Protective Effects of Grape Seed Extract on Lipopolysaccharide Exposure and Radiation-Induced Intestinal Mucosal Damage: Insights from an In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Seed Extract
2.2. Cell Culture
2.3. Treatments with GSE and LPS
2.4. MTT Assay
2.5. Irradiation of Cell Culture
2.6. Cell Permeability Assay
2.7. Analysis of Oxidative Stress
2.8. Statistical Analysis
3. Results
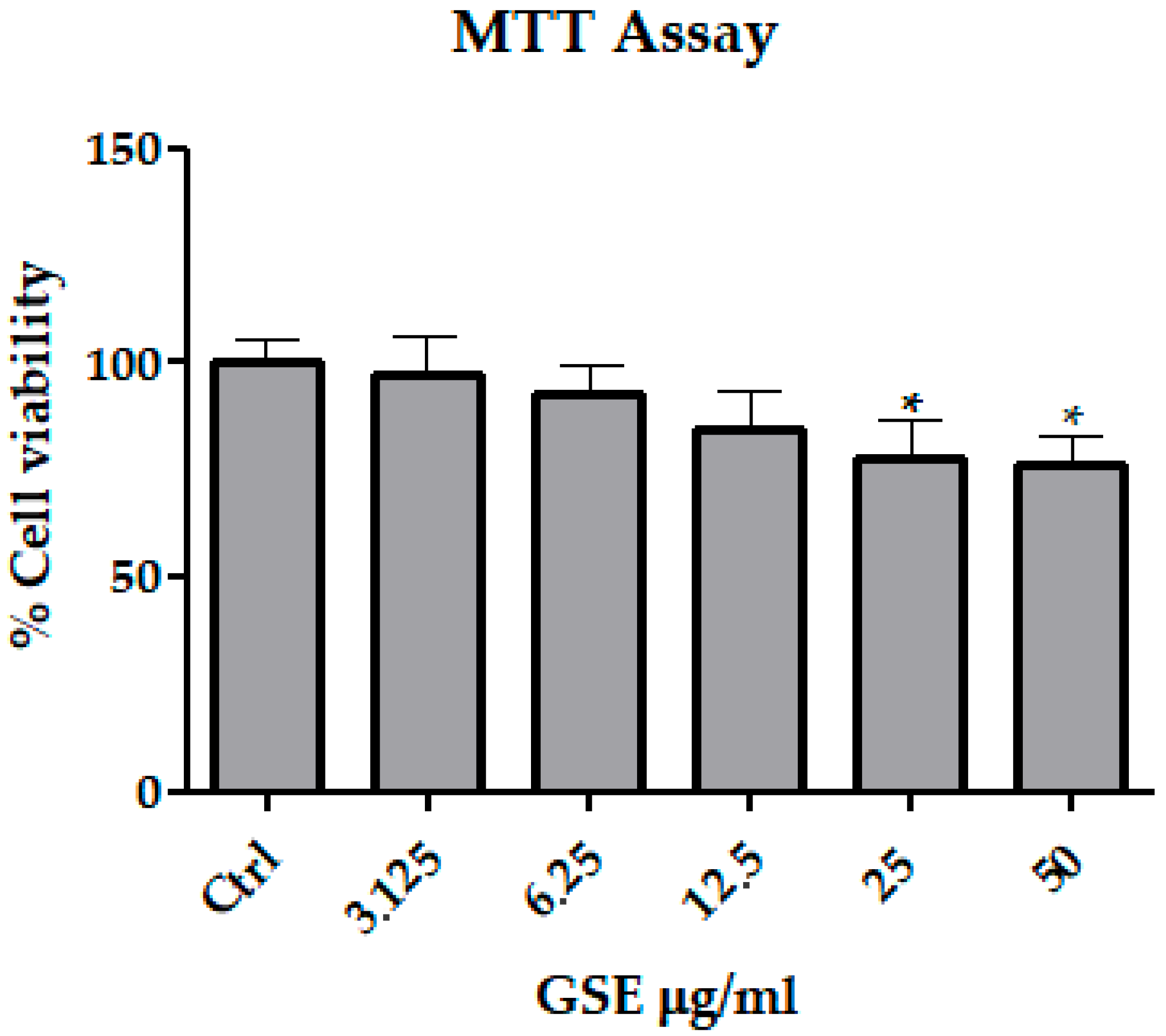
3.1. MTT Assay
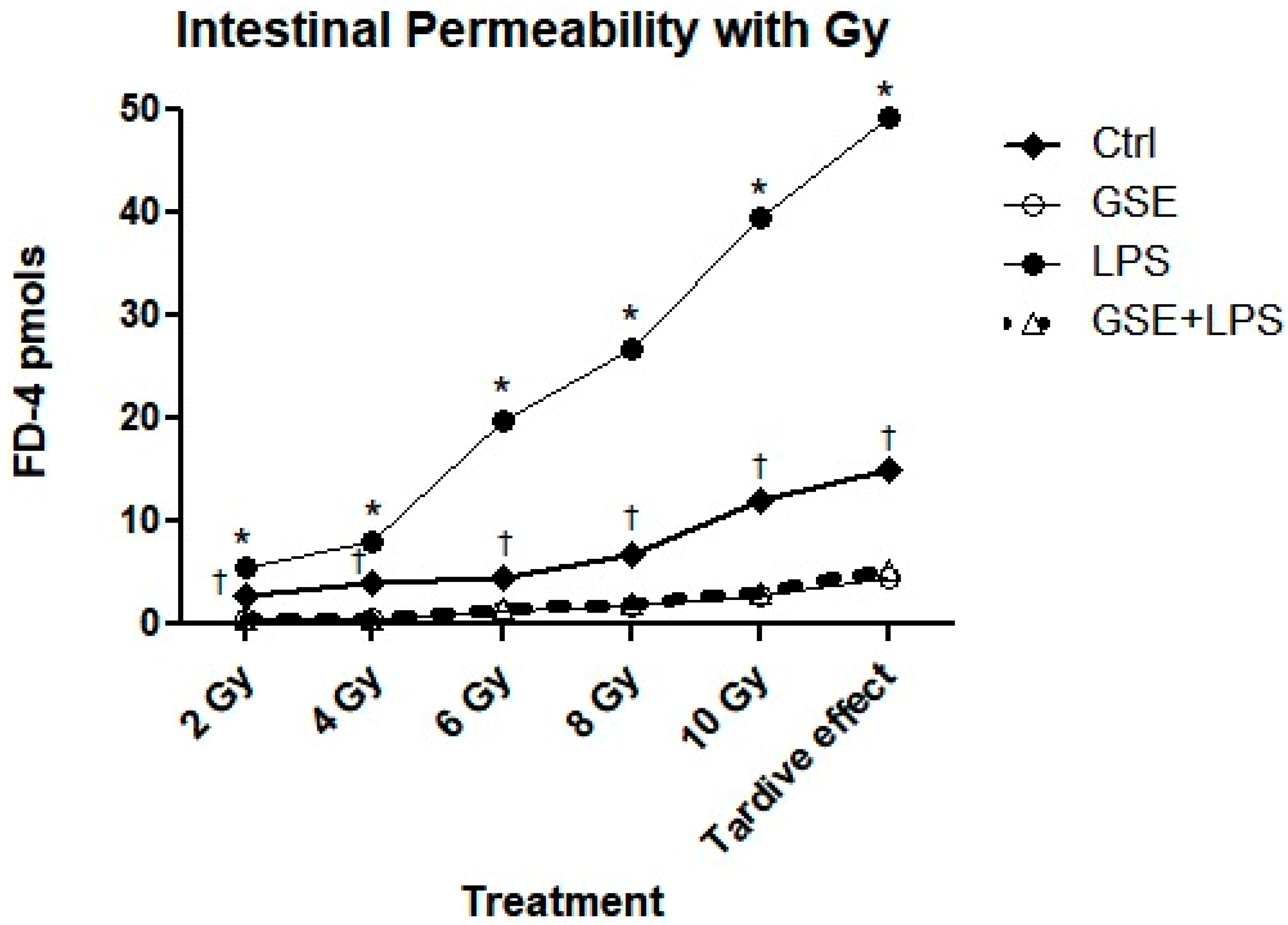
3.2. FD-4 Permeability Analysis
3.3. Analysis of ROS Production Following Treatments and Irradiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Garg, S.; Zheng, J.; Wang, J.; Authier, S.; Pouliot, M.; Hauer-Jensen, M. Segmental Differences in Radiation-Induced Alterations of Tight Junction-Related Proteins in Non-Human Primate Jejunum, Ileum and Colon. Radiat. Res. 2015, 185, 50–59. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Li, X.; Wang, L.; Hu, L.; Li, A.; Zhou, J. JAC4 Protects from X-ray Radiation-Induced Intestinal Injury by JWA-Mediated Anti-Oxidation/Inflammation Signaling. Antioxidants 2022, 11, 1067. [Google Scholar] [CrossRef]
- Fiorino, C.; Guckemberger, M.; Schwarz, M.; van der Heide, U.A.; Heijmen, B. Technology-driven research for radiotherapy innovation. Mol. Oncol. 2020, 14, 1500–1513. [Google Scholar] [CrossRef]
- Altomare, A.; Fiore, M.; D’Ercole, G.; Imperia, E.; Nicolosi, R.M.; Della Posta, S.; Pasqua, G.; Cicala, M.; De Gara, L.; Ramella, S.; et al. Protective Role of Natural Compounds Under Radiation-Induced Injury. Nutrients 2022, 14, 5374. [Google Scholar] [CrossRef]
- Mulinacci, N.; Valletta, A.; Pasqualetti, V.; Innocenti, M.; Giuliani, C.; Bellumori, M.; De Angelis, G.; Carnevale, A.; Locato, V.; Di Venanzio, C.; et al. Effects of ionizing radiation on bioactive plant extracts useful for preventing oxidative damages. Nat. Prod. Res. 2019, 33, 1106–1114. [Google Scholar] [CrossRef]
- Qiu, X.; Dong, K.; Guan, J.; He, J. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int. Immunopharmacol. 2020, 84, 106517. [Google Scholar] [CrossRef]
- Azmoonfar, R.; Khosravi, H.; Rafieemehr, H.; Mirzaei, F.; Dastan, D.; Ghiasvand, M.R.; Khorshidi, L.; Pashaki, A.S. Radioprotective effect of Malva sylvestris L. against radiation-induced liver, kidney and intestine damages in rat: A histopathological study. Biochem. Biophys. Rep. 2023, 34, 101455. [Google Scholar] [CrossRef]
- Khan, S.A.; Wingard, J.R. Infection and mucosal injury in cancer treatment. J. Natl. Cancer Inst. Monogr. 2001, 29, 31–36. [Google Scholar] [CrossRef]
- Saada, H.N.; Said, U.Z.; Meky, N.H.; Azime, A.S.A. Grape seed extract Vitis vinifera protects against radiation-induced oxidative damage and metabolic disorders in rats. Phytother. Res. 2009, 23, 434–438. [Google Scholar] [CrossRef]
- Kitsiou, M.; Purk, L.; Gutierrez-Merino, J.; Karatzas, K.A.; Klymenko, O.V.; Velliou, E. A Systematic Quantitative Determination of the Antimicrobial Efficacy of Grape Seed Extract against Foodborne Bacterial Pathogens. Foods 2023, 12, 929. [Google Scholar] [CrossRef]
- Simonetti, G.; Santamaria, A.R.; D’Auria, F.D.; Mulinacci, N.; Innocenti, M.; Cecchini, F.; Pericolini, E.; Gabrielli, E.; Panella, S.; Antonacci, D.; et al. Evaluation of anti-Candida activity of Vitis vinifera L. seed extracts obtained from wine and table cultivars. Biomed. Res. Int. 2014, 2014, 127021. [Google Scholar] [CrossRef]
- Simonetti, G.; D’Auria, F.D.; Mulinacci, N.; Innocenti, M.; Antonacci, D.; Angiolella, L.; Santamaria, A.R.; Valletta, A.; Donati, L.; Pasqua, G. Anti-Dermatophyte and Anti-Malassezia Activity of Extracts Rich in Polymeric Flavan-3-ols Obtained from Vitis vinifera Seeds. Phytother. Res. 2017, 31, 124–131. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, R.M.; Bonincontro, G.; Imperia, E.; Badiali, C.; De Vita, D.; Sciubba, F.; Dugo, L.; Guarino, M.P.L.; Altomare, A.; Simonetti, G.; et al. Protective Effect of Procyanidin-Rich Grape Seed Extract against Gram-Negative Virulence Factors. Antibiotics 2023, 12, 1615. [Google Scholar] [CrossRef] [PubMed]
- Targhi, R.G.; Banaei, A.; Saba, V. Radioprotective effect of grape seed extract against gamma irradiation in mouse bone marrow cells. J. Cancer Res. Ther. 2019, 15, 512–516. [Google Scholar] [CrossRef]
- El-Desouky, W.; Hanafi, A.; Abbas, M.M. Radioprotective effect of green tea and grape seed extracts mixture on gamma irradiation induced immune suppression in male albino rats. Int. J. Radiat. Biol. 2017, 93, 433–439. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health; Springer: Berlin/Heidelberg, Germany, 2015; pp. 103–111. [Google Scholar]
- Gori, M.; Altomare, A.; Cocca, S.; Solida, E.; Ribolsi, M.; Carotti, S.; Rainer, A.; Francesconi, M.; Morini, S.; Cicala, M.; et al. Palmitic Acid Affects Intestinal Epithelial Barrier Integrity and Permeability In Vitro. Antioxidants 2020, 9, 417. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L.; Donaghy, H.; Stoner, S.P.; Wheeler, H.R.; Diakos, C.I.; Howell, V.M. Differential effects of radiation fractionation regimens on glioblastoma. Radiat. Oncol. 2022, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Possenti, L.; Mecchi, L.; Rossoni, A.; Sangalli, V.; Bersini, S.; Cicchetti, A.; Costantino, M.L.; Candrian, C.; Arrigoni, C.; Rancati, T.; et al. Radiobiological Studies of Microvascular Damage through In Vitro Models: A Methodological Perspective. Cancers 2021, 13, 1182. [Google Scholar] [CrossRef]
- Gruber, S.; Dörr, W. Tissue reactions to ionizing radiation-Oral mucosa. Mutat. Res. Rev. Mutat. Res. 2016, 770, 292–298. [Google Scholar] [CrossRef]
- Muganu, M.; Paolocci, M.; Primiceri, S.; Tartaglia, R.; Benucci, I.; Cerreti, M.; D’Onofrio, C.; Paolacci, A.R.; Bignami, C. Intra-Varietal Variability of Romanesco Variety (Vitis vinifera L.). BIO Web Conf. 2019, 13, 01006. [Google Scholar] [CrossRef]
- Dinicola, S.; Cucina, A.; Pasqualato, A.; D’Anselmi, F.; Proietti, S.; Lisi, E.; Pasqua, G.; Antonacci, D.; Bizzarri, M. Antiproliferative and Apoptotic Effects Triggered by Grape Seed Extract (GSE) versus Epigallocatechin and Procyanidins on Colon Cancer Cell Lines. Int. J. Mol. Sci. 2012, 13, 651. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Oxidative stress of alternariol in Caco-2 cells. Toxicol. Lett. 2014, 229, 458–464. [Google Scholar] [CrossRef]
- Araki, Y.; Sugihara, H.; Hattori, T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol. Rep. 2006, 16, 1357–1362. [Google Scholar] [CrossRef]
- Koch, J.; Mönch, D.; Maaß, A.; Gromoll, C.; Hehr, T.; Leibold, T.; Schlitt, H.J.; Dahlke, M.H.; Renner, P. Three-dimensional cultivation increases chemo- and radioresistance of colorectal cancer cell lines. PLoS ONE 2021, 16, e0244513. [Google Scholar] [CrossRef]
- Moyes, S.M.; Killick, E.M.; Morris, J.F.; Kadhim, M.A.; Hill, M.A.; Carr, K.E. Changes produced by external radiation in parameters influencing intestinal permeability and microparticle uptake in vitro. Int. J. Radiat. Biol. 2008, 84, 467–486. [Google Scholar] [CrossRef]
- Guardamagna, I.; Lonati, L.; Savio, M.; Stivala, L.A.; Ottolenghi, A.; Baiocco, G. An Integrated Analysis of the Response of Colorectal Adenocarcinoma Caco-2 Cells to X-Ray Exposure. Front. Oncol. 2021, 11, 688919. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Ramanath, V.; Kuttan, R. Protection against Whole Body γ-Irradiation Induced Oxidative Stress and Clastogenic Damage in Mice by Ginger Essential Oil. Asian Pac. J. Cancer Prev. 2016, 17, 1325–1332. [Google Scholar] [CrossRef]
- Catalioto, R.M.; Festa, C.; Triolo, A.; Altamura, M.; Maggi, C.A.; Giuliani, S. Differential Effect of Ethanol and Hydrogen Peroxide on Barrier Function and Prostaglandin E2 Release in Differentiated Caco-2 Cells: Selective Prevention by Growth Factors. J. Pharm. Sci. 2009, 98, 713–727. [Google Scholar] [CrossRef]
- Safdari, B.; Sia, T.; Wattchow, D.; Smid, S. Effects of pro-inflammatory cytokines, lipopolysaccharide and COX-2 mediators on human colonic neuromuscular function and epithelial permeability. Cytokine 2016, 83, 231–238. [Google Scholar] [CrossRef]
- Hirotani, Y.; Ikeda, K.; Kato, R.; Myotoku, M.; Umeda, T.; Ijiri, Y.; Tanaka, K. Protective effects of lactoferrin against intestinal mucosal damage induced by lipopolysaccharide in human intestinal Caco-2 cells. Yakugaku Zasshi 2008, 128, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef]
- Sonis, S.T.; Eilers, J.P.; Epstein, J.B.; LeVeque, F.G.; Liggett, W.H.; Mulagha, M.T.; Peterson, D.E.; Rose, A.H.; Schubert, M.M.; Spijkervet, F.K.; et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer 1999, 85, 2103–2113. [Google Scholar] [CrossRef]
- Keefe, D.M.; Sonis, S.T.; Bowen, J.M. Emerging drugs for chemotherapy-induced mucositis. Expert. Opin. Emerg. Drugs 2008, 13, 511–522. [Google Scholar] [CrossRef]
- Dowlath, M.J.H.; Karuppannan, S.K.; Sinha, P.; Dowlath, N.S.; Arunachalam, K.D.; Ravindran, B.; Chang, S.W.; Nguyen-Tri, P.; Nguyen, D.D. Effects of radiation and role of plants in radioprotection: A critical review. Sci. Total Environ. 2021, 779, 146431. [Google Scholar] [CrossRef]
- Alfouzan, A.F. Radiation therapy in head and neck cancer. Saudi Med. J. 2021, 42, 247–254. [Google Scholar] [CrossRef]
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Radic. Biol. Med. 2006, 40, 1603–1614. [Google Scholar] [CrossRef]
- Cheah, K.Y.; Howarth, G.S.; Bastian, S.E. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS ONE 2014, 9, e85184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Babini, G.; Morini, J.; Barbieri, S.; Baiocco, G.; Ivaldi, G.B.; Liotta, M.; Tabarelli de Fatis, P.; Ottolenghi, A. A Co-culture Method to Investigate the Crosstalk Between X-ray Irradiated Caco-2 Cells and PBMC. J. Vis. Exp. 2018, 131, 56908. [Google Scholar]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14, 1175. [Google Scholar] [CrossRef]
- Santini, V. Amifostine: Chemotherapeutic and radiotherapeutic protective effects. Expert. Opin. Pharmacother. 2001, 2, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Ammar, R.M.; Aziz-Kalbhenn, E.; Rabini, S.; Klauck, S.M.; Dawood, M.; Saedd, M.E.M.; Kampf, C.J.; Efferth, T. Anti-inflammatory and tight junction protective activity of the herbal preparation STW 5-II on mouse intestinal organoids. Phytomedicine 2021, 88, 153589. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazaly, M.; El Hazek, R.M.; Khayyal, M.T. Protective effect of the herbal preparation, STW 5, against intestinal damage induced by gamma radiation in rats. Int. J. Radiat. Biol. 2015, 91, 150–156. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altomare, A.; Fiore, M.; Imperia, E.; D’Ercole, G.; Spagnuolo, L.; De Gara, L.; Pasqua, G.; Cicala, M.; Ramella, S.; Guarino, M.P.L. Protective Effects of Grape Seed Extract on Lipopolysaccharide Exposure and Radiation-Induced Intestinal Mucosal Damage: Insights from an In Vitro Study. Microbiol. Res. 2025, 16, 176. https://doi.org/10.3390/microbiolres16080176
Altomare A, Fiore M, Imperia E, D’Ercole G, Spagnuolo L, De Gara L, Pasqua G, Cicala M, Ramella S, Guarino MPL. Protective Effects of Grape Seed Extract on Lipopolysaccharide Exposure and Radiation-Induced Intestinal Mucosal Damage: Insights from an In Vitro Study. Microbiology Research. 2025; 16(8):176. https://doi.org/10.3390/microbiolres16080176
Chicago/Turabian StyleAltomare, Annamaria, Michele Fiore, Elena Imperia, Gabriele D’Ercole, Ludovica Spagnuolo, Laura De Gara, Gabriella Pasqua, Michele Cicala, Sara Ramella, and Michele Pier Luca Guarino. 2025. "Protective Effects of Grape Seed Extract on Lipopolysaccharide Exposure and Radiation-Induced Intestinal Mucosal Damage: Insights from an In Vitro Study" Microbiology Research 16, no. 8: 176. https://doi.org/10.3390/microbiolres16080176
APA StyleAltomare, A., Fiore, M., Imperia, E., D’Ercole, G., Spagnuolo, L., De Gara, L., Pasqua, G., Cicala, M., Ramella, S., & Guarino, M. P. L. (2025). Protective Effects of Grape Seed Extract on Lipopolysaccharide Exposure and Radiation-Induced Intestinal Mucosal Damage: Insights from an In Vitro Study. Microbiology Research, 16(8), 176. https://doi.org/10.3390/microbiolres16080176








