Serratia marcescens Strain VIRS2 Isolated from Saline Soil Enhances Rice Growth and Salt Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Endophytic Isolates
2.2. Salt-Tolerant and IAA-Producing Analysis
2.3. 16S rRNA Sequencing
2.4. Screening for Plant Growth-Promotion Properties
2.5. Rice Growth-Promotion Analysis
In Vitro Conditions
Hydroponic Conditions
2.6. Physiological and Biochemical Analysis
2.7. Whole Genome Sequence and Annotation of VIRS2
2.8. Relative Gene Expression Analysis
2.9. Data Statistical Analysis
3. Results
3.1. Selection of Potential Strains for Growth Promotion and Salt Tolerance in Rice
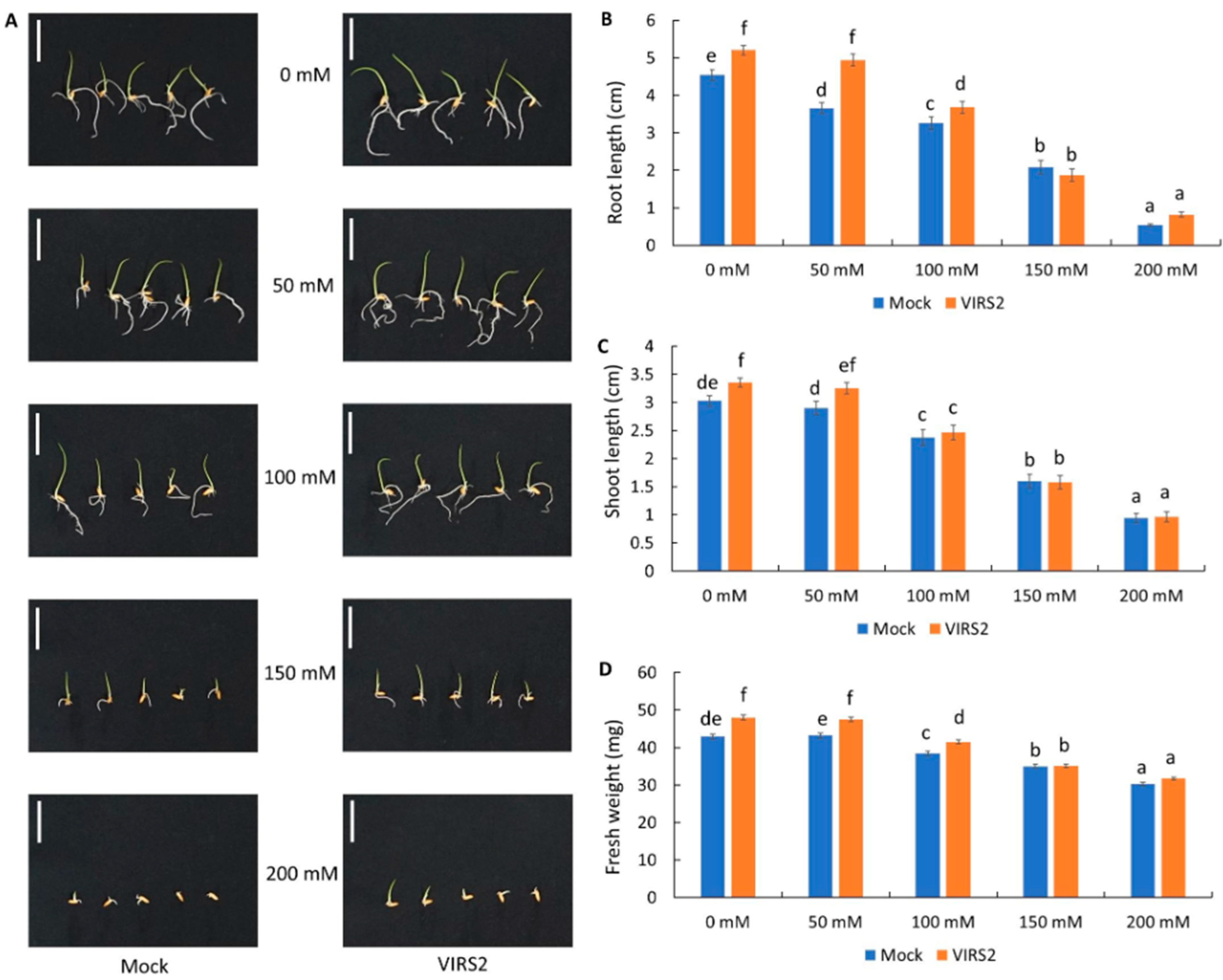
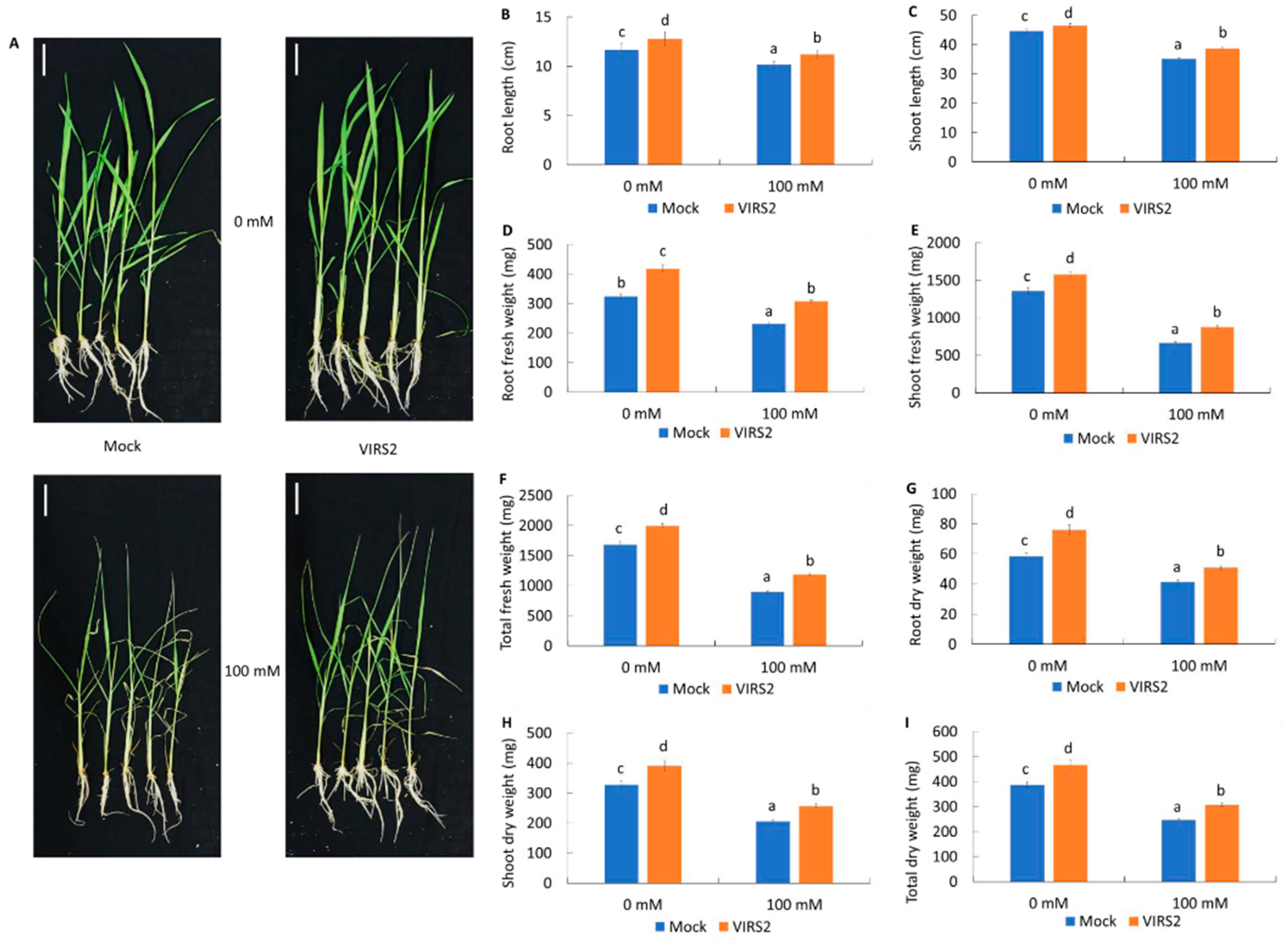
3.2. VIRS2 Enhanced Rice Growth Under Saline Conditions
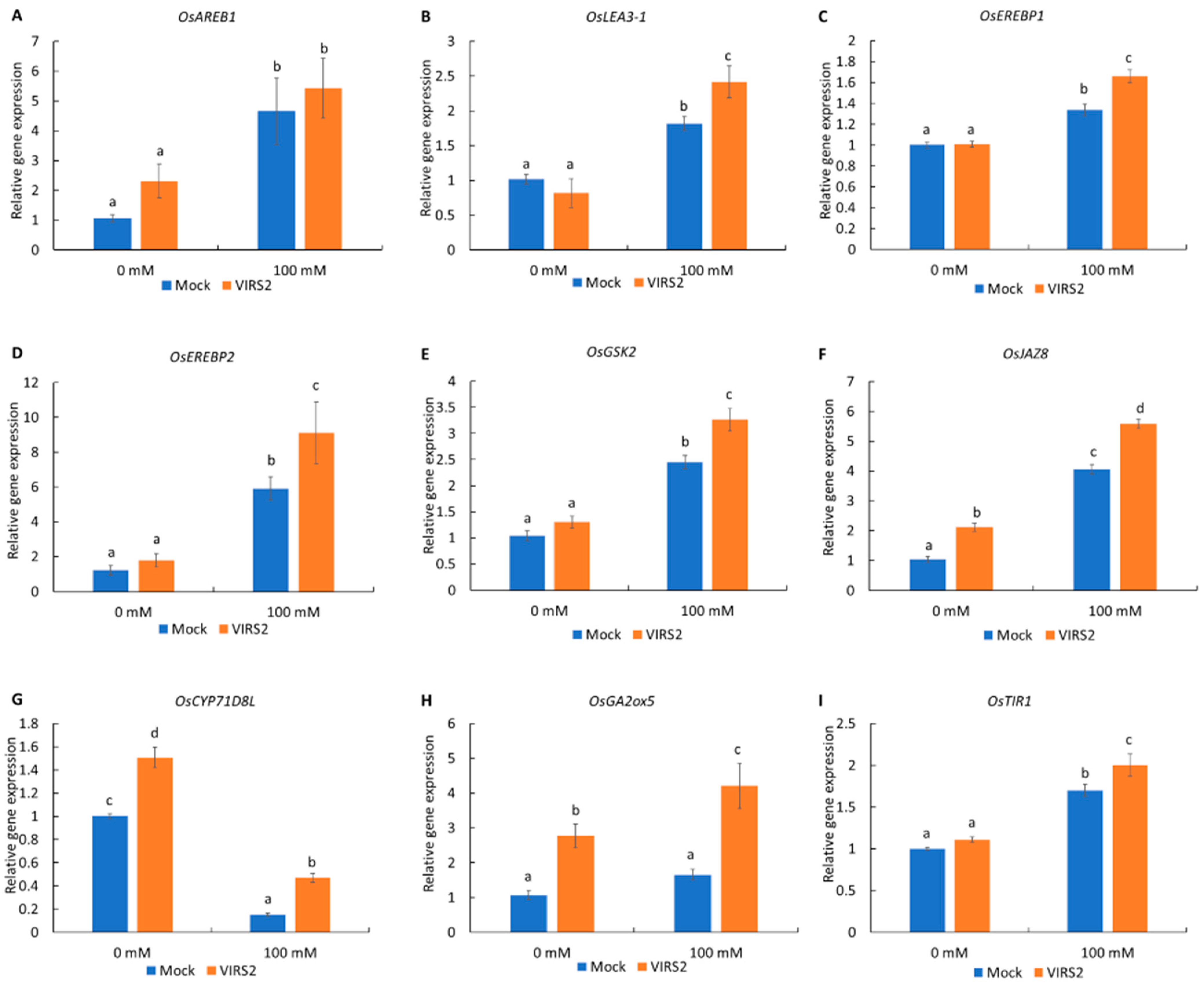
3.3. VIRS2 Affected the Expression of Plant Growth and Stress-Related Genes in Rice
3.4. Genome Properties of VIRS2
4. Discussion
4.1. Serratia Marcescens VIRS2 Carries Biochemical Characteristics of PGPR
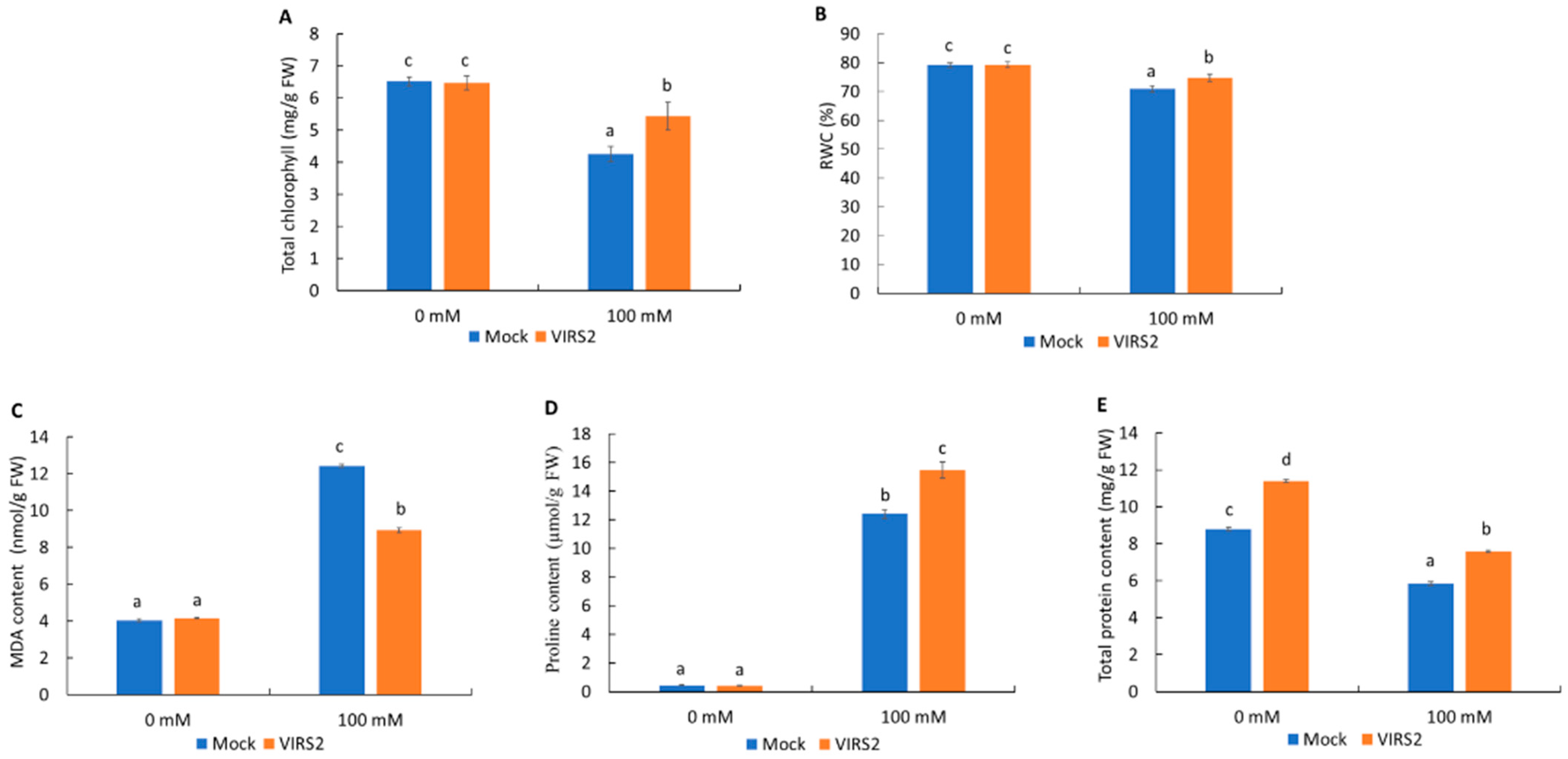
4.2. VIRS2 Shows Potential for Rice Growth Promotion and Salt Tolerance
4.3. VIRS2 Changes the Expression of Stress-Response-Related Genes in Rice
4.4. A Vast Number of PGP and Stress-Tolerant Genes Are Present in the VIRS2 Genome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ½MS | Half-strength Murashige and Skoog |
| ABA | Abscisic acid |
| ACC | 1-aminocyclopropane-1-carboxylate |
| ANOVA | Analysis of variance |
| BR | Brassinosteroids |
| Ca | Calcium |
| CAS | Chrome Azurol S |
| CK | Cytokinin |
| DAT | Days after treatment |
| DMRT | Duncan Multiple Range Test |
| ETH | Ethylene |
| EPS | Exopolysaccharide |
| GA | Gibberellic acid |
| IAA | Indole-3-acetic acid |
| IAM | Indole-3-acetamide |
| JA | Jasmonic acid |
| P | Phosphate |
| PGPR | Plant growth-promoting rhizobacteria |
| K | Potassium |
| KD18 | Khang Dan 18 |
| LB | Luria Broth |
| LEA | Late Embryogenesis Abundant |
| MDA | Malondialdehyde |
| MR | Mekong River |
| ROS | Reactive oxygen species |
| RWC | Relative water content |
| SA | Salicylic acid |
| ST-PGPR | Salt-tolerant PGPR |
| TAM | Tryptamine |
References
- Hashim, N.; Ali, M.M.; Mahadi, M.R.; Abdullah, A.F.; Wayayok, A.; Kassim, M.S.M.; Jamaluddin, A. Smart farming for sustainable rice production: An insight into application, challenge, and future prospect. Rice Sci. 2024, 31, 47–61. [Google Scholar] [CrossRef]
- Le Toan, T.; Huu, N.; Simioni, M.; Phan, H.; Arai, H.; Mermoz, S.; Bouvet, A.; de Eccher, I.; Diallo, Y.; Duong, T.H. Agriculture in Viet Nam under the impact of climate change. In Climate Change in Viet Nam, Impacts and Adaptation. A COP26 Assessment Report of the GEMMES Viet Nam Project 2021; Agence Française de Développement: Paris, France, 2021. [Google Scholar]
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M.M. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 2020, 66, 144–160. [Google Scholar] [CrossRef]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M.; El-Shehawi, A.M. Bacillus tequilensis Strain ‘UPMRB9’ improves biochemical attributes and nutrient accumulation in different rice varieties under salinity stress. PLoS ONE 2021, 16, e0260869. [Google Scholar] [CrossRef] [PubMed]
- Basak, N.; Rai, A.K.; Sundha, P.; Meena, R.L.; Bedwal, S.; Yadav, R.K.; Sharma, P.C. Assessing soil quality for rehabilitation of salt-affected agroecosystem: A comprehensive review. Front. Environ. Sci. 2022, 10, 935785. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Hoang, M.T. Impacts of climate change on inundation and salinity intrusion of cuu long delta. VNU J. Sci. Earth Environ. Sci. 2011, 27, 112–118. [Google Scholar]
- Rogers, M.E.; Colmer, T.D.; Frost, K.; Henry, D.; Cornwall, D.; Hulm, E.; Hughes, S.; Snowball, R.; Nichols, P.G.H.; Craig, A.D. Erratum to: The influence of nacl salinity and hypoxia on aspects of growth in Trifolium species. Crop Pasture Sci. 2010, 61, 1049–1050. [Google Scholar] [CrossRef]
- Razzaque, S.; Elias, S.M.; Haque, T.; Biswas, S.; Jewel, G.N.A.; Rahman, S.; Weng, X.; Ismail, A.M.; Walia, H.; Juenger, T.E. Gene expression analysis associated with salt stress in a reciprocally crossed rice population. Sci. Rep. 2019, 9, 8249. [Google Scholar] [CrossRef]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.; Guan, C. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant 2020, 42, 42. [Google Scholar] [CrossRef]
- Manh Tuong, H.; Garcia Mendez, S.; Vandecasteele, M.; Willems, A.; Luo, D.; Beirinckx, S.; Goormachtig, S. Stenotrophomonas sp. SRS1 promotes growth of arabidopsis and tomato plants under salt stress conditions. Plant Soil 2022, 473, 547–571. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Y.; Zhou, J.L.; Wang, G.; Mo, Y.; Ling, Y.; Huang, Y.; Zhang, Y.; Hu, H.; Wang, Y. RL-WG26 Mediated salt stress tolerance in rice seedlings: A new insight into molecular mechanisms. Plant Stress 2024, 11, 100306. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of plant growth promoting rhizobacteria in grain legumes: Growth promotion and crop production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ayaz, M.; Mu, G.; Hussain, A.; Yuanyuan, Q.; Yu, C.; Xu, Y.; Manghwar, H.; Gu, Q.; Wu, H.; et al. Revealing plant growth-promoting mechanisms of bacillus strains in elevating rice growth and its interaction with salt stress. Front. Plant Sci. 2022, 13, 994902. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus Pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Mahmud, F.A.; Islam, M.A.; Rubel, M.H.; Mukharjee, S.K.; Kumar, M.; Bhattacharya, P.; Ahmed, F. Effects of halotolerant rhizobacteria on rice seedlings under salinity stress. Sci. Total Environ. 2023, 892, 163774. [Google Scholar] [CrossRef]
- Hamada, M.A.; Soliman, E.R.S. Characterization and genomics identification of key genes involved in denitrification-dnra-nitrification pathway of plant growth-promoting rhizobacteria (Serratia marcescens OK482790). BMC Microbiol. 2023, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Nordstedt, N.P.; Jones, M.L. Genomic Analysis of Serratia Plymuthica MBSA-MJ1: A plant growth promoting rhizobacteria that improves water stress tolerance in greenhouse ornamentals. Front. Microbiol. 2021, 12, 653556. [Google Scholar] [CrossRef]
- Guo, L.; Han, C.; Liu, T.; Wang, Y.; Sun, P.; Pang, Q.; Zhang, X.; Xiang, W.; Zhao, J. Integrated Physiological, Biochemical and Transcriptomic Analyses Reveal the Mechanism of Salt Tolerance Induced by a Halotolerant Serratia sp. NTN6 in Maize. Environ. Exp. Bot. 2024, 221, 105724. [Google Scholar] [CrossRef]
- Turhan, E.; Kiran, S.; Ates, Ç.; Ates, O.; Kusvuran, S.; Ellialtioglu, S.S. Ameliorative effects of inoculation with Serratia marcescens and grafting on growth of eggplant seedlings under salt stress. J. Plant Nutr. 2020, 43, 594–603. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zhang, M.; Li, X.; Wang, M.; Li, L.; Li, X.; Ding, Z.; Tian, H. Serratia marcescens PLR enhances lateral root formation through supplying plr-derived auxin and enhancing auxin biosynthesis in Arabidopsis. J. Exp. Bot. 2022, 73, 3711–3725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, J.; Hao, X.; Feng, G.; Shen, Y.; Wang, G.; Chen, Z. Serratia marcescens LYGN1 reforms the rhizosphere microbial community and promotes cucumber and pepper growth in plug seedling cultivation. Plants 2024, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192–195. [Google Scholar] [CrossRef] [PubMed]
- AlAli, H.A.; Khalifa, A.; Almalki, M. Plant growth-promoting rhizobacteria from Ocimum basilicum improve growth of Phaseolus vulgaris and Abelmoschus esculentus. South Afr. J. Bot. 2021, 139, 200–209. [Google Scholar] [CrossRef]
- Mir, M.I.; Hameeda, B.; Quadriya, H.; Kumar, B.K.; Ilyas, N.; Kee Zuan, A.T.; El Enshasy, H.A.; Dailin, D.J.; Kassem, H.S.; Gafur, A.; et al. Multifarious Indigenous diazotrophic rhizobacteria of rice (Oryza sativa L.) rhizosphere and their effect on plant growth promotion. Front. Nutr. 2022, 8, 781764. [Google Scholar] [CrossRef]
- Amri, M.; Rjeibi, M.R.; Gatrouni, M.; Mateus, D.M.; Asses, N.; Pinho, H.J.; Abbes, C. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms 2023, 11, 783. [Google Scholar] [CrossRef]
- Rana, G.; Mandal, T.; Mandal, N.; Sakha, D.; Meikap, B. Calcite solubilization by bacteria: A novel method of environment pollution control. Geomicrobiol. J. 2015, 32, 846–852. [Google Scholar] [CrossRef]
- Sun, F.; Ou, Q.; Wang, N.; Guo, Z.; Ou, Y.; Li, N.; Peng, C. Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob. Ecol. Conserv. 2020, 23, e01141. [Google Scholar] [CrossRef]
- Louden, B.C.; Haarmann, D.; Lynne, A.M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef]
- Jhuma, T.A.; Rafeya, J.; Sultana, S.; Rahman, M.T.; Karim, M.M. Isolation of endophytic salt-tolerant plant growth-promoting rhizobacteria from Oryza sativa and evaluation of their plant growth-promoting traits under salinity stress condition. Front. Sustain. Food Syst. 2021, 5, 687531. [Google Scholar] [CrossRef]
- Abdelwahed, S.; Trabelsi, E.; Saadouli, I.; Kouidhi, S.; MASMOUDI, A.-S.; Cherif, A.; Mnif, W.; Mosbah, A. A New Pioneer colorimetric micro-plate method for the estimation of ammonia production by plant growth promoting rhizobacteria (PGPR). Main Group Chem. 2022, 21, 55–68. [Google Scholar] [CrossRef]
- Bado, S.; Forster, B.P.; Ghanim, A.M.A.; Jankowicz-Cieslak, J.; Berthold, G.; Luxiang, L. Protocol for Screening for salt tolerance in rice. In Protocols for Pre-Field Screening of Mutants for Salt Tolerance in Rice, Wheat and Barley; Springer International Publishing: Cham, Switzerland, 2016; pp. 21–31. ISBN 978-3-319-26588-9. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Determination of chlorophyll. In Plant-Microbe Interactions; Springer Protocols Handbooks; Springer: New York, NY, USA, 2021; pp. 145–146. ISBN 978-1-07-161079-4. [Google Scholar]
- Ly, L.K.; Ho, T.M.; Bui, T.P.; Nguyen, L.T.; Phan, Q.; Le, N.T.; Khuat, L.T.M.; Le, L.H.; Chu, H.H.; Pham, N.B.; et al. CRISPR/Cas9 targeted mutations of OsDSG1 gene enhanced salt tolerance in rice. Funct. Integr. Genom. 2024, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Zhao, M. Whole-genome analysis revealed the growth-promoting mechanism of endophytic bacterial strain Q2H1 in potato plants. Front. Microbiol. 2022, 13, 1035901. [Google Scholar] [CrossRef] [PubMed]
- Rosete Ambriz, S.A.; Alejo Hernandez, M.A.; Sanchez Cruz, N.; Ceapa, C.D. Genome mining of Streptomyces for the discovery of low-resistance antibiotics. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cho, G.-S.; Stein, M.; Brinks, E.; Rathje, J.; Lee, W.; Suh, S.H.; Franz, C.M. Serratia nevei sp. nov. and Serratia bockelmannii sp. nov., isolated from fresh produce in germany and reclassification of Serratia Marcescens subsp. sakuensis ajithkumar et al. 2003 as a later heterotypic synonym of Serratia marcescens subsp. Marcescens. Syst. Appl. Microbiol. 2020, 43, 126055. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Khamna, S.; Yokota, A.; Peberdy, J.F.; Lumyong, S. Indole-3-acetic acid production by Streptomyces sp. isolated from some thai medicinal plant rhizosphere soils. Eurasian J. Biosci. 2010, 4, 23–32. [Google Scholar] [CrossRef]
- Devi, K.A.; Pandey, P.; Sharma, G.D. Plant growth-promoting endophyte Serratia marcescens AL2-16 enhances the growth of Achyranthes Aspera L., a Medicinal Plant. HAYATI J. Biosci. 2016, 23, 173–180. [Google Scholar] [CrossRef]
- Kulkova, I.; Wróbel, B.; Dobrzyński, J. Serratia spp. as plant growth-promoting bacteria alleviating salinity, drought, and nutrient imbalance stresses. Front. Microbiol. 2024, 15, 1342331. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Afridi, M.S.; Javed, M.A.; Sumaira; Suleman, F.; Nadeem, M.; Ali, S.; Alwahibi, M.S.; Elshikh, M.S.; et al. Bacterial-mediated salinity stress tolerance in maize (Zea mays L.): A Fortunate way toward sustainable agriculture. ACS Omega 2023, 8, 20471–20487. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative water content, proline, and antioxidant enzymes in leaves of long shelf-life tomatoes under drought stress and rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Ullah, I.; Amaral, A.T.D., Jr.; Alatawi, A. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Zhang, X.; Long, Y.; Chen, X.; Zhang, B.; Xin, Y.; Li, L.; Cao, S.; Liu, F.; Wang, Z.; Huang, H.; et al. A NAC transcription factor OsNAC3 positively regulates ABA response and salt tolerance in rice. BMC Plant Biol. 2021, 21, 546. [Google Scholar] [CrossRef]
- Jisha, V.; Dampanaboina, L.; Vadassery, J.; Mithöfer, A.; Kappara, S.; Ramanan, R. Overexpression of an AP2/ERF type transcription factor OsEREBP1 Confers biotic and abiotic stress tolerance in rice. PLoS ONE 2015, 10, e0127831. [Google Scholar] [CrossRef]
- Serra, T.S.; Figueiredo, D.D.; Cordeiro, A.M.; Almeida, D.M.; Lourenço, T.; Abreu, I.A.; Sebastián, A.; Fernandes, L.; Contreras-Moreira, B.; Oliveira, M.M.; et al. OsRMC, a negative regulator of salt stress response in rice, is regulated by two AP2/ERF transcription factors. Plant Mol. Biol. 2013, 82, 439–455. [Google Scholar] [CrossRef]
- He, Y.; Hong, G.; Zhang, H.; Tan, X.; Li, L.; Kong, Y.; Sang, T.; Xie, K.; Wei, J.; Li, J. The OsGSK2 kinase integrates brassinosteroid and jasmonic acid signaling by interacting with OsJAZ4. Plant Cell 2020, 32, 2806–2822. [Google Scholar] [CrossRef]
- Peethambaran, P.K.; Glenz, R.; Höninger, S.; Shahinul Islam, S.M.; Hummel, S.; Harter, K.; Kolukisaoglu, Ü.; Meynard, D.; Guiderdoni, E.; Nick, P.; et al. Salt-inducible expression of OsJAZ8 improves resilience against salt-stress. BMC Plant Biol. 2018, 18, 311. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ye, H.; Yao, R.; Zhang, T.; Xiong, L. OsJAZ9 acts as a transcriptional regulator in jasmonate signaling and modulates salt stress tolerance in rice. Plant Sci. 2015, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Wang, R.; Ou, X.; Fang, Z.; Tian, C.; Duan, J.; Wang, Y.; Zhang, M. OsTIR1 and OsAFB2 downregulation via OsmiR393 overexpression leads to more tillers, early flowering and less tolerance to salt and drought in rice. PLoS ONE 2012, 7, e30039. [Google Scholar] [CrossRef]
- Shan, C.; Mei, Z.; Duan, J.; Chen, H.; Feng, H.; Cai, W. OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt Stress. PLoS ONE 2014, 9, e87110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. J. Exp. Bot. 2020, 71, 1160–1170. [Google Scholar]
- Matteoli, F.P.; Passarelli-Araujo, H.; Reis, R.J.A.; Da Rocha, L.O.; De Souza, E.M.; Aravind, L.; Olivares, F.L.; Venancio, T.M. Genome sequencing and assessment of plant growth-promoting properties of a Serratia marcescens strain isolated from vermicompost. BMC Genom. 2018, 19, 750. [Google Scholar] [CrossRef]
- Figueredo, E.F.; da Cruz, T.A.; de Almeida, J.R.; Batista, B.D.; Marcon, J.; de Andrade, P.A.M.; de Almeida Hayashibara, C.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The key role of indole-3-acetic acid biosynthesis by Bacillus thuringiensis RZ2MS9 in promoting maize growth revealed by the ipdC gene knockout mediated by the crispr-cas9 system. Microbiol. Res. 2023, 266, 127218. [Google Scholar] [CrossRef]
- Guerrieri, M.C.; Fiorini, A.; Fanfoni, E.; Tabaglio, V.; Cocconcelli, P.S.; Trevisan, M.; Puglisi, E. Integrated genomic and greenhouse assessment of a novel plant growth-promoting rhizobacterium for tomato plant. Front. Plant Sci. 2021, 12, 660620. [Google Scholar] [CrossRef]

| No. | PGP Traits | 0% | 2% | 4% | 6% | 8% |
|---|---|---|---|---|---|---|
| 1 | Nitrogen fixation (mm) | 8.66 ± 0.83 c | 3.23 ± 0.03 b | 2.15 ± 0.02 a | - | - |
| 2 | Phosphate solubilization (SI) | 1.19 ± 0.00 b | 1.12 ± 0.01 a | 1.14 ± 0.03 a | - | - |
| 3 | Potassium solubilization (SI) | 1.49 ± 0.04 b | 1.31 ± 0.01 a | - | - | - |
| 4 | Calcium solubilization (SI) | 1.84 ± 0.11 a | 2.72 ± 0.35 b | 1.30 ± 0.24 a | - | - |
| 5 | Siderophore (SI) | 1.70 ± 0.06 a | 1.34 ± 0.01 a | 2.05 ± 0.02 b | 2.10 ± 0.11 b | - |
| 6 | IAA production (µg/mL) | 137.66 ± 0.71 d | 108.16 ± 0.90 c | 87.33 ± 1.15 b | 73.62 ± 0.79 a | - |
| 7 | NH3 (µM) | 8.31 ± 0.91 d | 6.13 ± 0.67 c | 5.21 ± 0.57 bc | 3.38 ± 0.38 a | 3.93 ± 0.43 ab |
| 8 | Biofilm (OD550) | 0.29 ± 0.01 d | 0.10 ± 0.00 c | 0.06 ± 0.00 b | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| 9 | Exopolysacharite (g/L) | 6.10 ± 0.42 b | 3.41 ± 0.50 a | 9.16 ± 0.43 c | 8.42 ± 0.49 c | 3.97 ± 0.43 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, T.M.; Le, M.V.; Nguyen, H.H.T.; Phan, Q.; Bui, T.P.; Ly, L.K.; Lam, V.B.; Vandecasteele, M.; Goormachtig, S.; Chu, H.H.; et al. Serratia marcescens Strain VIRS2 Isolated from Saline Soil Enhances Rice Growth and Salt Tolerance. Microbiol. Res. 2025, 16, 97. https://doi.org/10.3390/microbiolres16050097
Ho TM, Le MV, Nguyen HHT, Phan Q, Bui TP, Ly LK, Lam VB, Vandecasteele M, Goormachtig S, Chu HH, et al. Serratia marcescens Strain VIRS2 Isolated from Saline Soil Enhances Rice Growth and Salt Tolerance. Microbiology Research. 2025; 16(5):97. https://doi.org/10.3390/microbiolres16050097
Chicago/Turabian StyleHo, Tuong M., Manh V. Le, Ha H. T. Nguyen, Quyen Phan, Thao P. Bui, Linh K. Ly, Van B. Lam, Michiel Vandecasteele, Sofie Goormachtig, Ha H. Chu, and et al. 2025. "Serratia marcescens Strain VIRS2 Isolated from Saline Soil Enhances Rice Growth and Salt Tolerance" Microbiology Research 16, no. 5: 97. https://doi.org/10.3390/microbiolres16050097
APA StyleHo, T. M., Le, M. V., Nguyen, H. H. T., Phan, Q., Bui, T. P., Ly, L. K., Lam, V. B., Vandecasteele, M., Goormachtig, S., Chu, H. H., & Do, P. T. (2025). Serratia marcescens Strain VIRS2 Isolated from Saline Soil Enhances Rice Growth and Salt Tolerance. Microbiology Research, 16(5), 97. https://doi.org/10.3390/microbiolres16050097






