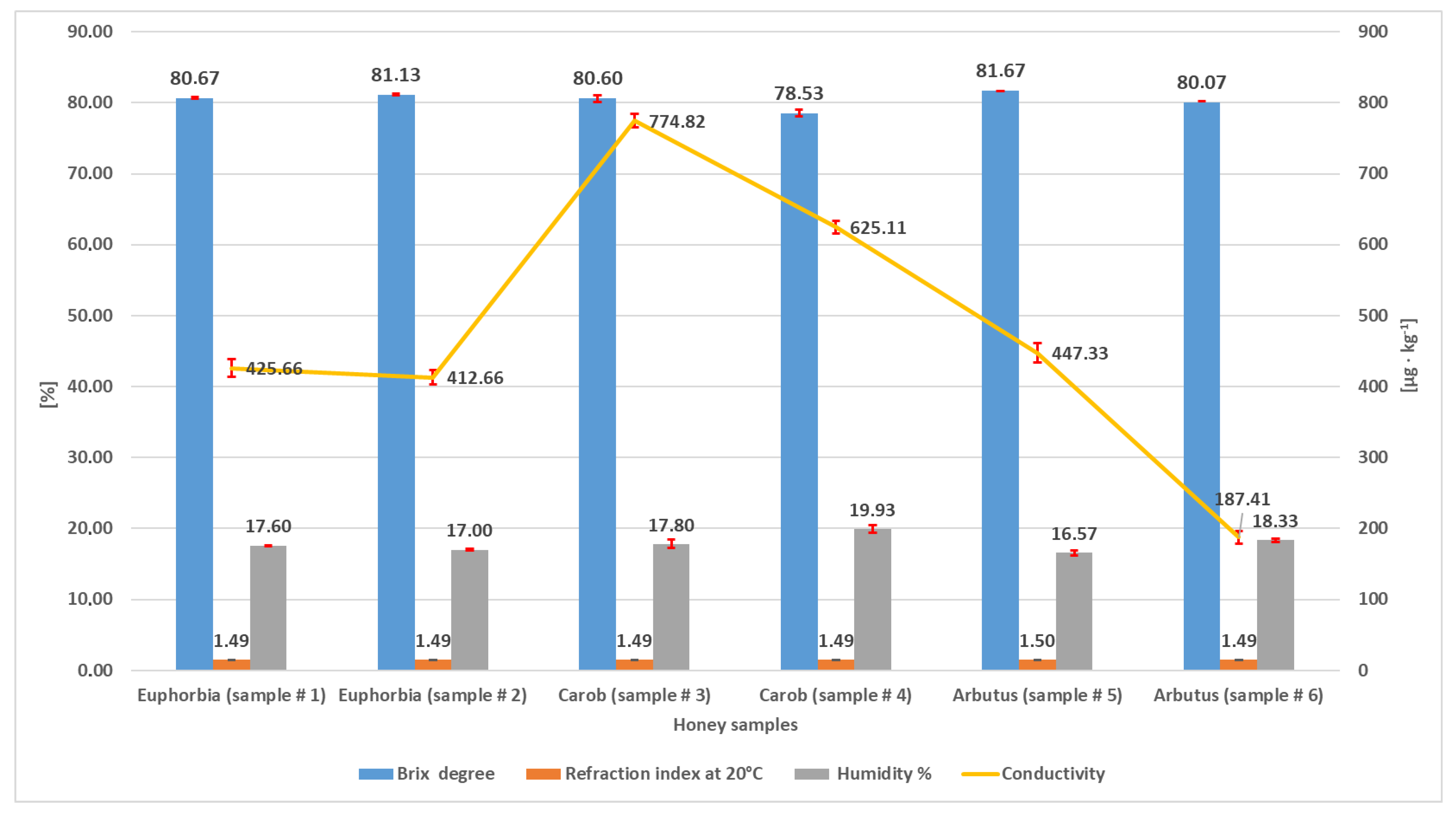
Relative humidity (or moisture) is an extremely important parameter to be assessed in honey analysis. It provides information on pedoclimatic conditions, soil characteristics, beekeepers’ manipulation, and post-harvest processing [36]. As can be seen from Figure 1, humidity ranged from 16.57% (sample #5, arbutus) to 19.93% (sample #4, carob), in compliance with the literature [37] and with Codex Alimentarius (humidity should be ≤20%) [1].
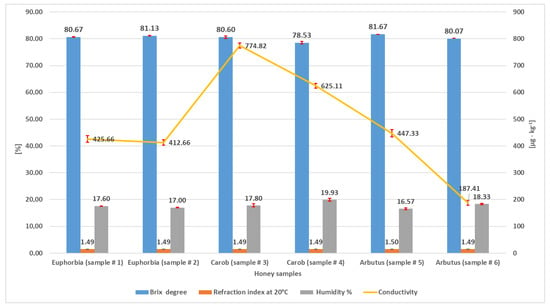
Figure 1.
Brix degrees, refractive index, humidity percentage, and conductivity measured in the three types of honey. Values are means of triplicate determinations.
3.1.2. Total Soluble Solids (TSS)
Moisture is reversely correlated to TSS, which is the expression of the content of sugars (predominantly) and minerals. Figure 1 shows that TSS values (°Brix) were in the range 78.53–81.67, with the minimum found in carob (sample #4) and the maximum in strawberry tree (sample #5). These values are in accordance with the literature for commercial honeys [38]. Generally, for values > 80 °Brix and <20% water, a honey is considered of high quality and displays a better stability during storage. According to this, the lowest quality sample in our set was carob honey (#4).
3.1.3. Refractive Index (RI)
The analysis carried out with the refractometer gave the same value for the whole set of samples, with only a slight fluctuation for sample #5 (1.49°/1.5°). These data are in agreement with those reported for authentic honeys [39].
3.1.4. Acidity and pH
Free acidity (FA) in honey is given by the presence of organic acids, such as tartaric, oxalic, and acetic. These acids are in a variable state of equilibrium between their free and combined form, with the latter represented by lactones. For this reason, another parameter, total acidity (TA), which takes into account both FA and combined acidity (CA), is generally measured, in order to neutralize FA and LA fluctuations. Table 2 reports the FA, CA, and TA values for honey samples. Euphorbia reported values much lower compared to other honeys of the same species produced in Morocco, namely 24 meq·kg−1 vs. 50 meq·kg−1 [37]. High accordance was found between the actual data and those reported in the literature for carob samples [40]. No previous data on acidity could be found for arbutus samples. The pH values are reported in Table 2. Although not yet regulated, this parameter expresses the ability of honey to fight microbial contamination. In fact, microorganisms generally need a neutral environment, while in honey the pH ranges between 3 and 5. As shown in Table 2, the pH range in our samples was 4.37–4.81; this finding is comparable to that published for Moroccan honeys [37].

Table 2.
Data obtained from the determination of acidity in the six honey samples.
3.1.5. Diastase
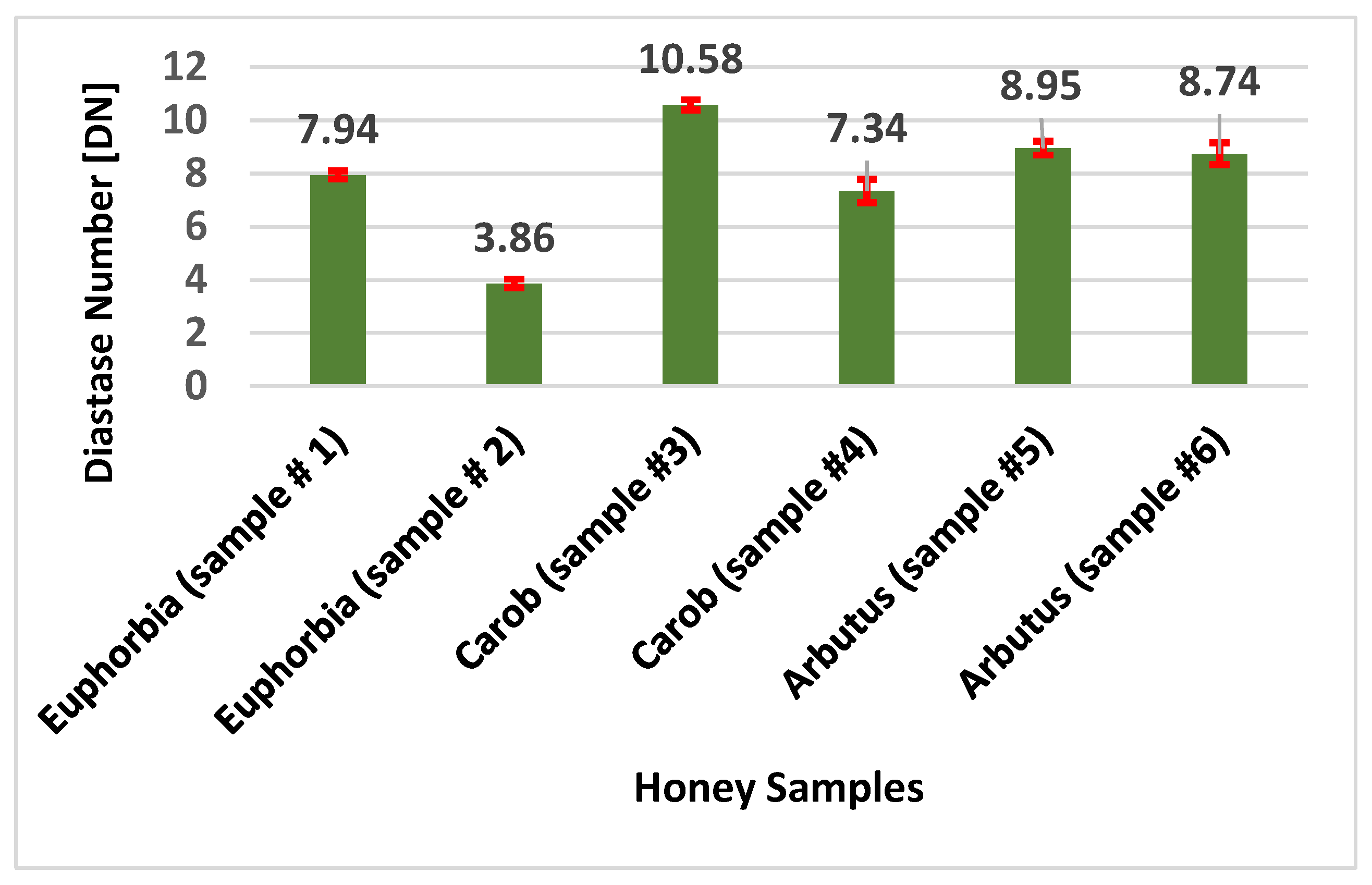
The diastase activity was evaluated for each honey sample investigated. In Figure 2, the results of experimental determinations are expressed as diastase number (DN), which is defined as the amount of enzyme that converts 0.01 g of starch to the prescribed end-point in one hour at 40 °C under the conditions of the test [41]. The honey samples analyzed showed DN values between 3.86 (Euphorbia #2) and 10.58 (Carob #3). Owing to its heat sensitivity, diastase (α and β amylase) may be considered as a valid indicator of honey quality; moreover, this enzyme enriches the nutraceutical function of honey [42]. Diastase is capable of breaking down glycosidic linkages in oligo and polysaccharides, i.e., starch into simple sugars. Diastase content is particularly influenced by storage conditions (including high temperature) and the decrystallization process. In fact, heating represents one of the crucial steps during commercial processing, because it prevents the undesirable crystallization, reduces moisture content, and eliminates the microorganisms responsible for fermentation and spoilage [43]. A low level of diastase is an indication of inappropriate heat treatment and fraudulent practices related to the use of industrial sugars, as in the case where honey bees are fed with glucose [44]. In 50% of honey samples investigated the DN values were above and below (sample #1) 8, which corresponds to the legal limit of DN fixed by Honey Quality and International Regulation Standards [45]. Values below this threshold, such as those found in both Euphorbia samples and Carob #4, may be related to human manipulation. A novel and interesting approach consists of the correlation between diastase activity and honey geobotanical origin [46]. Looking at the literature, the DN values measured in twelve carob honeys coming from Sicily were, on average, equal to 19.93 ± 2.81; this is significantly higher than the DN levels of this study [40]. Comparable values (on average 10.53 ± 0.81) were previously detected in several carob honeys coming from Morocco [47]. This comparison supports the hypothesis that diastase levels might be correlated to the geographical origin of honey. However, in the present work, DN values for Euphorbia samples lower than those reported for other Moroccan Euphorbia honeys (on average 12.67 ± 0.76 and 37.40 ± 1.51, respectively) were found [47,48].
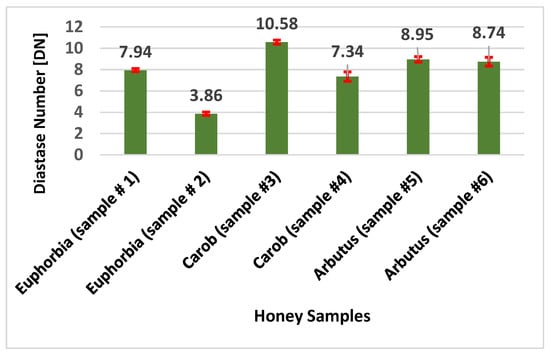
Figure 2.
Evaluation of diastase activity in honey samples.
3.2. Multi-Element Analysis
3.2.1. Macroelements
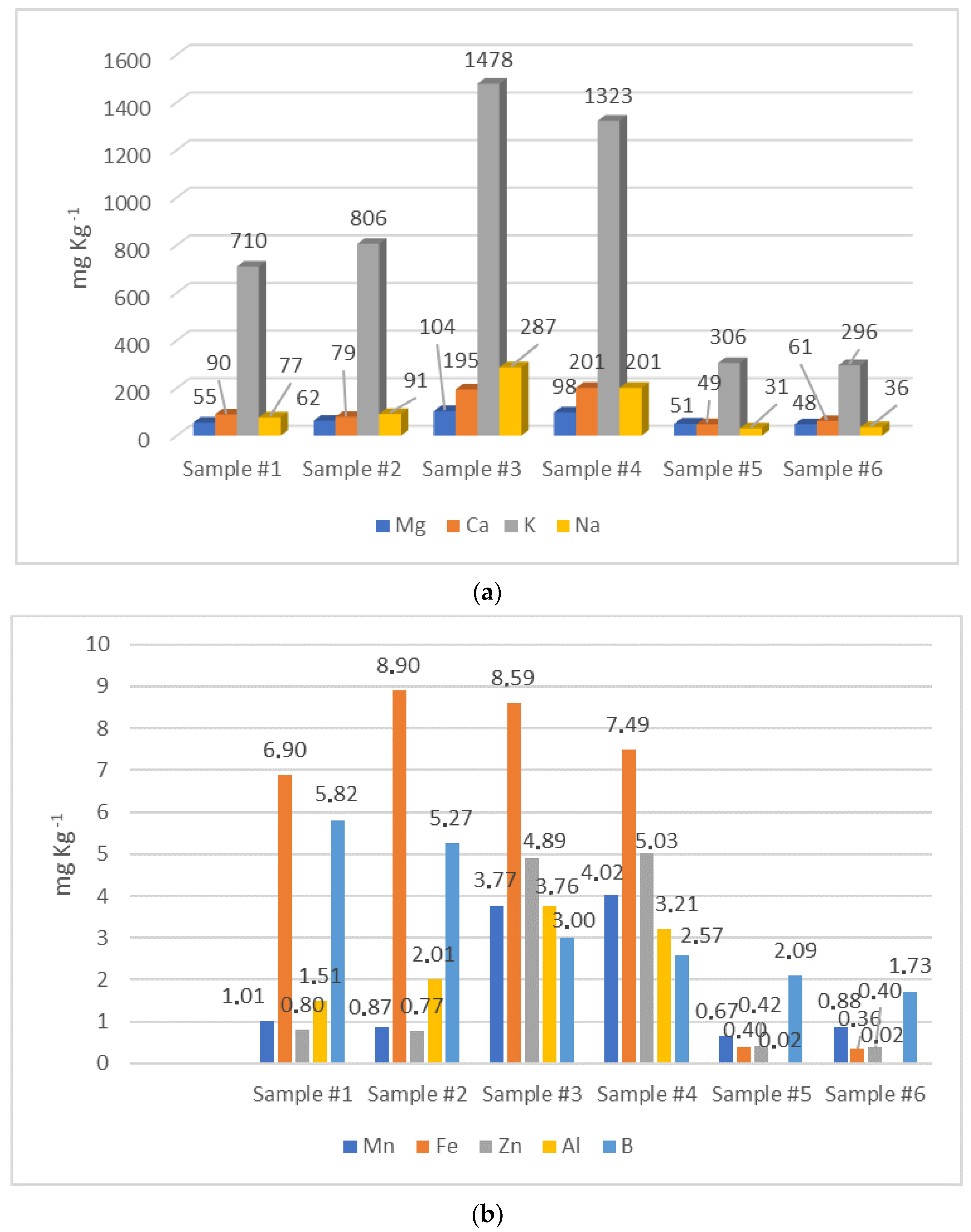
The mineral profile is significantly useful for the evaluation of the nutritional value of honey. Moreover it can be considered as a potential indicator of geographical origin as well as an important biomarker for environmental pollution with heavy metals [49,50]. The soil composition, the botanical origin, along with anthropogenic factors (beekeeping practices), environmental pollution, and honey processing, exert a significant influence on the mineral profiles of honey. In fact, the elements are translocated to plants and flowers through the root system, pass to the nectar, and then to the honey obtained from it [51]. In this work, the concentration of 21 elements was determined. The mean levels of macro (Na, Mg, Ca, and K) and micro (Al, Mn, Zn, B, and Fe) elements are represented in Figure 3a,b, respectively. The mean content of the remaining micro elements (Li, Ba, Ni, Se, Sb, Sr, Cr, Cu, Hg, As, Cd, and Pb) are reported in Table 3. The dominant element found in all honey samples was potassium. Both carob samples showed the highest levels of K, Na, Ca, and Mg, while both arbutus samples showed the lowest concentrations of the same elements. The macroelements determined in this work were also the most abundant as found in various unifloral honeys from Spain and Italy [52,53].
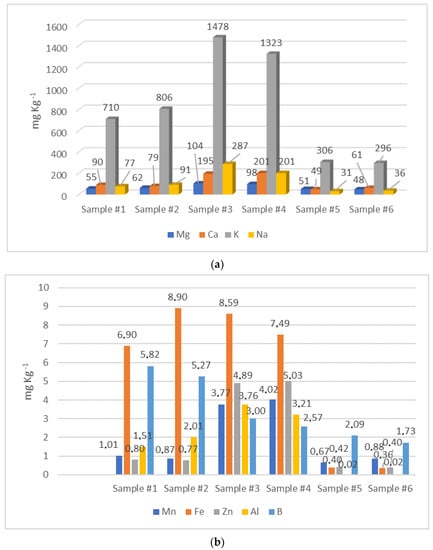
Figure 3.
Macro (a) and micro (b) elements determined in honey samples by means of ICP-MS.

Table 3.
Contents of microelements (expressed as mean ± standard deviation) in the investigated honey samples. Each sample was analyzed in triplicate (n = 3).
Comparable mean values of Ca (126.11 mg∙kg−1), K (1882.22 mg∙kg−1), and Mg (53.51 mg∙kg−1) were detected in several arbutus Croatian honeys [47]. Moroccan carob honey showed much higher concentrations of Na, which varied in the range of 367.52 to 855.24 mg∙kg−1, and a comparable level of K, with a wide variation ranging from 644.02 mg∙kg−1 to 1883.15 mg∙kg−1 [54]. In the same work, higher Ca values, ranging from 129.35 mg∙kg−1 to 688.43 mg∙kg−1, were detected [47]. Regarding Euphorbia honey, the mean levels of K (334.31 mg∙kg−1), Na (40.22 mg∙kg−1), and Mg (41.21 mg∙kg−1) in Moroccan honey were all lower than our estimation [48]. On the contrary, the average Ca content (117.91 mg∙kg−1) was higher than that found in the present work [48]. In all three varieties of honey investigated, the Mg concentrations exceeded the maximum limit set by the Codex Alimentarius: 25 mg∙kg−1 of Mg in honey [1]; it was also higher than that reported for multifloral honey from the Mediterranean area, probably due to the abundant presence of this element in the soil of North Africa [55].
3.2.2. Microelements
Quantitative Analysis
Microelements such as Fe, B, Mn, Zn, Al, Cu, Li, Ba, Se, Cr, and Ni are essential for a wide range of physiological processes and have certain nutritional benefits. However, these elements have a specific range of intake; excessive exposure may induce acute and chronic toxicity [56]. The concentrations of Fe, B, Zn, Mn, and Al were significantly higher in both carob samples, while they were very low in arbutus honey samples. Euphorbia samples were in the middle, with Fe and B levels comparable to those of carob honey, while Al, Mn and Zn levels were similar to those found in arbutus honeys. In all samples, Fe concentrations were below the maximum limit allowed in honey (15 mg∙kg−1) and fixed by the Codex Alimentarius [1]. In addition, lower amounts of Fe, Mn, Zn, and Al in both Euphorbia samples were found compared to the values reported in previous studies on the same variety of honey [48]. The contents of Fe and Zn in both carob samples were much higher than those measured elsewhere for Moroccan carob honey [54]. It is interesting to note that the carob samples investigated displayed significant levels of zinc. The latter is known to be involved in numerous metabolic pathways in humans by actively taking part in the proper functioning of the endocrine and exocrine pancreas, spermatogenesis, and testosterone metabolism [57]. Concerning arbutus honey, higher mean values of Al (2.23 mg∙kg−1), B (8.36 mg∙kg−1), Fe (2.84 mg∙kg−1), Mn (0.572 mg∙kg−1), and Zn (2.20 mg∙kg−1) were detected in the same type of honey but produced in Croatia [47]. All the three varieties of honey here investigated were a poor source of Se when compared to Turkish and Spanish honeys of different botanical origin, where a concentration of Se in a range from 0.020 to 0.927 mg∙kg−1 was assessed [58].
Toxic Elements
Hg, Pb, As, and Cd are regarded as potentially toxic elements. In particular, Pb, Cd, and Hg have been included in the European Regulation that sets maximum levels of certain contaminants in foodstuffs [59]. The European Union Directive 2014/63/EU does not mention contaminants such as potentially toxic elements. Only recently did the European Commission issue a Regulation that introduces the maximum admitted level for Pb content in honey, set at 100 μg∙kg−1 [60]. In this work, the levels of Hg and As were below the LOD in all samples. The contents of Pb were in a range between 0.023 mg∙kg−1 (Euphorbia sample #1) and 0.067 mg∙kg−1 (carob sample #4), while the concentration of Cd was 0.010 mg∙kg−1 (Euphorbia sample #1) and 0.030 mg∙kg−1 (carob sample #4).
Nutritional Value
A nutritional and risk estimation of honey consumption was made for the analyzed honeys, relying on the most recent EFSA data on Dietary Reference Values (DRV) for essential elements and on the Tolerable Intake (TI) and Benchmark Doses (BMD) for non-essential elements. Calculation was based on the daily average consumption data for the Moroccan population (14.1 g/day) [61]. The nutritional contribution of elements from the three varieties of honey investigated was, on average, low, with DRV ranging from 0.0001% for Zn, Fe, B, Mn, Cu, Se, and Sr, to 0.8% for K. The EFSA Panel on contaminants in the food chain proposed a set of non-essential (toxic or potentially toxic) element intake levels expressed as kg of body weight and defined as tolerable weekly intake (TWI), tolerable daily intake (TDI), or benchmark dose (BMD), which are considered safe or free of risk of adverse health effects [62,63]. Regarding the exposure assessment of non-essential elements, the contribution given by regular consumption of these three types of honey to the dietary intake is considered negligible. Therefore, the consumption of these varieties of honey can be considered safe for human health.
3.3. Volatiles Distribution
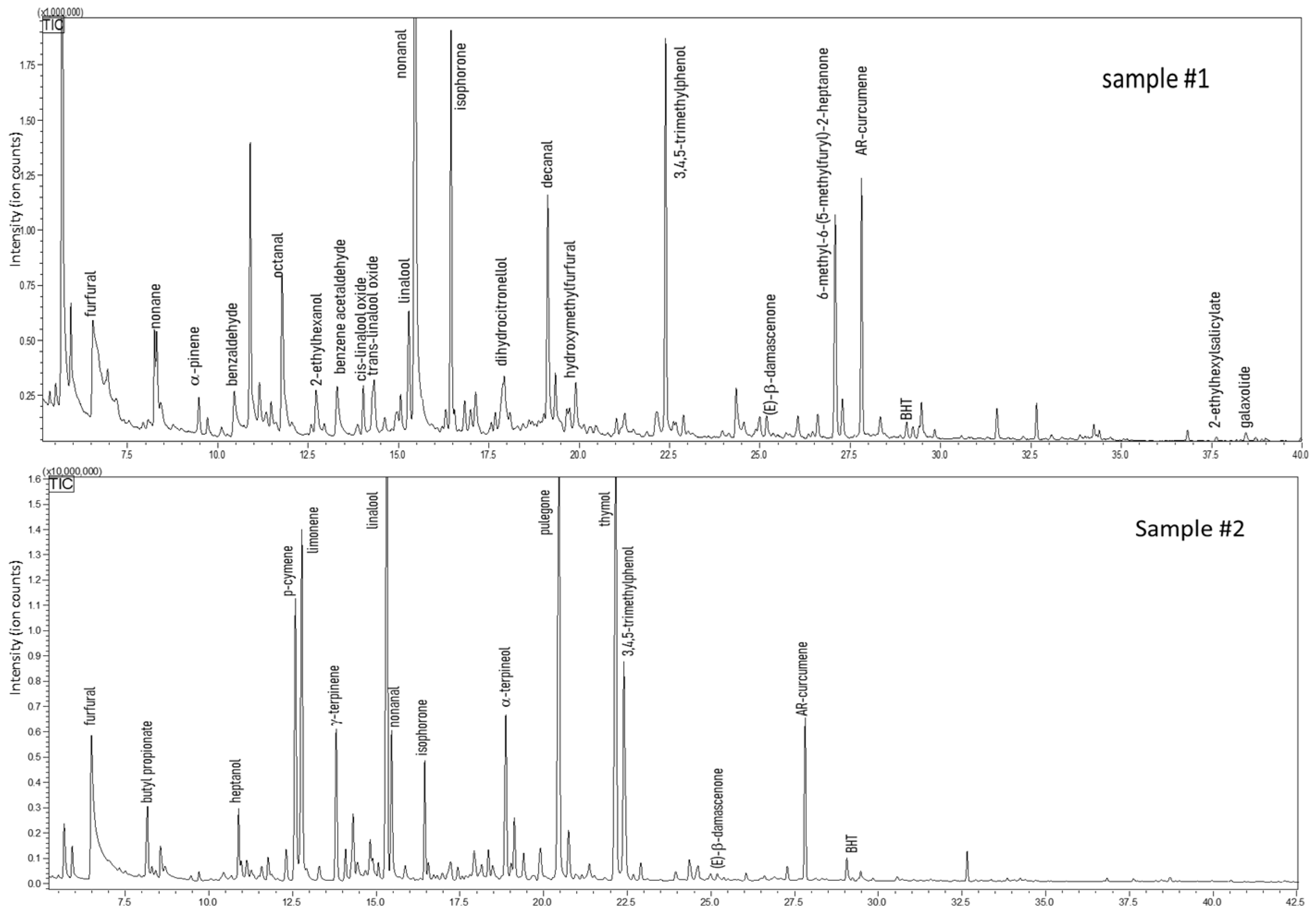
A great part of the metabolic pathways in food is affected by environmental conditions (e.g., temperature, humidity, and light exposure, among others). These biochemical reactions end up in the production of metabolites, part of which are volatile. For this reason, the analysis of the volatile fraction of honey is an important tool not only for descriptive purposes but also for obtaining information on its processing and manufacturing and hence its quality [64]. As an example, heat treatment triggers Maillard reactions, whose products are low boiling pyrazines and furans released into the headspace. SPME sample preparation techniques coupled with GC allowed the determination of a rich volatile profile for each honey sample. Figure 4 shows the GC fingerprints of Euphorbia honey, namely samples #1 and #2. As can be derived from Table 4, the two Euphorbia honeys share around 35% constituents. Within this fraction, worthy of mention are octane, nonanal, and decanal, which were quantified as 22%, 19.2%, and 9.8% in sample #1; furfural, p-cymene, and linalool, present at 6.1%, 9.2%, and 12.6% in sample #2. Although identified in both samples, these volatiles show remarkable differences in terms of quantity. In addition, numerous compounds were found only in one sample at a noticeable level. In particular, sample #2 reported a variety of terpenoids, such as limonene, p-cymene, α-terpinene, (Z)-β-ocimene, γ-terpinene, which provides a composition similar to a citrus essential oil. However, a comparison of the actual data with previous reports is not feasible, due to the lack of publications on Euphorbia honey analyzed by means of SPME-GC. Nonetheless, the presence in Euphorbia honey of some volatiles treated as honey markers must be emphasized. These are furfural, benzaldehyde, nonanal, isophorone, and decanal [64].
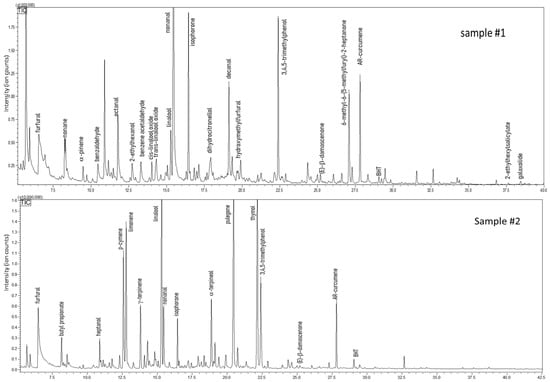
Figure 4.
GC-MS profiles of honey samples obtained from Euphorbiaceae plant family.

Table 4.
Volatile distribution determined in honey samples by means of HS-SPME-GC. Values are means of triplicate analyses and are expressed as raw area percentage ± standard deviation.
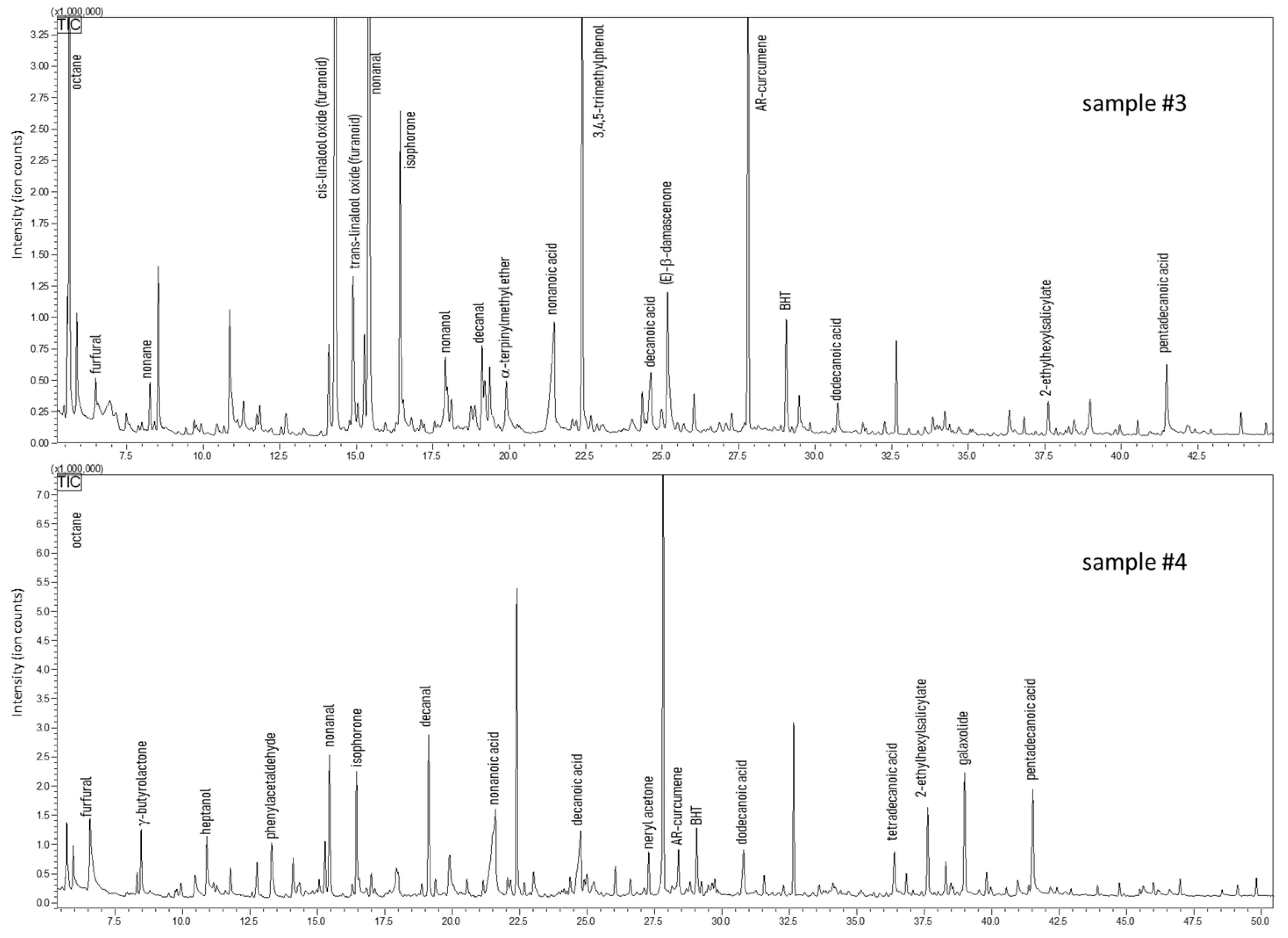
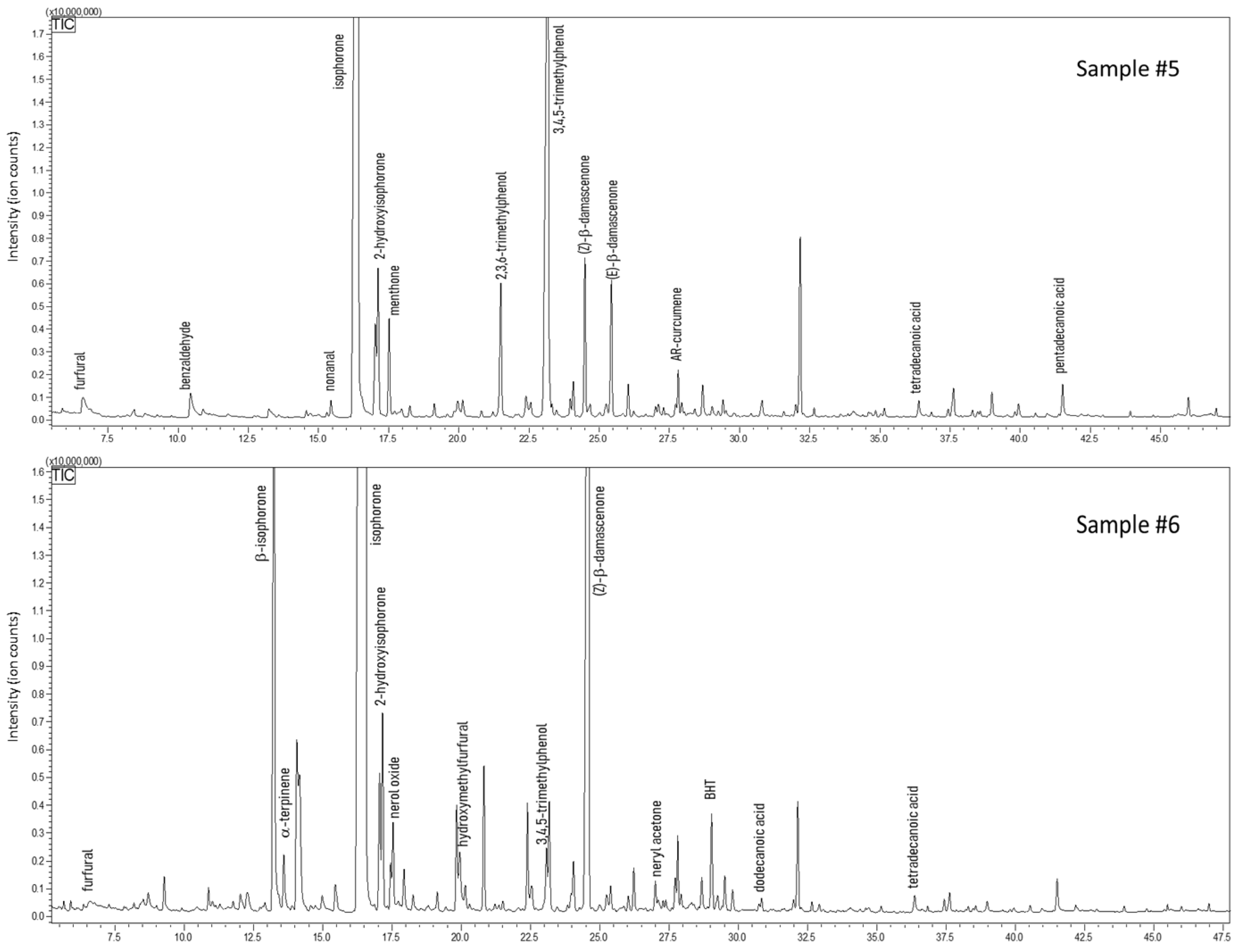
The analysis carried out on carob honey samples evidenced a 34% fraction of volatiles shared by samples #3 and #4, whose chromatograms are shown in Figure 5. The typical volatile markers of honey were determined also in this case in both carob samples, e.g., furfural, benzaldehyde, linalool, and isophorone. Conversely, specific compounds were present only in one sample, such as linalool oxides, methyl salicylate (a balsamic substance), hexanol, 2-ethylhexanol, and (E)-β-damascenone (sample #3); whereas γ-butyrolactone, terpenoids, hydroxymethylfurfural, and octanal were determined only in sample #4, which also reported a higher amount and a variety of aldehydes/acids (i.e., decanal). From a literature survey, only one paper could be found that focuses on the volatile composition of carob honey; less than twenty compounds of our study find confirmation [65]. For instance, furfural, benzaldehyde, phenylacetaldehyde, damascenone, and methyl anthranilate were major volatiles identified in both the works. However, a considerable number of volatile markers have not been reported, in particular terpenoids and their oxygenated derivatives [65]. This mismatch can be easily justified by the different technique used for volatile investigation, namely headspace analysis. This extraction procedure lacks sensitivity toward low concentrated molecules. About 36% was the fraction of common volatiles shared by the two samples of arbutus honeys, samples #5 and #6, whose chromatograms are shown in Figure 6. Compared to the other honey samples, arbutus showed a better quantitative matching when considering common couples of compounds. Only for 3,4,5-trimethylphenol (TMP) and (Z)-β-damascenone a remarkable difference was observed—6.95% vs. 0.30% for TMP, and 2.78% vs. 13.9% for (Z)-β-damascenone, in sample #3 and #4, respectively. Four volatiles were determined only in this type of honey, namely ethyl lactate, β-isophorone, and two monoterpenoids. Surprisingly, with the exception of sample #5, in all the other honey samples butylated hydroxytoluene (BHT) was detected. This phenol is categorized as a synthetic antioxidant additive and must be reported on the label when added to food and cosmetics [66]. However, in none of the honeys where it was found was BHT labelled, even though such honeys were declared as natural and pure. Hydroxymethylfurfural (HMF) is a product of Maillard reactions, generally found in honey after heat treatment or a long period of storage. HMF was detected at low levels in four samples, while a conspicuous amount was determined in carob honey (sample #4).
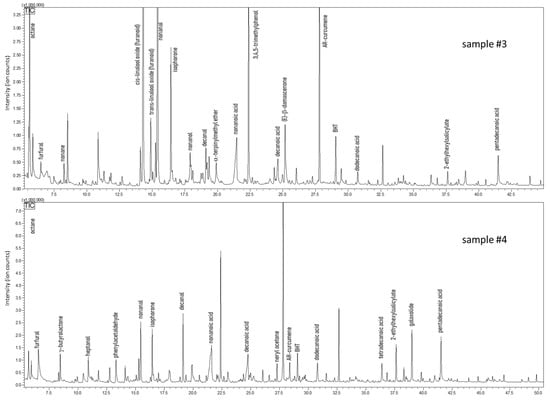
Figure 5.
GC-MS profiles of carob honeys (Ceratonia siliqua).
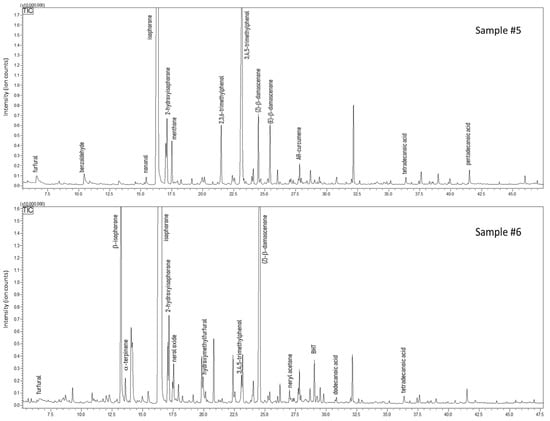
Figure 6.
GC-MS profiles of honey samples obtained from strawberry tree (Arbutus unedo).
The main conclusions that can be addressed from SPME-GC analysis are the following: in order to establish botanical/geographical markers, it is mandatory to analyze a statistically significant number of samples. In fact, as SPME is a sensitive technique, it was possible to establish the presence of very low concentrations of volatiles, as shown in previous studies [67,68,69]. This allowed emphasis of a remarkable variability of sources for the making of honey, despite what was labelled by the producer. In other words, the technique showed that bees do not suck nectar or honeydew strictly from one source.
4. Conclusions
The three varieties of Moroccan honey investigated revealed physico-chemical profiles and volatile fingerprints that place them within the commercial category. The physico-chemical parameters of the analyzed samples showed compatibility with the values reported in the Codex Alimentarius for genuine honeys. In particular, humidity ranged from 16.6% to 19.9%, while the inversely correlated TSS values ranged from 78.5° to 81.7° Brix. According to the grading system of the US Department of Agriculture, honeys with such values have better stability during storage [70]. With respect to diastase activity, four samples out of six showed DN values (mean value 8.67) above 8, the minimum threshold fixed by the Honey Quality and International Regulation Standard [45]. The monitoring of this parameter provided useful information on the quality of the product, in particular with regard to the storage and handling conditions of the product but also its geobotanical origin. Through multi-element analysis, a variety of micro- and macroelements were determined; in particular, Na (120.5 mg·kg−1), K (819.8 mg·kg−1), and Mg (69.6 mg·kg−1) displayed concentrations comparable to those of other commercial honeys from Morocco [51]. In terms of exposure to potentially toxic elements, the contribution given by regular consumption of these three types of honey to the dietary intake is negligible. Finally, for the first time the volatile fingerprint was assessed in the species investigated, highlighting a rich composition (total quantified fraction, from 88.72 ± 3.66% to 97.42 ± 2.24%) with characteristic presence of markers in many cases (i.e., furfural, isophorone, and damascenone). To this end, the SPME preconcentration technique was shown to be a suitable and sensitive technique. Significant differences were found in the volatile fraction of the same types of honey, suggesting the importance of further investigation on a wider collection of samples. Indeed, honey is a product of bee metabolism, and the techniques used for its sampling and analysis (SPME-GC-MS) are adequately sensitive to point out that, beyond the variability due to the animal origin, other factors must be taken into account, such as geographical origin, time of harvest, manufacturing procedures, and storage conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jeta1010001/s1. Table S1: Analytical parameters for method validation in multi-element analysis.
Author Contributions
Conceptualization, R.C.; methodology, R.M., V.N., C.C. and R.C.; software, R.M. and V.N.; validation, R.M., V.N. and R.C.; formal analysis, R.M., V.N., C.C. and R.C.; investigation, R.M. and R.C.; resources, R.M. and R.C.; data curation, R.M. and R.C.; writing—original draft preparation, R.V. and R.C.; writing—review and editing, R.V. and R.C.; visualization, N.C.; supervision, N.C.; project administration, N.C. and S.Z.; funding acquisition, N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors acknowledge the support of the Erasmus + programme of the European Union  .
.
 .
.Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO; WHO. Codex Alimentarius—International Food Standards; Standard for honey CXS 12-1981. Adopted in 1981, amended in 2019; FAO: Rome, Italy; WHO: Genewa, Switzerland, 2019. [Google Scholar]
- Alvarez-Suarez, J.; Giampieri, F.; Battino, M. Honey as a Source of Dietary Antioxidants: Structures, Bioavailability and Evidence of Protective Effects Against Human Chronic Diseases. CMC 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Muzi, M.G.; Canini, A. Acacia Honey and Chrysin Reduce Proliferation of Melanoma Cells through Alterations in Cell Cycle Progression. Int. J. Oncol. 2010, 37, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with High Levels of Antioxidants Can Provide Protection to Healthy Human Subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Statista. Available online: www.statista.com (accessed on 20 September 2022).
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on Their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, K.B.; Jovetić, M.S.; Tešić, Ž.L. Physicochemical Parameters as a Tool for the Assessment of Origin of Honey. J. AOAC Int. 2017, 100, 840–851. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vázquez, M. Honeydew Honeys: A Review on the Characterization and Authentication of Botanical and Geographical Origins. J. Agric. Food Chem. 2018, 66, 2523–2537. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.-U. Application of Analytical Methods in Authentication and Adulteration of Honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Mădaş, N.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Bonta, V.; Bobiş, O.; Fauconnier, M.L.; Francis, F.; Haubruge, E.; Nguyen, K.B. Volatile Profile and Physico-Chemical Analysis of Acacia Honey for Geographical Origin and Nutritional Value Determination. Foods 2019, 8, 445. [Google Scholar] [CrossRef]
- Piasenzotto, L.; Gracco, L.; Conte, L. Solid phase microextraction (SPME) applied to honey quality control. J. Sci. Food Agric. 2003, 83, 1037–1044. [Google Scholar] [CrossRef]
- Soria, A.C.; Sanz, J.; Martínez-Castro, I. SPME followed by GC–MS: A powerful technique for qualitative analysis of honey volatiles. Eur. Food Res. Technol. 2009, 228, 579–590. [Google Scholar] [CrossRef]
- Andrade, P.; Ferreres, F.; Amaral, M.T. Analysis of Honey Phenolic Acids by HPLC, Its Application to Honey Botanical Characterization. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 2281–2288. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC–LTQ/Orbitrap/MS/MS) Study of Phenolic Profile of Serbian Poplar Type Propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Biesaga, M.; Pyrzyńska, K. Stability of bioactive polyphenols from honey during different extraction methods. Food Chem. 2013, 136, 46–54. [Google Scholar] [CrossRef]
- Giordano, A.; Retamal, M.; Fuentes, E.; Ascar, L.; Velásquez, P.; Rodríguez, K.; Montenegro, G. Rapid Scanning of the Origin and Antioxidant Potential of Chilean Native Honey Through Infrared Spectroscopy and Chemometrics. Food Anal. Methods 2019, 12, 1511–1519. [Google Scholar] [CrossRef]
- Svecnjak, L.; Prđun, S.; Rogina, J.; Bubalo, D.; Jerkovic, I. Characterization of Satsuma mandarin (Citrus unshiu Marc.) nectar-to-honey transformation pathway using FTIR-ATR spectroscopy. Food Chem. 2017, 232, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Bunaciu, A.A.; Aboul-Enein, H.Y. Honey Discrimination Using Fourier Transform-Infrared Spectroscopy. Chemistry 2022, 4, 848–854. [Google Scholar] [CrossRef]
- López, B.; Latorre, M.J.; Fernández, M.I.; García, M.A.; García, S.; Herreroa, C. Chemometric classification of honeys according to their type based on quality control data. Food Chem. 1996, 55, 281–287. [Google Scholar] [CrossRef]
- Anguebes-Franseschi, F.; Abatal, M.; Pat, L.; Flores, A.; Córdova Quiroz, A.V.; Ramírez-Elias, M.A.; San Pedro, L.; May Tzuc, O.; Bassam, A. Raman Spectroscopy and Chemometric Modeling to Predict Physical-Chemical Honey Properties from Campeche, Mexico. Molecules 2019, 24, 4091. [Google Scholar] [CrossRef]
- Yücel, Y.; Sultanoğlu, P. Characterization of Hatay honeys according to their multi-element analysis using ICP-OES combined with chemometrics. Food Chem. 2013, 140, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Antonova, O.; Calvo, J.; Seifert, A. Rapid Detection of Thermal Treatment of Honey by Chemometrics-Assisted FTIR Spectroscopy. Foods 2021, 10, 2892. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Biluca, F.C.; Braghini, F.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Phenolic Composition and Biological Activities of Stingless Bee Honey: An Overview Based on Its Aglycone and Glycoside Compounds. Food Res. Int. 2021, 147, 110553. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality, Composition and Health-Protective Properties of Citrus Honey: A Review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef] [PubMed]
- Bobis, O.; Moise, A.R.; Ballesteros, I.; Reyes, E.S.; Durán, S.S.; Sánchez-Sánchez, J.; Cruz-Quintana, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Eucalyptus Honey: Quality Parameters, Chemical Composition and Health-Promoting Properties. Food Chem. 2020, 325, 126870. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.; Gasparrini, M.; Forbes-Hernández, T.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Wedmore, E.B. The Accurate Determination of the Water Content of Honeys: Part I. Introduction and Results. Bee World 1955, 36, 197–206. [Google Scholar] [CrossRef]
- Harmonized Methods of the International Honey Commission. Available online: www.ihc-platform.net/ihcmethods2009.pdf (accessed on 2 December 2022).
- Louveaux, J.; Pourtallier, M.P.; Vorwohl, G. Méthodes d’analyses des miels. Conductivité. Bull. Apic. 1973, 16, 1–3. [Google Scholar]
- AOAC. Official Methods of Analysis, Acidity of Honey; AOAC: Rockwille, MD, USA, 1990; Volume 962, p. 1033. [Google Scholar]
- Phadebas. Available online: https://www.phadebas.com/products/honey-diastase-test-products/ (accessed on 2 December 2022).
- U.S. EPA. Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry; U.S. EPA: Washington, DC, USA, 1998.
- Machado De-Melo, A.A.; de Almeida-Muradian, L.B.; Sancho, M.T.; Pascual-Maté, A. Composition and Properties of Apis Mellifera Honey: A Review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Bettar, I.; González-Miret, M.L.; Hernanz, D.; Marconi, A.; Heredia, F.J.; Terrab, A. Characterisation of Moroccan Spurge (Euphorbia) Honeys by Their Physicochemical Characteristics, Mineral Contents and Colour. Arab. J. Chem. 2019, 12, 2052–2060. [Google Scholar] [CrossRef]
- Shafiiq Kinoo, M.; Fawzi Mahomoodally, M.; Puchooa, D. Anti-Microbial and Physico-Chemical Properties of Processed and Raw Honeys of Mauritius. Adv. Infect. Dis. 2012, 2, 25–36. [Google Scholar] [CrossRef]
- Nikolova, K.; Panchev, I.; Sainov, S.; Gentscheva, G.; Ivanova, E. Selected Physical Properties of Lime Bee Honey in Order to Discriminate between Pure Honey and Honey Adulterated with Glucose. Int. J. Food Prop. 2012, 15, 1358–1368. [Google Scholar] [CrossRef]
- Ferrauto, G.; Pavone, P. Palynological, Physico-Chemical and Organoleptic Characteristics of Carob Tree (Ceratonia siliqua L.) Honey from Sicily. Int. J. Food Sci. Technol. 2013, 48, 1596–1602. [Google Scholar] [CrossRef]
- Schade, J.E.; Marsh, G.L.; Eckert, J.E. Diastase activity and hydroxy-methyl-furfural in honey and their usefulness in detecting heat alteration. J. Food Sci. 1958, 23, 446–463. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis-Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Singh, I.; Singh, S. Honey Moisture Reduction and Its Quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Gürbüz, S.; Çakıcı, N.; Mehmetoğlu, S.; Atmaca, H.; Demir, T.; Arıgül Apan, M.; Atmaca, Ö.F.; Güney, F. Physicochemical Quality Characteristics of Southeastern Anatolia Honey, Turkey. Int. J. Anal. Chem. 2020, 2020, 8810029. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R.; et al. Honey Quality and International Regulatory Standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Bicudo de Almeida-Muradian, L.; Monika Barth, O.; Dietemann, V.; Eyer, M.; Freitas, A.D.S.D.; Martel, A.-C.; Marcazzan, G.L.; Marchese, C.M.; Mucignat-Caretta, C.; Pascual-Maté, A.; et al. Standard Methods for Apis mellifera Honey Research. J. Apic. Res. 2020, 59, 1–62. [Google Scholar] [CrossRef]
- Lovaković, B.T.; Lazarus, M.; Brčić Karačonji, I.; Jurica, K.; Živković Semren, T.; Lušić, D.; Brajenović, N.; Pelaić, Z.; Pizent, A. Multi-Elemental Composition and Antioxidant Properties of Strawberry Tree (Arbutus unedo L.) Honey from the Coastal Region of Croatia: Risk-Benefit Analysis. J. Trace Elem. Med. Biol. 2018, 45, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Boutoub, O.; El-Guendouz, S.; Estevinho, L.M.; Paula, V.B.; Aazza, S.; El Ghadraoui, L.; Rodrigues, B.; Raposo, S.; Carlier, J.; Costa, M.C.; et al. Antioxidant Activity and Enzyme Inhibitory Potential of Euphorbia Resinifera and E. Officinarum Honeys from Morocco and Plant Aqueous Extracts. Environ. Sci. Pollut. Res. 2021, 28, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Cicero, N.; Gervasi, T.; Durazzo, A.; Lucarini, M.; Macrì, A.; Nava, V.; Giarratana, F.; Tardugno, R.; Vadalà, R.; Santini, A. Mineral and Microbiological Analysis of Spices and Aromatic Herbs. Foods 2022, 11, 548. [Google Scholar] [CrossRef]
- Mottese, A.F.; Naccari, C.; Vadalà, R.; Bua, G.D.; Bartolomeo, G.; Rando, R.; Cicero, N.; Dugo, G. Traceability of Opuntia ficus-indica L. Miller by ICP-MS multi-element profile and chemometric approach. J. Sci. Food Agric. 2018, 98, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef]
- Fernandeztorres, R.; Perezbernal, J.; Bellolopez, M.; Callejonmochon, M.; Jimenezsanchez, J.; Guiraumperez, A. Mineral Content and Botanical Origin of Spanish Honeys. Talanta 2005, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and Trace Elements in Different Honey Types Produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Kriaa, W.; Lyoussi, B.; Makni, M. Ceratonia siliqua Honeys from Morocco: Physicochemical Properties, Mineral Contents, and Antioxidant Activities. J. Food Drug Anal. 2018, 26, 67–73. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Badeka, A.; Papastephanou, C.; Kontominas, M.G. Nutritional Aspects and Botanical Origin Recognition of Mediterranean Honeys Based on the “mineral Imprint’’ with the Application of Supervised and Non-Supervised Statistical Techniques. Eur. Food Res. Technol. 2019, 245, 1939–1949. [Google Scholar] [CrossRef]
- Tariba, B. Metals in Wine—Impact on Wine Quality and Health Outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Hosseinzadeh Colagar, A. Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar] [PubMed]
- Yücel, Y.; Sultanoğlu, P. Determination of Industrial Pollution Effects on Citrus Honeys with Chemometric Approach. Food Chem. 2012, 135, 170–178. [Google Scholar] [CrossRef]
- FAOSTAT. Production Database from the Food and Agriculture Organization of the United Nations. 2019. Available online: http://www.fao.org/faostat (accessed on 19 August 2022).
- European Commission. 2015/1005 amending Regulation (EC) no 1881/2006 as regards maximum levels of lead in certain foodstuffs. Off. J. Eur. Union 2015, 58, 9–14. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2014/63/EU of the European Parliament and of the Council of 15 May 2014 amending Council Directive 2001/110/EC relating to honey. Off. J. Eur. Union 2014, L164, 1–5. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on the risks to public health related to the presence of arsenic in food. EFSA J. 2009, 7, 1351. [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on lead in food. EFSA J. 2010, 8, 1570. [Google Scholar]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.K.; Kaparakou, E.; Kanakis, C.; Pappas, C.; Tarantilis, P. The Use of SPME-GC-MS IR and Raman Techniques for Botanical and Geographical Authentication and Detection of Adulteration of Honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef]
- Ozcan-Sinir, G.; Copur, O.U.; Barringer, S.A. Botanical and Geographical Origin of Turkish Honeys by Selected-ion Flo-tube Mass Spectrometry and Chemometrics. J. Sci. Food Agric. 2020, 100, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- European Commission Regulation (EC). No. 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union 2008, L354, 16–33. [Google Scholar]
- Costa, R.; Mondello, L. Methods in Flavor and Fragrance Analysis. In Kirk-Othmer Encyclopedia; John Wiley & sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Costa, R.; De Grazia, S.; Grasso, E.; Trozzi, A. Headspace-Solid-Phase Microextraction-Gas Chromatography as Analytical Methodology for the Determination of Volatiles in Wild Mushrooms and Evaluation of Modifications Occurring during Storage. J. Anal. Methods Chem. 2015, 2015, 951748. [Google Scholar] [CrossRef]
- Tardugno, R.; Cicero, N.; Costa, R.; Nava, V.; Vadalà, R. Exploring Lignans, a Class of Health Promoting Compounds, in a Variety of Edible Oils from Brazil. Foods 2022, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- USDA. Extracted Honey Grading Manual; Standards for Honey Grading; USDA: Washington, DC, USA, 1985. Available online: https://www.ams.usda.gov/sites/default/files/media/Extracted_Honey_Inspection_Instructions%5B1%5D.pdf (accessed on 15 December 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).