State-of-the-Art on the Impact of Bimodal Acoustic Stimulation on Speech Perception in Noise in Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria and Eligibility
2.2. Search Strategy
2.3. Study Selection and Data Extraction
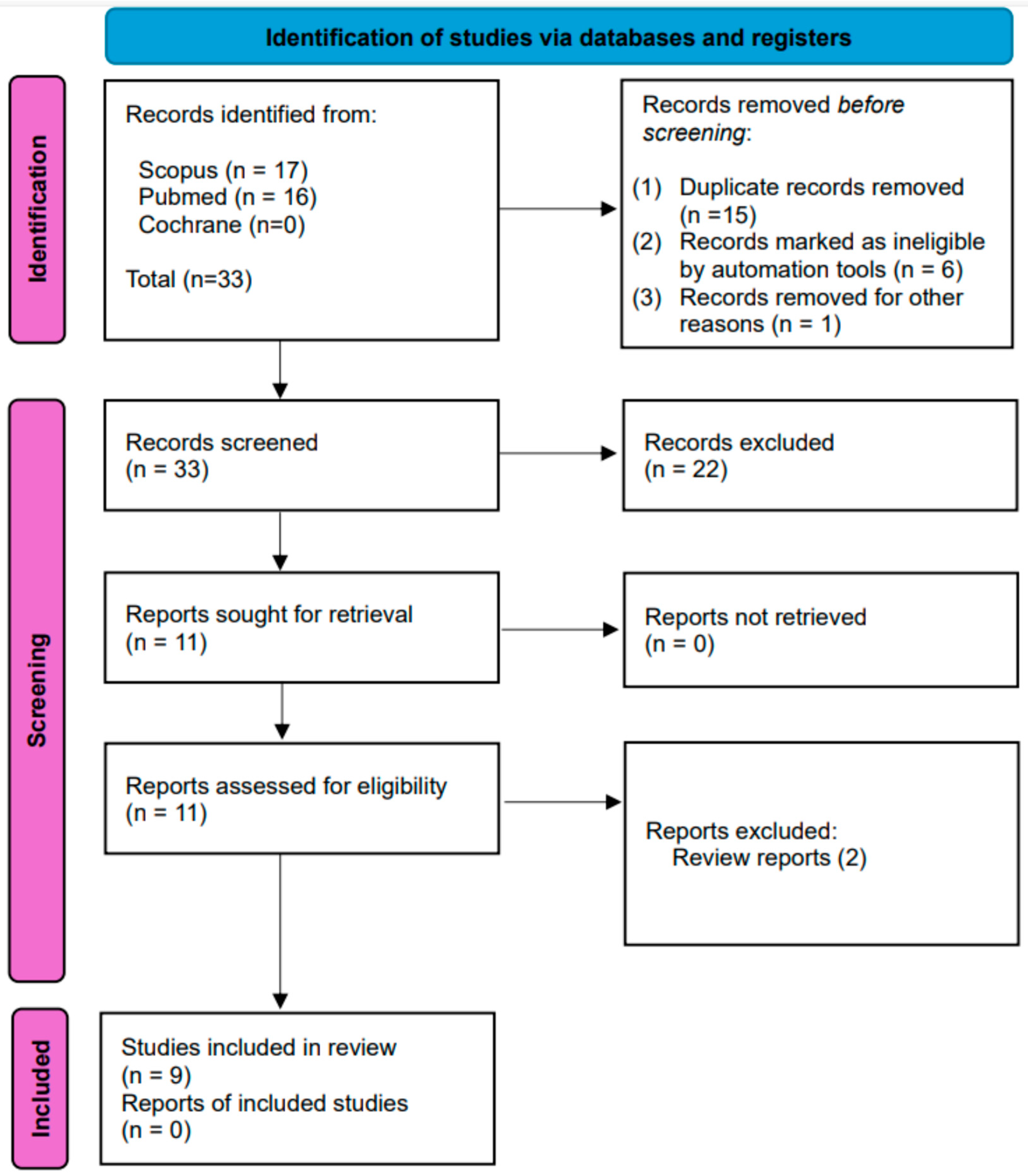
3. Results
The Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, S.; Sahwan, M.; Metryka, A.; Kluk, K.; Bruce, I.A. The benefits of preserving residual hearing following cochlear implantation: A systematic review. Int. J. Audiol. 2021, 60, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.T.M.; Carvalho, A.; Tsuji, R.K.; Bento, R.F.; Goffi-Gomez, M.V.S. Balancing the Loudness in Speech Processors and Contralateral Hearing Aids in Users of Unilateral Cochlear Implants. Int. Arch. Otorhinolaryngol. 2021, 25, e235–e241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angermeier, J.; Hemmert, W.; Zirn, S. Clinical Feasibility and Familiarization Effects of Device Delay Mismatch Compensation in Bimodal CI/HA Users. Trends Hear. 2023, 27, 23312165231171987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Y.; Gifford, R.H. Objective measure of binaural processing: Acoustic change complex in response to interaural phase differences. Hear. Res. 2024, 448, 109020. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, M.; Taşdemir, İ.; Çiprut, A. Listening Effort in Prelingual Cochlear Implant Recipients: Effects of Spectral and Temporal Auditory Processing and Contralateral Acoustic Hearing. Otol. Neurotol. 2022, 43, e1077–e1084. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.H.; Sunderhaus, L.W.; Dawant, B.M.; Labadie, R.F.; Noble, J.H. Cochlear implant spectral bandwidth for optimizing electric and acoustic stimulation (EAS). Hear. Res. 2022, 426, 108584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mertens, G.; Andries, E.; Clement, C.; Cochet, E.; Hofkens-Van den Brandt, A.; Jacquemin, L.; Joossen, I.; Vermeersch, H.; Lammers, M.J.; Van Rompaey, V.; et al. Contralateral hearing aid use in adult cochlear implant recipients: Retrospective analysis of auditory outcomes. Int. J. Audiol. 2024, 63, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Whitaker, G.; Lee, Y.S. Effects of the Configuration of Hearing Loss on Consonant Perception between Simulated Bimodal and Electric Acoustic Stimulation Hearing. J. Am. Acad. Audiol. 2021, 32, 521–527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, Y.S.; Straw, S. Interactions Between Slopes of Residual Hearing and Frequency Maps in Simulated Bimodal and Electric-Acoustic Stimulation Hearing. J. Speech Lang. Hear. Res. 2024, 67, 282–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dourado, R.P.B.; Caldas, F.F.; Cardoso, C.C.; Santos, D.C.D.; Bahmad, F., Jr. Benefits of Bimodal Stimulation to Speech Perception in Noise and Silence. Int. Arch. Otorhinolaryngol. 2023, 27, e645–e653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelsall, D.; Lupo, J.; Biever, A. Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study. Am. J. Otolaryngol. 2021, 42, 102773. [Google Scholar] [CrossRef] [PubMed]
- Stronks, H.C.; Briaire, J.J.; Frijns, J.H.M. The Temporal Fine Structure of Background Noise Determines the Benefit of Bimodal Hearing for Recognizing Speech. J. Assoc. Res. Otolaryngol. 2020, 21, 527–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancini, P.; Dincer D’Alessandro, H.; Portanova, G.; Atturo, F.; Russo, F.Y.; Greco, A.; de Vincentiis, M.; Giallini, I.; De Seta, D. Bimodal cochlear implantation in elderly patients. Int. J. Audiol. 2021, 60, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid. Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Au, A.; Dowell, R.C. An overview of factors affecting bimodal and electric-acoustic stimulation (EAS) speech understanding outcomes. Hear. Res. 2023, 431, 108736. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, B.M.; Schuchman, G.; Bernstein, J.G. Trimodal speech perception: How residual acoustic hearing supplements cochlear-implant consonant recognition in the presence of visual cues. Ear Hear. 2015, 36, e99–e112. [Google Scholar] [CrossRef] [PubMed]
- Zirn, S.; Hemmert, W.; Roth, S.; Müller, F.U.; Angermeier, J. Unterschiedliche Stimulationszeitpunkte bei bimodaler Versorgung mit Hörgerät und Cochleaimplantat: Übersichtsartikel zu Quantifizierung und Kompensation Interaural stimulation timing mismatch in listeners provided with a cochlear implant and a hearing aid: A review focusing on quantification and compensation. HNO 2023, 71, 513–520. (In Germany)Erratum in: HNO 2023, 71, 521 [Google Scholar] [CrossRef] [PubMed]
- Ciorba, A.; Guidi, M.P.; Skarżyński, P.H.; Bianchini, C.; Rosignoli, M.; Mazzoli, M.; Pelucchi, S.; Hatzopoulos, S. Rehabilitation of Severe to Profound Sensorineural Hearing Loss in Adults: Audiological Outcomes. Ear Nose Throat J. 2021, 100 (Suppl. 3), 215S–219S. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.; Jürgens, T.; Williges, B.; Kollmeier, B.; Wiebe, K.; Galindo, J.; Wesarg, T. Speech Intelligibility and Spatial Release From Masking Improvements Using Spatial Noise Reduction Algorithms in Bimodal Cochlear Implant Users. Trends Hear. 2021, 25, 23312165211005931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sampathkumar, R.; Kaehne, A.; Kumar, N.; Kameswaran, M.; Irving, R. Systematic review of cochlear implantation in adults with asymmetrical hearing loss. Cochlear Implant. Int. 2021, 22, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.H.; Dorman, M.F. Bimodal Hearing or Bilateral Cochlear Implants? Ask the Patient. Ear Hear. 2019, 40, 501–516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duret, S.; Bigand, E.; Guigou, C.; Marty, N.; Lalitte, P.; Bozorg Grayeli, A. Participation of Acoustic and Electric Hearing in Perceiving Musical Sounds. Front. Neurosci. 2021, 15, 558421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Firszt, J.B.; Reeder, R.M.; Holden, L.K.; Dwyer, N.Y. Asymmetric Hearing Study Team. Results in Adult Cochlear Implant Recipients With Varied Asymmetric Hearing: A Prospective Longitudinal Study of Speech Recognition, Localization, and Participant Report. Ear Hear. 2018, 39, 845–862. [Google Scholar] [CrossRef] [PubMed]
- Firszt, J.B.; Holden, L.K.; Dwyer, N.Y.; Reeder, R.M.; Strube, M.J. Asymmetric Hearing Study Team. Asymmetric Hearing Loss in Adult Cochlear Implant Recipients: Results and Recommendations From a Multisite Prospective Clinical Trial. Ear Hear. 2023, 44, 1140–1156. [Google Scholar] [CrossRef]
- Thompson, N.J.; Dillon, M.T.; Buss, E.; Rooth, M.A.; King, E.R.; Bucker, A.L.; McCarthy, S.A.; Deres, E.J.; O’Connell, B.P.; Pillsbury, H.C., III; et al. Subjective Benefits of Bimodal Listening in Cochlear Implant Recipients with Asymmetric Hearing Loss. Otolaryngol. Head Neck Surg. 2020, 162, 933–941. [Google Scholar] [CrossRef]
- Noble, W.; Gatehouse, S. Interaural asymmetry of hearing loss, Speech, Spatial and Qualities of Hearing Scale (SSQ) disabilities, and handicap. Int. J. Audiol. 2004, 43, 100–114. [Google Scholar] [CrossRef]
- Forli, F.; Berrettini, S.; Bruschini, L.; Canelli, R.; Lazzerini, F. Cochlear implantation in patients with asymmetric hearing loss: Reporting and discussing the benefits in speech perception, speech reception threshold, squelch abilities, and patients’ reported outcomes. J. Laryngol. Otol. 2022, 136, 964–969. [Google Scholar] [CrossRef]
- Mason, M.; Kokkinakis, K. Perception of consonants in reverberation and noise by adults fitted with bimodal devices. J. Speech Lang. Hear. Res. 2014, 57, 1512–1520. [Google Scholar] [CrossRef][Green Version]
- Dorman, M.F.; Loiselle, L.H.; Cook, S.J.; Yost, W.A.; Gifford, R.H. Sound Source Localization by Normal-Hearing Listeners, Hearing-Impaired Listeners and Cochlear Implant Listeners. Audiol. Neurotol. 2016, 21, 127–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Firszt, J.B.; Holden, L.K.; Skinner, M.W.; Tobey, E.A.; Peterson, A.; Gaggl, W.; Runge-Samuelson, C.L.; Ashley, W.P. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear. 2004, 25, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.H.; Dorman, M.F.; McKarns, S.A.; Spahr, A.J. Combined electric and contralateral acoustic hearing: Word and sentence recognition with bimodal hearing. J. Speech Lang. Hear. Res. 2007, 50, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Blamey, P.; Artieres, F.; Başkent, D.; Bergeron, F.; Beynon, A.; Burke, E.; Dillier, N.; Dowell, R.; Fraysse, B.; Gallégo, S.; et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: An update with 2251 patients. Audiol. Neurotol. 2013, 18, 36–47. [Google Scholar] [CrossRef]
- Firszt, J.B.; Holden, L.K.; Reeder, R.M.; Cowdrey, L.; King, S. Cochlear Implantation in Adults With Asymmetric Hearing Loss. Ear Hear. 2012, 33, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, I.; Bebear, J.P.; Marx, M.; Fraysse, B.; Truy, E.; Lina-Granade, G.; Mondain, M.; Sterkers-Artières, F.; Bordure, P.; Robier, A.; et al. Predictive factors of cochlear implant outcomes in the elderly. Audiol. Neurotol. 2014, 19 (Suppl. 1), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Sucher, C.M.; McDermott, H.J. Bimodal stimulation: Benefits for music perception and sound quality. Cochlear Implant. Int. 2009, 10 (Suppl. 1), 96–99. [Google Scholar] [CrossRef] [PubMed]
- Farinetti, A.; Roman, S.; Mancini, J.; Baumstarck-Barrau, K.; Meller, R.; Lavieille, J.P.; Triglia, J.M. Quality of life in bimodal hearing users (unilateral cochlear implants and contralateral hearing aids). Eur. Arch. Otorhinolaryngol. 2015, 272, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.; Forli, F.; Guglielmi, V.; De Corso, E.; Paludetti, G.; Berrettini, S.; Fetoni, A.R. A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngol. Ital. 2016, 36, 155–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anzivino, R.; Conti, G.; Di Nardo, W.; Fetoni, A.R.; Picciotti, P.M.; Marra, C.; Guglielmi, V.; Fortunato, S.; Forli, F.; Paludetti, G.; et al. Prospective Evaluation of Cognitive Functions After Rehabilitation With Cochlear Implant or Hearing Aids: Preliminary Results of a Multicentric Study on Elderly Patients. Am. J. Audiol. 2019, 28, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Mosnier, I.; Lahlou, G.; Flament, J.; Mathias, N.; Ferrary, E.; Sterkers, O.; Bernardeschi, D.; Nguyen, Y. Benefits of a contralateral routing of signal device for unilateral Naída CI cochlear implant recipients. Eur. Arch. Otorhinolaryngol. 2019, 276, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Johansson, B.; Magnusson, L.; Lyxell, B.; Ellis, R.J. Speech Recognition and Cognitive Skills in Bimodal Cochlear Implant Users. J. Speech Lang. Hear. Res. 2017, 60, 2752–2763. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.A.; Firszt, J.B.; Rotz, L.A.; Hammes, D.; Reeder, R.; Willis, M. Cochlear implants in infants and toddlers. Ann. Otol. Rhinol. Laryngol. 2000, 109, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; O’Donoghue, G. Bilateral cochlear implantation: An evidence-based medicine evaluation. Laryngoscope 2007, 117, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.; Henkin, Y.; Kral, A. Asymmetric Hearing During Development: The Aural Preference Syndrome and Treatment Options. Pediatrics 2015, 136, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.; Kral, A. Animal and human studies on developmental monaural hearing loss. Hear. Res. 2019, 380, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Nittrouer, S.; Chapman, C. The effects of bilateral electric and bimodal electric—Acoustic stimulation on language development. Trends Amplif. 2009, 13, 190–205. [Google Scholar] [CrossRef]
- Gifford, R.H. Bilateral cochlear implants or bimodal hearing for children with bilateral sensorineural hearing loss. Curr. Otorhinolaryngol. Rep. 2020, 8, 385–394. [Google Scholar] [CrossRef]
- Killan, C.F.; Hoare, D.J.; Katiri, R.; Pierzycki, R.H.; Adams, B.; Hartley, D.E.; Ropar, D.; Kitterick, P.T. A Scoping Review of Studies Comparing Outcomes for Children With Severe Hearing Loss Using Hearing Aids to Children With Cochlear Implants. Ear Hear. 2022, 43, 290–304. [Google Scholar] [CrossRef]
- Sturm, J.J.; Kuhlmey, M.; Alexiades, G.; Hoffman, R.; Kim, A.H. Comparison of speech performance in bimodal versus bilateral cochlear implant users. Laryngoscope 2021, 131, E1322–E1327. [Google Scholar] [CrossRef]
- Laria, C.; Auletta, G.; Riccardi, P.; Papa, C.; Malesci, R.; Franzé, A.; Marciano, E. Very good performance with bimodal stimulation in a like-hybrid modality in a patient with profound bilateral sensorineural hearing loss with low-frequencies preservation. Am. J. Otolaryngol. 2014, 35, 70–72. [Google Scholar] [CrossRef][Green Version]
- Warren, S.E.; Dunbar, M.N. Bimodal hearing in individuals with severe-to-profound hearing loss: Benefits, challenges, and management. In Seminars in Hearing; Thieme Medical Publishers: New York, NY, USA, 2018; Volume 39, pp. 405–413. [Google Scholar]
- Welch, C.; Dillon, M.T.; Pillsbury, H.C. Electric and acoustic stimulation in cochlear implant recipients with hearing preservation. In Seminars in Hearing; Thieme Medical Publishers: New York, NY, USA, 2018; Volume 39, pp. 414–427. [Google Scholar]
- Vroegop, J.L.; Goedegebure, A.; van der Schroeff, M.P. How to optimally fit a hearing aid for bimodal cochlear implant users: A systematic review. Ear Hear. 2018, 39, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Boroujeni, M.; Dajani, H.R.; Giguère, C. Perception of Prosody in Hearing-Impaired Individuals and Users of Hearing Assistive Devices: An Overview of Recent Advances. J. Speech Lang. Hear. Res. 2023, 66, 775–789. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Strings | Number of Results |
|---|---|---|
| PubMed Central | (acoustic bimodal stimulation on speech perception in noise) AND ((“1 October 2020”[Date—Publication]: “17 July 2024”[Date—Publication])) | 16 |
| Cochrane | acoustic bimodal stimulation on speech perception in noise in Title Abstract Keyword—with Cochrane Library publication date Between Oct 2020 and Jul 2024 (Word variations have been searched) | 0 |
| Scopus | TITLE-ABS-KEY (acoustic AND bimodal AND stimulation AND on AND speech AND perception AND in AND noise) AND PUBYEAR > 2019 AND PUBYEAR < 2025 | 17 |
| First Author, Title (IT/EN), Journal, Year | Database | Topic Aim of the Study | Type of Study, Sample Size, Tools | Results |
|---|---|---|---|---|
| Fan Y, Objective measure of binaural processing: Acoustic change complex in response to interaural phase differences. Hear Res. 2024 [4] | Pubmed | To investigate the feasibility of using an objective measure of interaural phase difference (IPD) sensitivity via the acoustic change complex (ACC) to assess candidacy for cochlear implantation with preserved low-frequency acoustic hearing, focusing on its correlation with behavioral interaural time difference (ITD) sensitivity and its potential clinical application. | Type of study: Experimental study. Sample size: Ten adult listeners with normal hearing. Tools: Behavioral and objective measures of binaural cue sensitivity using the acoustic change complex (ACC) with imposed IPD at 250 Hz and 1000 Hz. | (1) ACC amplitude increases with IPD; (2) Significant correlation between ACC-based IPD sensitivity at 250 Hz and behavioral ITD sensitivity; (3) Higher sensitivity to IPDs observed at 250 Hz compared to 1000 Hz, suggesting potential clinical utility for identifying EAS candidacy. |
| Gifford RH, Cochlear implant spectral bandwidth for optimizing electric and acoustic stimulation (EAS). Hear Res. 2022 [6] | Pubmed | The study explores the impact of different crossover frequencies in electric and acoustic stimulation (EAS) on speech recognition and listening difficulty in cochlear implant users with acoustic hearing preservation. It compares CI alone, BS (CI+HA), and best-aided EAS (CIHA+HA) listening conditions. | Type of study: Exploratory study. Sample size: 15 adult cochlear implant recipients with acoustic hearing preservation. Tools: Speech recognition test in semi-diffuse noise, subjective listening difficulty estimates. | The study found no significant effect of low-frequency cochlear implant (CI) cutoff on speech recognition or subjective listening difficulty across three listening conditions: CI alone, BS (CI+HA), and best-aided EAS (CIHA+HA). Both BS and best-aided EAS conditions showed significant benefits over CI alone, with additional benefits observed in best-aided EAS compared to BS hearing. Future research is needed to explore the efficacy of spiral ganglion (SG)-place-based fitting strategies for optimizing outcomes in experienced and newly activated EAS users. |
| Mertens G, Contralateral hearing aid use in adult cochlear implant recipients: retrospective analysis of auditory outcomes. Int J Audiol. 2024 [7] | Pubmed | To retrospectively investigate the frequency of usage of BS among cochlear implant (CI) users, as well as its clinical benefit relative to unilateral use. | Type of study: Retrospective study. Sample size: 103 adults Tools: Clinical Minimal Outcome Measurements test battery. | The preoperative contralateral residual hearing in the BS group was significantly better than that of the CI-only group. In both groups, speech perception in quiet and in noise improved after CI, with no significant difference between postoperative unimodal conditions. For the BS group, an additional significant improvement was found for the BS condition compared to the unimodal. |
| Yoon YS, Effects of the Configuration of Hearing Loss on Consonant Perception between Simulated Bimodal and Electric Acoustic Stimulation Hearing. J Am Acad Audiol. 2021 [8] | Pubmed | Investigating the impact of different configurations of hearing loss on speech perception benefits in BS and electric acoustic stimulation (EAS) hearing. To determine how consonant recognition is influenced by various patterns of hearing loss in simulated BS and EAS conditions using acoustic stimulation. | Type of study: Experimental study. Sample size: 20 adult subjects (10 per group). Tools: Acoustic simulation for consonant recognition, band-pass filtering for simulated hearing loss configurations, eight-channel noise vocoder for electric stimulation mimicking spectral mismatch. | Significant BS and EAS benefits occurred regardless of the configurations of hearing loss and hearing technology (BS vs. EAS). Place information was better transmitted in EAS hearing than in BS hearing. |
| Yoon YS, Interactions Between Slopes of Residual Hearing and Frequency Maps in Simulated Bimodal and Electric Acoustic Stimulation Hearing. J Speech Lang Hear Res. 2024 [9] | Pubmed | Topic: Investigating the influence of residual hearing slopes and cochlear implant frequency map settings on BS and electric acoustic stimulation (EAS) benefits in speech perception. Aim: To determine how different configurations of residual hearing and frequency map settings impact the benefits of BS and EAS stimulation in speech perception tasks. | Type of study: Experimental study Sample size: Adults with normal hearing. Tools: Sentence perception tests (unilateral and bilateral), acoustic stimulation with low-pass filters for hearing loss slopes, eight-channel sinewave vocoder for electric stimulation with different frequency map settings. | The largest BS/EAS benefit occurred with the shallow slope, and the smallest occurred with the steep slope. The effects of the slopes on BS/EAS benefit were greatest with the meet or gap map and the least with the overlap map. EAS benefit was greater than BS benefit at higher signal-to-noise ratios regardless of frequency map. |
| Dourado RPB, Benefits of Bimodal Stimulation to Speech Perception in Noise and Silence. Int Arch Otorhinolaryngol. 2023 [11] | Pubmed | To present whether bimodality still offers hearing benefits to the population who uses acoustic stimulation associated with electrical stimulation. | Type of study: Observational study. Sample size: 13 participants Tools: Hearing in Noise Test (HINT), Visual Analog Scale (VAS), four-tone means, Speech, Spatial, and Hearing Qualities questionnaire. | Individuals with an average hearing level between 50 and 70 dB showed better sentence recognition in both silence and noise. |
| Kelsall D, Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study. Am J Otolaryngol. 2021 [12] | Pubmed | To evaluate speech understanding outcomes in post-linguistically deafened adults with poor hearing performance despite well-fit hearing aids, comparing unilateral and bilateral/BS listening conditions preimplant and up to 12 months post-implant. | Type of study: Multicenter, prospective, repeated-measures, within-subject controlled study. Sample size: 100 post-linguistically deafened adults with bilateral moderate-to-profound sensorineural hearing loss. Tools: Speech recognition tests: monosyllabic consonant-nucleus-consonant (CNC) words in quiet; AzBio sentences in coincident noise at +5 dB and +10 dB SNR in implant ear and BS conditions. | Significant improvements in monosyllabic word scores in the implant ear only: 84% at 3 months, 93% at 6 months, and 97% at 12 months post-implant. Mean gain of 51% points for monosyllabic words and 32% points for sentences in noise at 12 months (p < 0.001). BS condition: 87% demonstrated improved monosyllabic word scores at 6 months, with a mean gain of 40% points (p < 0.001). Significant improvements in sentences in noise at 6- and 12-months post-implant in the BS condition (p < 0.001). |
| Stronks HC, The Temporal Fine Structure of Background Noise Determines the Benefit of Bimodal Hearing for Recognizing Speech. J Assoc Res Otolaryngol. 2020 [13] | Scopus | To investigate the role of temporal fine structure (TFS) and envelope cues in speech recognition in noise for cochlear implant (CI) users and BS listeners. | Type of study: Experimental study. Sample size: Not specified in the text. Tools: Speech recognition tests in steady-state (SS) noise, babble noise, and amplitude-modulated steady-state (AMSS) noise. | Babble noise was more detrimental to speech recognition than AMSS noise in CI-only conditions, contradicting initial hypotheses about TFS availability. BS benefit was observsed across noise types, indicating TFS dependence rather than envelope cues alone. |
| Mancini P, Bimodal cochlear implantation in elderly patients. Int J Audiol. 2021 [14] | Pubmed | Bimodal stimulation for asymmetric hearing loss in elderly adults, focusing on benefits in speech perception integration, particularly noise environments. | Type of study: retrospective clinical study. Sample size: 17 bimodal cochlear implant users. Tools: Speech audiometry in quiet and noise conditions using STARR and Matrix tests. | Bimodal Pure-tone threshold audiometry and speech perception in booth quiet and noise conditions significantly outperformed CI or HA alone. Age had a significant effect on bimodal STARR results, and bimodal STARR scores improved significantly compared to the better ear condition. |
| Author, Year, [Ref. num] | EPHPP Scores | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SB | D | C | B | DC | DO | Overall | |||||||
| R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 | ||
| Fan Y, 2024 [4] | W | W | W | W | W | M | W | W | S | S | S | S | W |
| Gifford RH, 2022 [6] | W | W | W | W | W | W | W | W | M | M | S | S | W |
| Mertens G, 2024 [7] | S | S | W | W | S | S | n/a | n/a | S | S | S | S | S |
| Yoon YS, 2021 [8] | M | S | W | W | W | W | W | W | M | W | S | S | W |
| Yoon YS, 2024 [9] | W | W | W | W | W | W | W | W | W | M | S | S | W |
| Dourado RPB, 2023 [11] | W | W | W | W | W | W | n/a | n/a | S | S | S | S | W |
| Kelsall D, 2021 [12] | S | S | M | M | S | S | W | W | S | S | S | S | S |
| Stronks HC, 2020 [13] | W | W | M | M | W | W | S | S | S | S | W | W | W |
| Mancini P, 2021 [14] | W | W | W | W | W | W | n/a | n/a | S | S | S | S | W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarella, A.; Notaro, A.; Laria, C.; Serra, N.; Genovese, E.; Malesci, R.; Auletta, G.; Fetoni, A.R. State-of-the-Art on the Impact of Bimodal Acoustic Stimulation on Speech Perception in Noise in Adults: A Systematic Review. Audiol. Res. 2024, 14, 914-927. https://doi.org/10.3390/audiolres14050077
Casarella A, Notaro A, Laria C, Serra N, Genovese E, Malesci R, Auletta G, Fetoni AR. State-of-the-Art on the Impact of Bimodal Acoustic Stimulation on Speech Perception in Noise in Adults: A Systematic Review. Audiology Research. 2024; 14(5):914-927. https://doi.org/10.3390/audiolres14050077
Chicago/Turabian StyleCasarella, Antonio, Anna Notaro, Carla Laria, Nicola Serra, Elisabetta Genovese, Rita Malesci, Gennaro Auletta, and Anna Rita Fetoni. 2024. "State-of-the-Art on the Impact of Bimodal Acoustic Stimulation on Speech Perception in Noise in Adults: A Systematic Review" Audiology Research 14, no. 5: 914-927. https://doi.org/10.3390/audiolres14050077
APA StyleCasarella, A., Notaro, A., Laria, C., Serra, N., Genovese, E., Malesci, R., Auletta, G., & Fetoni, A. R. (2024). State-of-the-Art on the Impact of Bimodal Acoustic Stimulation on Speech Perception in Noise in Adults: A Systematic Review. Audiology Research, 14(5), 914-927. https://doi.org/10.3390/audiolres14050077







