The Potential of Beneficial Microbes for Sustainable Alternative Approaches to Control Phytopathogenic Diseases
Abstract
1. Introduction
2. Diversity of Microbial Biological Control Agents (MBCAs)
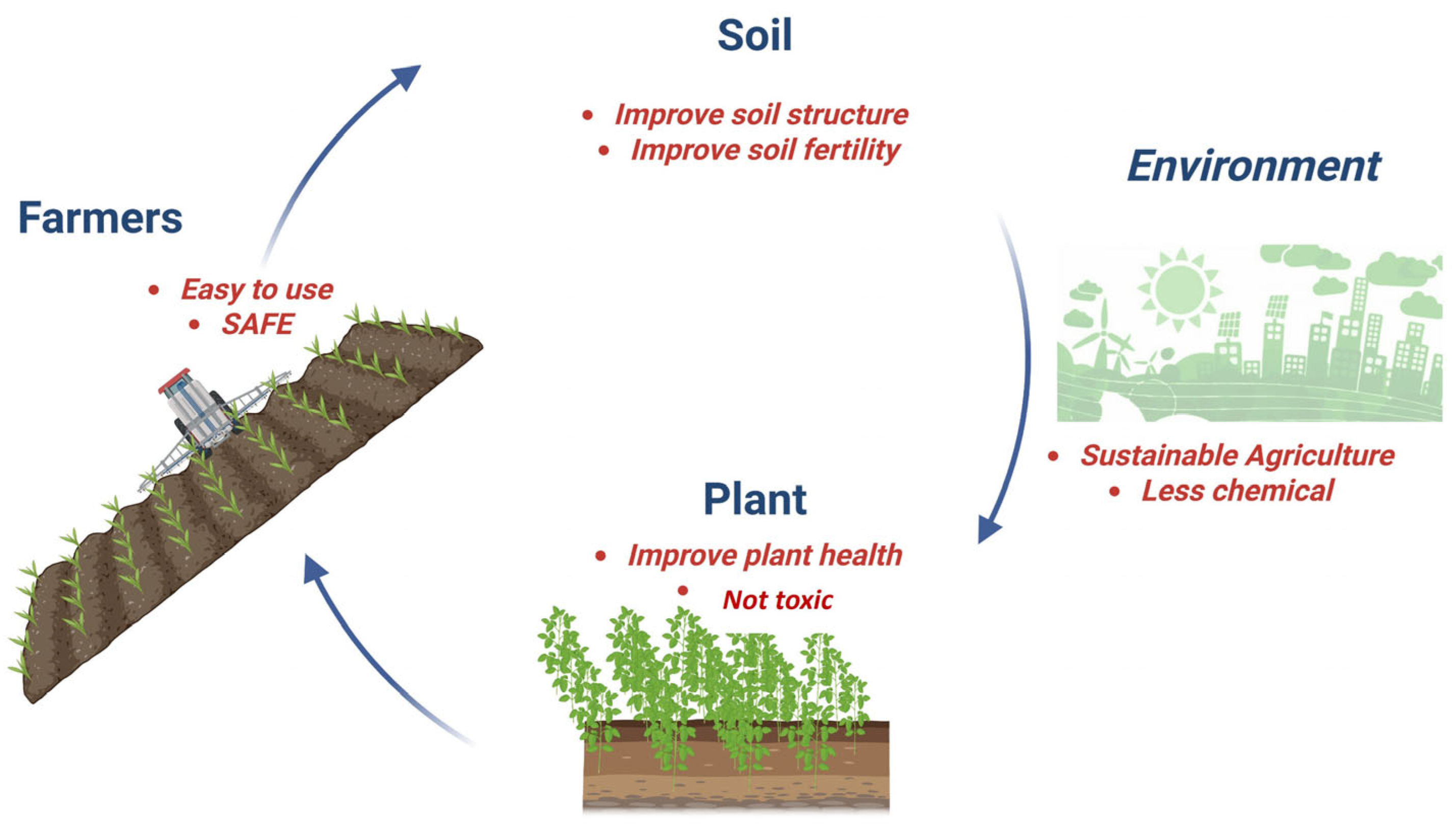
3. Advantages of Using Microbial Biological Control Agents (MBCAs)
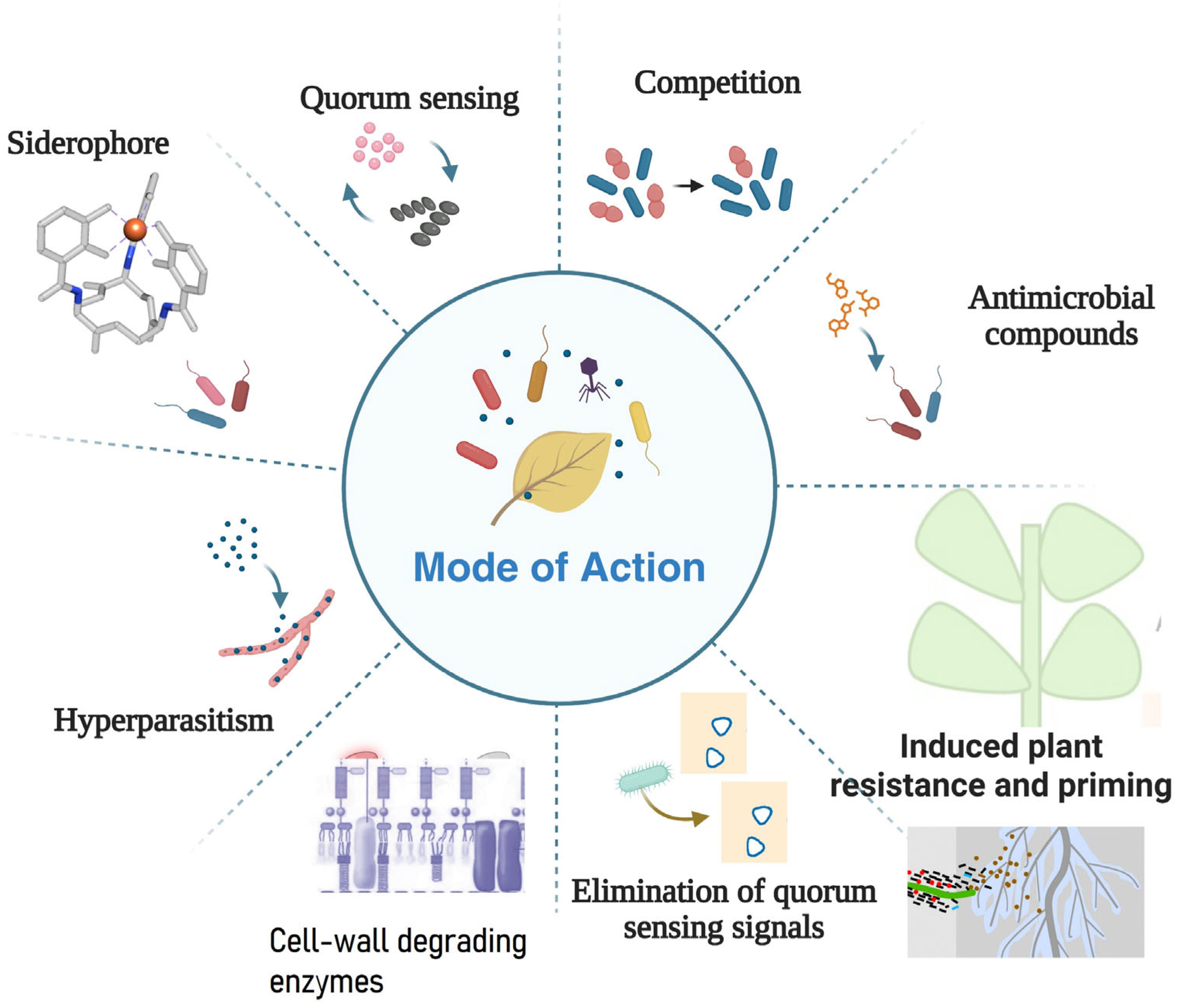
4. Mode of Action
4.1. Hyperparasitism
4.2. Antibiosis via Antimicrobial Metabolites
4.2.1. Antibiotics
4.2.2. Bacteriocins
4.3. Competition for Nutrients or Space
4.4. Elimination of Quorum-Sensing Signals
4.5. Siderophores
4.6. Production of Cell-Wall-Degrading Enzymes
4.7. Induced Plant Resistance and Priming
5. Formulation and Development of Biopesticides
6. Challenges of Biopesticides
6.1. MBCAs: From the Laboratory to the Field
6.2. Limited Number of Registered MBCA Products
6.3. Lack of Awareness and Knowledge of Biopesticides
6.4. Legislative Procedure and Commercialization
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Vero, S.; Garmendia, G.; Allori, E.; Sanz, J.M.; Gonda, M.; Alconada, T.; Cavello, I.; Dib, J.R.; Diaz, M.A.; Nally, C.; et al. Microbial Biopesticides: Diversity, Scope, and Mechanisms Involved in Plant Disease Control. Diversity 2023, 15, 457. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Legein, M.; Smets, W.; Vandenheuvel, D.; Eilers, T.; Muyshondt, B.; Prinsen, E.; Samson, R.; Lebeer, S. Modes of Action of Microbial Biocontrol in the Phyllosphere. Front. Microbiol. 2020, 11, 1619. [Google Scholar] [CrossRef]
- Monfil, V.O.; Casas-Flores, S. Chapter 32—Molecular Mechanisms of Biocontrol in Trichoderma spp. and Their Applications in Agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 429–453. Available online: https://www.sciencedirect.com/science/article/pii/B9780444595768000321 (accessed on 13 January 2024).
- Bilgrami, A.L.; Khan, A. Chapter 9—Biopesticidal potentials of predaceous and parasitic fungi. In Plant Nematode Biopesticides; Bilgrami, A.L., Khan, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 141–163. Available online: https://www.sciencedirect.com/science/article/pii/B9780128230060000097 (accessed on 14 January 2024).
- Boro, M.; Sannyasi, S.; Chettri, D.; Verma, A.K. Microorganisms in biological control strategies to manage microbial plant pathogens: A review. Arch. Microbiol. 2022, 204, 666. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Wang, X.; Zheng, L.; Liu, H. Inhibitory effects of Bacillus licheniformis BL06 on Phytophthora capsici in pepper by multiple modes of action. Biol. Control 2020, 144, 104210. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus thuringiensis strains to identify new potential biocontrol agents against Sclerotinia sclerotiorum and Plutellaxylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS line with gene Btcry1Ia encoding Cry1Ia toxin from Bacillus thuringiensis promotes integrated wheat defense against pathogen Stagonosporanodorum Berk. and greenbug Schizaphisgraminum Rond. Biol. Control 2020, 144, 104242. [Google Scholar] [CrossRef]
- Liang, Z.; Ali, Q.; Wang, Y.; Mu, G.; Kan, X.; Ren, Y.; Manghwar, H.; Gu, Q.; Wu, H.; Gao, X. Toxicity of Bacillus thuringiensis Strains Derived from the Novel Crystal Protein Cry31Aa with High Nematicidal Activity against Rice Parasitic Nematode Aphelenchoidesbesseyi. Int. J. Mol. Sci. 2022, 23, 8189. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, X.; Zhang, X.; Zhang, M.; Gu, Y.; Ali, Q.; Mohamed, M.S.R.; Xu, J.; Shi, J.; Gao, X.; et al. Mycosubtilin Produced by Bacillus subtilis ATCC6633 Inhibits Growth and Mycotoxin Biosynthesis of Fusarium graminearum and Fusarium verticillioides. Toxins 2021, 13, 791. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Zubair, M.; Farzand, A.; Mumtaz, F.; Khan, A.R.; Sheikh, T.M.M.; Haider, M.S.; Yu, C.; Wang, Y.; Ayaz, M.; Gu, Q.; et al. Novel Genetic Dysregulations and Oxidative Damage in Fusarium graminearum Induced by Plant Defense Eliciting Psychrophilic Bacillus atrophaeus TS1. Int. J. Mol. Sci. 2021, 22, 12094. [Google Scholar] [CrossRef]
- Yu, C.; Chen, H.; Zhu, L.; Song, Y.; Jiang, Q.; Zhang, Y.; Ali, Q.; Gu, Q.; Gao, X.; Borriss, R.; et al. Profiling of Antimicrobial Metabolites Synthesized by the Endophytic and Genetically Amenable Biocontrol Strain Bacillus velezensis DMW1. Microbiol. Spectr. 2023, 11, 0003823. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Hanif, A.; Tahir, H.A.S.; Gao, X. Marker assisted detection and LC-MS analysis of antimicrobial compounds in different Bacillus strains and their antifungal effect on Sclerotinia sclerotiorum. Biol. Control 2019, 133, 91–102. [Google Scholar] [CrossRef]
- Nifakos, K.; Tsalgatidou, P.C.; Thomloudi, E.-E.; Skagia, A.; Kotopoulis, D.; Baira, E.; Delis, C.; Papadimitriou, K.; Markellou, E.; Venieraki, A.; et al. Genomic Analysis and Secondary Metabolites Production of the Endophytic Bacillus velezensis Bvel1: A Biocontrol Agent against Botrytis cinerea Causing Bunch Rot in Post-Harvest Table Grapes. Plants 2021, 10, 1716. [Google Scholar] [CrossRef]
- Zhong, T.; Wang, Z.; Zhang, M.; Wei, X.; Kan, J.; Zalán, Z.; Wang, K.; Du, M. Volatile organic compounds produced by Pseudomonas fluorescens ZX as potential biological fumigants against gray mold on postharvest grapes. Biol. Control 2021, 163, 104754. [Google Scholar] [CrossRef]
- Ashajyothi, M.; Kumar, A.; Sheoran, N.; Ganesan, P.; Gogoi, R.; Subbaiyan, G.K.; Bhattacharya, R. Black pepper [Piper nigrum L.] associated endophytic Pseudomonas putida BP25 alters root phenotype and induces defense in rice [Oryza sativa L.] against blast disease incited by Magnaporthe oryzae. Biol. Control 2020, 143, 104181. [Google Scholar] [CrossRef]
- Wallace, R.L.; Hirkala, D.L.; Nelson, L.M. Efficacy of Pseudomonas fluorescens for control of Mucor rot of apple during commercial storage and potential modes of action. Can. J. Microbiol. 2018, 64, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Montes-Osuna, N.; Gómez-Lama Cabanás, C.; Valverde-Corredor, A.; Berendsen, R.L.; Prieto, P.; Mercado-Blanco, J. Assessing the Involvement of Selected Phenotypes of Pseudomonas simiae PICF7 in Olive Root Colonization and Biological Control of Verticillium dahliae. Plants 2021, 10, 412. [Google Scholar] [CrossRef]
- Agustí, L.; Bonaterra, A.; Moragrega, C.; Camps, J.; Montesinos, E. Biocontrol of Root Rot of Strawberry Caused by Phytophthora Cactorum with a Combination of Two Pseudomonas Fluorescens Strains. J. Plant Pathol. 2011, 93, 363–372. [Google Scholar]
- Kakembo, D.; Lee, Y.H. Analysis of traits for biocontrol performance of Pseudomonas parafulva JBCS1880 against bacterial pustule in soybean plants. Biol. Control 2019, 134, 72–81. [Google Scholar] [CrossRef]
- Ye, S.; Yan, R.; Li, X.; Lin, Y.; Yang, Z.; Ma, Y.; Ding, Z. Biocontrol potential of Pseudomonas rhodesiae GC-7 against the root-knot nematode Meloidogyne graminicola through both antagonistic effects and induced plant resistance. Front. Microbiol. 2022, 13, 1025727. [Google Scholar] [CrossRef]
- Sahebani, N.; Gholamrezaee, N. The biocontrol potential of Pseudomonas fluorescens CHA0 against root knot nematode [Meloidogyne javanica] is dependent on the plant species. Biol. Control 2021, 152, 104445. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bakr, R. Internal transcribed Spacers [ITS] based identification of Trichoderma isolates with a potential biocontrol activity against Macrophominaphaseolina, Aspergillus niger and Meloidogyne incognita. Afr. J. Microbiol. Res. 2018, 12, 715–722. [Google Scholar]
- Khalifa, E.S.Z.; Amer, G.A.A.; Bakr, R.A.; Hamad, A.S. Bio-control efficacy of Trichoderma against Strawberry charcoal rot disease. Egypt. J. Crop Prot. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Abdel-Lateif, K.S.; Bakr, R.A. Genetic diversity and biocontrol efficacy of indigenous Trichoderma isolates against Fusarium wilt of pepper. J. Basic Microbiol. 2020, 60, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Younesi, H.; Bazgir, E.; Darvishnia, M.; Chehri, K. Selection and control efficiency of Trichoderma isolates against Fusarium oxysporum f. sp. ciceris in Iran. Physiol. Mol. Plant Pathol. 2021, 116, 101731. [Google Scholar] [CrossRef]
- Fan, H.; Yao, M.; Wang, H.; Zhao, D.; Zhu, X.; Wang, Y.; Liu, X.; Duan, Y.; Chen, L. Isolation and effect of Trichoderma citrinoviride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC Microbiol. 2020, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavira, A.; Gea, F.J.; Santos, M. Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma asperellum biocontrol activity and induction of systemic defenses against Sclerotium cepivorum in onion plants under tropical climate conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- Díaz-Gutiérrez, C.; Arroyave, C.; Llugany, M.; Poschenrieder, C.; Martos, S.; Peláez, C. Trichoderma asperellum as a preventive and curative agent to control Fusarium wilt in Stevia rebaudiana. Biol. Control 2021, 155, 104537. [Google Scholar] [CrossRef]
- Qi, Q.; Fan, C.; Wu, H.; Sun, L.; Cao, C. Preparation of Trichoderma asperellum Microcapsules and Biocontrol of Cucumber Powdery Mildew. Microbiol. Spectr. 2023, 11, 0508422. [Google Scholar] [CrossRef]
- Saha, S.; Thosar, R.U.; Das, D.; Chavan, V. Strategic Management of Powdery Mildew of Grapes Using Fungicides and Bio-Control Agents. Int. J. Bio-Resour. Stress Manag. 2023, 14, 1028–1036. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest. Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Harman, G.E. Trichoderma—Not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Harman, G.E.; Kubicek, C.P. (Eds.) Trichoderma And Gliocladium, Volume 2: Enzymes, Biological Control and Commercial Applications, 1st ed.; CRC Press: Bristol, PA, USA; London, UK, 1998. [Google Scholar]
- Howell, C.R. Understanding the Mechanisms Employed by Trichoderma virens to Effect Biological Control of Cotton Diseases. Phytopathology 2006, 96, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Jeffries, P. Biology and ecology of mycoparasitism. Can. J. Bot. 1995, 73, 1284–1290. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, J.; Liang, X.; Zhan, G.; Jiang, S.; Kang, Z. Identification of a Novel Alternaria alternata Strain Able to Hyperparasitize Puccinia striiformis f. sp. tritici, the Causal Agent of Wheat Stripe Rust. Front. Microbiol. 2017, 8, 71. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2017.00071 (accessed on 13 January 2024). [CrossRef] [PubMed]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017, 5, 1005–1026. [Google Scholar] [CrossRef]
- Reithner, B.; Ibarra-Laclette, E.; Mach, R.L.; Herrera-Estrella, A. Identification of Mycoparasitism-Related Genes in Trichoderma atroviride. Appl. Environ. Microbiol. 2011, 77, 4361–4370. [Google Scholar] [CrossRef]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a biocontrol agent [BCA] of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef]
- Ali, A.A.; Eldeeb, A.; Ramadan, M.M.; El-Ashry, R.M. Nematicidal Effect of Three Trichoderma spp. on the Suitability of Tomato Plants for Meloidogyne incognita Reproduction. Egypt. J. Agronematology 2022, 21, 59–78. [Google Scholar] [CrossRef]
- Bakr, R.A.; Mahdy, M.E.; Mousa, E.S.M. Evaluation of Pasteuria penetrans application methods on controlling of root-knot nematode infecting eggplant. Egypt. J. Crop. Prot. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Öztürk, L.; Behmand, T.; Avcı, G.G.; Bozbuğa, R.; Mirik, M.; Elekcioğlu, İ.H. Survey of Pasteuria, the parasitic bacterial group to plant parasitic nematodes in Turkey. Egypt. J. Biol. Pest Control 2020, 30, 64. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ji, X.; Feng, Y.; Li, X.; Zou, C.; Xu, J.; Ren, Y.; Mi, Q.; Wu, J.; et al. Genomic and proteomic analyses of the fungus Arthrobotrysoligospora provide insights into nematode-trap formation. PLOS Pathog. 2011, 7, 1002179. [Google Scholar] [CrossRef] [PubMed]
- Nordbring-Hertz, B. Morphogenesis in the nematode-trapping fungus Arthrobotrysoligospora—An extensive plasticity of infection structures. Mycologist 2004, 18, 125–133. [Google Scholar] [CrossRef]
- Bhat, A.A.; Shakeel, A.; Waqar, S.; Handoo, Z.A.; Khan, A.A. Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes. Plants 2023, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Uysal, G.; Demir, D.; Çalişkan, S.; Sevindik, E.; Çağlayan, K. Use of Arthrobotrys spp. in biocontrol of the root-knot nematode Meloidogyne incognita. Eur. J. Biol. Res. 2023, 13, 173–180. [Google Scholar]
- Cesa-Luna, C.; Baez, A.; Quintero-Hernández, V.; De La Cruz-Enríquez, J.; Castañeda-Antonio, M.D.; Muñoz-Rojas, J. The importance of antimicrobial compounds produced by beneficial bacteria on the biocontrol of phytopathogens. Acta Biológica Colomb. 2020, 25, 140–154. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Hamid, S.; Lone, R.; Mohamed, H.I. Production of Antibiotics from PGPR and Their Role in Biocontrol of Plant Diseases. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Mohamed, H.I., El-Beltagi, H.E.D.S., Abd-Elsalam, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 441–461. [Google Scholar] [CrossRef]
- Hou, Q.; Kolodkin-Gal, I. Harvesting the complex pathways of antibiotic production and resistance of soil bacilli for optimizing plant microbiome. FEMS Microbiol. Ecol. 2020, 96, fiaa142. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth-promoting rhizobacteria [PGPR]: A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Mishra, J.; Arora, N.K. Secondary metabolites of fluorescent pseudomonads in biocontrol of phytopathogens for sustainable agriculture. Appl. Soil Ecol. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Cao, P.; Li, C.; Wang, H.; Yu, Z.; Xu, X.; Wang, X.; Zhao, J.; Xiang, W. Community Structures and Antifungal Activity of Root-Associated Endophytic Actinobacteria in Healthy and Diseased Cucumber Plants and Streptomyces sp. HAAG3-15 as a Promising Biocontrol Agent. Microorganisms 2020, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Han, X.; Li, X.; Zhang, X.; Wang, H.; Zhang, L.; Cao, P.; Wu, Y.; Wang, X.; Zhao, J.; et al. A Streptomyces sp. NEAU-HV9: Isolation, Identification, and Potential as a Biocontrol Agent against Ralstonia Solanacearum of Tomato Plants. Microorganisms 2020, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Glandorf, D.C.M.; Verheggen, P.; Jansen, T.; Jorritsma, J.-W.; Smit, E.; Leeflang, P.; Wernars, K.; Thomashow, L.S.; Laureijs, E.; Thomas-Oates, J.E.; et al. Effect of Genetically Modified Pseudomonas putida WCS358r on the Fungal Rhizosphere Microflora of Field-Grown Wheat. Appl. Environ. Microbiol. 2001, 67, 3371–3378. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Vlami, M.; de Souza, J.T. Antibiotic production by bacterial biocontrol agents. Antonie Van Leeuwenhoek 2002, 81, 537–547. [Google Scholar] [CrossRef]
- Almoneafy, A.A.; Moustafa-Farag, M.; Mohamed, H.I. The Auspicious Role of Plant Growth-Promoting Rhizobacteria in the Sustainable Management of Plant Diseases. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Mohamed, H.I., El-Beltagi, H.E.D.S., Abd-Elsalam, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 251–283. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Kannojia, P.; Choudhary, K.K.; Srivastava, A.K.; Singh, A.K. Chapter Four—PGPR Bioelicitors: Induced Systemic Resistance [ISR] and Proteomic Perspective on Biocontrol. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 67–84. Available online: https://www.sciencedirect.com/science/article/pii/B9780128158791000045 (accessed on 14 January 2024).
- Fernando, W.G.D.; Nakkeeran, S.; Zhang, Y. Biosynthesis of Antibiotics by PGPR and its Relation in Biocontrol of Plant Diseases. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 67–109. [Google Scholar] [CrossRef]
- Dou, K.; Lu, Z.; Wu, Q.; Ni, M.; Yu, C.; Wang, M.; Li, Y.; Wang, X.; Xie, H.; Chen, J.; et al. MIST: A Multilocus Identification System for Trichoderma. Appl. Environ. Microbiol. 2020, 86, 01532-20. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Rozen, D.E.; Kamilova, F. Wars between microbes on roots and fruits. F1000Research 2017, 6, 343. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, Z.; Tan, D.; Yang, J.; Zhou, Q.; Zeng, F.; Zhang, M.; Bie, Q.; Chen, C.; Xue, Y.; et al. Atrichodermones A-C, three new secondary metabolites from the solid culture of an endophytic fungal strain, Trichoderma atroviride. Fitoterapia 2017, 123, 18–22. [Google Scholar] [CrossRef]
- Song, Y.P.; Miao, F.P.; Fang, S.T.; Yin, X.L.; Ji, N.Y. Halogenated and Nonhalogenated Metabolites from the Marine-Alga-Endophytic Fungus Trichoderma asperellum cf44-2. Mar. Drugs. Mar. Drugs. 2018, 16, 266. [Google Scholar] [CrossRef]
- Wang, C.; Zolotarskaya, O.Y.; Nair, S.S.; Ehrhardt, C.J.; Ohman, D.E.; Wynne, K.J.; Yadavalli, V.K. Real-Time Observation of Antimicrobial Polycation Effects on Escherichia coli: Adapting the Carpet Model for Membrane Disruption to Quaternary Copolyoxetanes. Langmuir 2016, 32, 2975–2984. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, X.; Kong, F.-D.; Huang, H.-M.; Zhao, Y.-N.; Liu, M.; Wang, Z.-P.; Han, J. Overexpression of Global Regulator Talae1 Leads to the Discovery of New Antifungal Polyketides From Endophytic Fungus Trichoderma afroharzianum. Front. Microbiol. 2020, 11, 622785. [Google Scholar] [CrossRef] [PubMed]
- Kawada, M.; Yoshimoto, Y.; Kumagai, H.; Someno, T.; Momose, I.; Kawamura, N.; Isshiki, K.; Ikeda, D. PP2A inhibitors, harzianic acid and related compounds produced by fungus strain F-1531. J. Antibiot. 2004, 57, 235–237. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Zaid, R.; Lawry, R.; Hermosa, R.; Monte, E.; Horwitz, B.A.; Mukherjee, P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal. Biol. Rev. 2018, 32, 62–85. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, R.; Hermosa, R.; Vizcaino, J.; Sanz, L.; Monte, E.; Gutierrez, S. Secondary metabolites produced by Trichoderma and their importance in the biocontrol process. Microorg.nd. Enzym. Biocontrol. 2005, 1–22. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J.S.; López-Bucio, J. Chapter 36—Enhanced Plant Immunity Using Trichoderma. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 495–504. Available online: https://www.sciencedirect.com/science/article/pii/B9780444595768000369 (accessed on 15 January 2024).
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in vivo Models. Front. Microbiol. 2021, 12, 630695. [Google Scholar] [CrossRef] [PubMed]
- Chikindas, M.L.; Weeks, R.; Drider, D.; Chistyakov, V.A.; Dicks, L.M. Functions and emerging applications of bacteriocins. Curr. Opin. Biotechnol. 2018, 49, 23–28. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I.; et al. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Hammami, R.; Fernandez, B.; Lacroix, C.; Fliss, I. Anti-infective properties of bacteriocins: An update. Cell Mol. Life Sci. CMLS 2013, 70, 2947–2967. [Google Scholar] [CrossRef]
- Hanchi, H.; Hammami, R.; Gingras, H.; Kourda, R.; Bergeron, M.G.; Ben Hamida, J.; Ouellette, M.; Fliss, I. Inhibition of MRSA and of Clostridium difficile by durancin 61A: Synergy with bacteriocins and antibiotics. Future Microbiol. 2017, 12, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Lucas, R.; Abriouel, H.; Grande Burgos, M.J.; Pérez Pulido, R. Bacteriocins. In Decontamination of Fresh and Minimally Processed Produce; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 317–332. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118229187.ch18 (accessed on 13 January 2024).
- Nazari, M.; Smith, D.L. A PGPR-Produced Bacteriocin for Sustainable Agriculture: A Review of Thuricin 17 Characteristics and Applications. Front. Plant Sci. 2020, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The Ecological Role of Volatile and Soluble Secondary Metabolites Produced by Soil Bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. Int. J. Exp. Plant Biol. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, U. Chapter Four—PGPR Interaction: An Ecofriendly Approach Promoting the Sustainable Agriculture System. In Advances in Botanical Research; Bais, H., Sherrier, J., Eds.; Plant Microbe Interactions, Volume 75; Academic Press: Cambridge, MA, USA, 2015; pp. 81–113. Available online: https://www.sciencedirect.com/science/article/pii/S0065229615000671 (accessed on 13 January 2024).
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Faizan, S.; Hussain, A. Role of Microorganisms as Biofertilizers. In Microbiota and Biofertilizers: A Sustainable Continuum for Plant and Soil Health [Internet]; Hakeem, K.R., Dar, G.H., Mehmood, M.A., Bhat, R.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 83–98. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS ONE 2013, 8, 55731. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Liu, W.; Zheng, X. Biocontrol of postharvest gray and blue mold decay of apples with Rhodotorulamucilaginosa and possible mechanisms of action. Int. J. Food Microbiol. 2011, 146, 151–156. [Google Scholar] [CrossRef]
- Pantelides, I.S.; Tjamos, S.E.; Striglis, I.A.; Chatzipavlidis, I.; Paplomatas, E.J. Mode of action of a non-pathogenic Fusarium oxysporum strain against Verticillium dahliae using Real Time QPCR analysis and biomarker transformation. Biol. Control 2009, 50, 30–36. [Google Scholar] [CrossRef]
- Heredia-Ponce, Z.; Gutiérrez-Barranquero, J.A.; Purtschert-Montenegro, G.; Eberl, L.; de Vicente, A.; Cazorla, F.M. Role of extracellular matrix components in the formation of biofilms and their contribution to the biocontrol activity of Pseudomonas chlororaphis PCL1606. Environ. Microbiol. 2021, 23, 2086–2101. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Chapter 15—Rhizosphere biology☆☆This chapter is a revision of the third edition chapter by P. Marschner. pp. 369–388. Elsevier Ltd. In Marschner’s Mineral Nutrition of Plants, 4th, ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 587–614. Available online: https://www.sciencedirect.com/science/article/pii/B9780128197738000046 (accessed on 13 January 2024). [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Singh, I. Biocontrol of Soilborne Root Pathogens: An Overview. In Root Biology; Giri, B., Prasad, R., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 181–220. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L.; et al. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Aslanli, A.; Maslova, O.; Lyagin, I. Quorum Sensing as a Trigger That Improves Characteristics of Microbial Biocatalysts. Microorganisms 2023, 11, 1395. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.; Zhang, S.; Yang, T.; Li, Z.; Wang, B.; Sun, H.; Zhang, M.; Li, X. Quorum sensing: Cell-to-cell communication in Saccharomyces cerevisiae. Front. Microbiol. 2023, 14, 1250151. [Google Scholar] [CrossRef]
- Moradi, F.; Hadi, N. Quorum-quenching activity of some Iranian medicinal plants. New Microbes New Infect. 2021, 42, 100882. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, R.; Jin, L.; Zhu, W.; Ji, Y.; Xu, X.; Zhu, L. The regulation of N-acyl-homoserine lactones [AHLs]-based quorum sensing on EPS secretion via ATP synthetic for the stability of aerobic granular sludge. Sci. Total Environ. 2019, 673, 83–91. [Google Scholar] [CrossRef]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial Quorum Sensing During Infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Ramadan, E.M.; AbdelHafez, A.A.; Hassan, E.A.; Saber, F.M. Plant growth promoting rhizobacteria and their potential for biocontrol of phytopathogens. Afr. J. Microbiol. Res. 2016, 10, 486–504. [Google Scholar]
- Glick, B.R. Biocontrol of Bacteria and Fungi. In Beneficial Plant-Bacterial Interactions; Glick, B.R., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 181–230. [Google Scholar] [CrossRef]
- Rapti, S.; Boyatzis, S.C.; Rivers, S.; Pournou, A. Siderophores and their Applications in Wood, Textile, and Paper Conservation. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 301–339. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator–Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore—A boon to agricultural sciences. Biol. Control 2020, 144, 104214. [Google Scholar] [CrossRef]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of Action of Microbial Biocontrol Agents against Botrytis cinerea. J. Fungi 2021, 7, 1045. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Management of Plant Pathogens with Microorganisms. Proc. Natl. Acad. Sci. USA 2014, 80, 443. [Google Scholar] [CrossRef]
- Jadhav, H.; Sayyed, R. Hydrolytic Enzymes of Rhizospheric Microbes in Crop Protection. MOJ Cell Sci. Rep. 2016, 3, 135–136. [Google Scholar]
- Jadhav, H.P.; Shaikh, S.S.; Sayyed, R.Z. Role of Hydrolytic Enzymes of Rhizoflora in Biocontrol of Fungal Phytopathogens: An Overview. In Rhizotrophs: Plant Growth Promotion to Bioremediation; Springer: Singapore, 2017; pp. 183–203. [Google Scholar] [CrossRef]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- Ajuna, H.B.; Lim, H.-I.; Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Yun, J.-Y.; Ahn, Y.S. The Prospect of Hydrolytic Enzymes from Bacillus Species in the Biological Control of Pests and Diseases in Forest and Fruit Tree Production. Int. J. Mol. Sci. 2023, 24, 16889. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: Their potential in antifungal biocontrol. J. Microbiol. 2012, 50, 103–111. [Google Scholar] [CrossRef]
- Reddy, E.C.; Reddy, G.S.; Goudar, V.; Sriramula, A.; Swarnalatha, G.V.; Al Tawaha, A.R.M.; Sayyed, R.Z. Hydrolytic Enzyme Producing Plant Growth-Promoting Rhizobacteria [PGPR] in Plant Growth Promotion and Biocontrol. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Sayyed, R.Z., Uarrota, V.G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 303–312. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, D.; Liang, Z.; Huang, K.; Wu, X. Antagonistic activity of Bacillus subtilis CW14 and its β-glucanase against Aspergillus ochraceus. Food Control 2022, 131, 108475. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N.K. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285. [Google Scholar] [CrossRef]
- Choub, V.; Ajuna, H.B.; Won, S.-J.; Moon, J.-H.; Choi, S.-I.; Maung, C.E.H.; Kim, C.-W.; Ahn, Y.S. Antifungal Activity of Bacillus velezensis CE 100 against Anthracnose Disease [Colletotrichum gloeosporioides] and Growth Promotion of Walnut [Juglans regia L.] Trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Hou, Y.; Xia, X.; Zhu, Z.; Huang, A.; Feng, S.; Li, P.; Shi, L.; Dong, P. Isolation and Screening of Antagonistic Endophytes against Phytophthora infestans and Preliminary Exploration on Anti-oomycete Mechanism of Bacillus velezensis 6-5. Plants 2023, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci 2015, 6, 573. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2015.00573 (accessed on 5 December 2023). [CrossRef] [PubMed]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling Crosstalk between Salicylic Acid and Ethylene/Jasmonate in Plant Defense: Do We Understand What They Are Whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef]
- Prajapati, S.; Kumar, N.; Kumar, S.; Lakharan, L.; Maurya, S. Biological control a sustainable approach for plant diseases management: A review. J. Pharmacogn. Phytochem. 2020, 9, 1514–1523. [Google Scholar]
- Nega, A. Review on Concepts in Biological Control of Plant Pathogens. J. Biol. Agric. Healthc. 2014, 4, 33. [Google Scholar]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Gonzalez-Mendoza, D.; Zayed, O. Cell-free culture filtrate of Trichoderma longibrachiatum AD-1 as alternative approach to control Fusarium solani and induce defense response Phaseolus vulgaris L. plants. Rhizosphere 2023, 25, 100648. [Google Scholar] [CrossRef]
- Risoli, S.; Cotrozzi, L.; Sarrocco, S.; Nuzzaci, M.; Pellegrini, E.; Vitti, A. Trichoderma-Induced Resistance to Botrytis cinerea in Solanum Species: A Meta-Analysis. Plants 2022, 11, 180. [Google Scholar] [CrossRef]
- Butu, M.; Rodino, S.; Butu, A. Chapter 3—Biopesticide formulations—Current challenges and future perspectives. In Biopesticides; Woodhead Publishing: Sawston, UK, 2022; pp. 19–29. Available online: https://www.sciencedirect.com/science/article/pii/B9780128233559000109 (accessed on 5 December 2023).
- Teixidó, N.; Usall, J.; Torres, R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Keswani, C.; Bisen, K.; Singh, V.; Sarma, B.K.; Singh, H.B. Formulation Technology of Biocontrol Agents: Present Status and Future Prospects. In Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 35–52. [Google Scholar] [CrossRef]
- Chaudhary, T.; Dixit, M.; Gera, R.; Shukla, A.K.; Prakash, A.; Gupta, G.; Shukla, P. Techniques for improving formulations of bioinoculants. 3 Biotech. 2020, 10, 199. [Google Scholar] [CrossRef]
- Tamreihao, K.; Ningthoujam, D.S.; Nimaichand, S.; Singh, E.S.; Reena, P.; Singh, S.H.; Nongthomba, U. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol. Res. 2016, 192, 260–270. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2017, 11, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, D.K.; Gawande, S.J.; Soumia, P.S.; Krishna, R.; Vaishnav, A.; Ade, A.B. Biocontrol strategies: An eco-smart tool for integrated pest and diseases management. BMC Microbiol. 2022, 22, 324. [Google Scholar] [CrossRef] [PubMed]
- Essiedu, J.A.; Adepoju, F.O.; Ivantsova, M.N. Benefits and limitations in using biopesticides: A review. AIP Conf. Proc. 2020, 2313, 080002. [Google Scholar]
- Lengai, G.M.W.; Muthomi, J.W. Biopesticides and Their Role in Sustainable Agricultural Production. J. Biosci. Med. 2018, 6, 7–41. [Google Scholar] [CrossRef]
- Akutse, K.; Subramanian, S.; Maniania, N.; Dubois, T.; Ekesi, S. Biopesticide Research and Product Development in Africa for Sustainable Agriculture and Food Security—Experiences From the International Centre of Insect Physiology and Ecology [icipe]. Front. Sustain. Food Syst. 2020, 4, 563016. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The status of biological control and recommendations for improving uptake for the future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Sundh, I.; Eilenberg, J. Why has the authorization of microbial biological control agents been slower in the EU than in comparable jurisdictions? Pest Manag. Sci. 2021, 77, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef]
- Wilson, M.J.; Jackson, T.A. Progress in the commercialisation of bionematicides. BioControl 2013, 58, 715–722. [Google Scholar] [CrossRef]
| MBCAs | Target Pathogen | Host Plant | References |
|---|---|---|---|
| Bacillus licheniformisBL06 | Phytophthora capsici | Pepper | [13] |
| Bacillus thuringiensis | Sclerotinia sclerotiorum | Sclerotiniose/Brassica campestris L. | [14] |
| Bacillus subtilis26DCryChS | Stagonosporanodorum Berk. | Wheat | [15] |
| Bacillus thuringiensisGBAC46 | Aphelenchoidesbesseyi | Rice | [16] |
| Bacillus SubtilisATCC6633 | Fusarium graminearum and Fusarium verticillioides | Wheat and Maize | [17] |
| Bacillus atrophaeusGBSC56 | Meloidogyne incognita | Tomato | [18] |
| Bacillus atrophaeusTS1 | Fusarium graminearum | Wheat | [19] |
| Bacillus velezensisDMW1 | Phytophthora sojaeand Ralstonia solanacearum | Tomato and Soybean | [20] |
| Bacillus amyloliquefaciensEZ1509 | Sclerotinia sclerotiorum | Rape Seed and Tabaco | [21] |
| Bacillus velezensisBvel1 | Botrytis cinerea | Pepper and Grape | [22] |
| Pseudomonas fluorescensZX | Botrytis cinerea | Grapes | [23] |
| Pseudomonas putidaBP25 | Magnaporthe oryzae | Rice | [24] |
| Pseudomonas fluorescens | Mucor piriformis | Apple | [25] |
| Pseudomonas simiae PICF7 | Verticillium dahliae | Olive plants | [26] |
| Pseudomonas fluorescensEPS817 | Phytophthora cactorum | Strawberry plants | [27] |
| Pseudomonas parafulva JBCS1880 | Xanthomonas axonopodispv. glycines | Soybean | [28] |
| Pseudomonas rhodesiaeGC-7 | Meloidogyne graminicola | Rice | [29] |
| Pseudomonas fluorescens CHA0 | Meloidogyne javanica | Tomato and Cucumber | [30] |
| Species | Target Pathogen | Disease | Crop | Reference |
|---|---|---|---|---|
| T. harzianum, T. asperellum, T. longibrachiatum | Meloidogyne incognita | Root-knot nematode | Tomato | [31] |
| T. viride | Macrophominaphaseolina | Charcoal rot | Strawberry | [32] |
| T. harzianum, T. asperellum, T. longibrachiatum, T. viride. | Fusarium oxysporum f. sp. capsici | Fusarium wilt | Pepper | [33] |
| T. longibrachiatum, T. harzianum | Fusarium oxysporum f. sp. ciceris | Fusarium wilt | Chickpea | [34] |
| T. citrinoviride | M. incognita | Root-knot | Tomato | [35] |
| T. longibrachiatum | Pythium ultimum | Damping-off, root rot | Melon | [36] |
| T. asperellum | Sclerotium cepivorum | White rot | Onion | [37] |
| T. asperellum | F. oxysporum | Fusarium wilt | Stevia | [38] |
| T. asperellum | Sphaerothecafuliginea | Powdery Mildew | Cucumber | [39] |
| T. afroharzanium | Plasmopara viticola, Uncinula necator | Downy mildew, Powdery mildew | Grapes | [40] |
| Secondary Metabolites | Trichoderma spp. | Biological Activity | References | ||||
|---|---|---|---|---|---|---|---|
| Antifungal | Antibacterial | Anti-Nematode | Cytotoxic | Mycotoxin | |||
| Atrichodermone A, B, C | T. atroviride | + | [76] | ||||
| bisabolan-1,10,11-triol Dechlorotrichodenone-C | T. asperellum | + | [77] | ||||
| Cerebroside A, D | T. saturnisporum Trichoderma spp. | + | [78] | ||||
| cycloneodiol oxide Epicycloneodiol oxide | T. harzianum T. koningiopsis | + | [79] | ||||
| Harzianopyridone | T. harzianum | + | [80] | ||||
| Koninginin C | T. koningii | + | [81] | ||||
| 6-pentyl-2H-pyran-2-one | T. atroviride T. harzianum T. koningii T. viride | + | + | [82] | |||
| Mycotoxin T2 | T. lignorum | + | + | [83] | |||
| Koninginin E, B, A | T. harzianum T. koningii | + | [84] | ||||
| Nafuredin | T. citrinoviride Trichoderma sp. | + | [79] | ||||
| Trichodermin | T. polysporum T. sporulosum T. virens, T. reesei | + | + | [83] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, R.; Abdelmoteleb, A.; Mendez-Trujillo, V.; Gonzalez-Mendoza, D.; Hewedy, O. The Potential of Beneficial Microbes for Sustainable Alternative Approaches to Control Phytopathogenic Diseases. Microbiol. Res. 2025, 16, 105. https://doi.org/10.3390/microbiolres16050105
Bakr R, Abdelmoteleb A, Mendez-Trujillo V, Gonzalez-Mendoza D, Hewedy O. The Potential of Beneficial Microbes for Sustainable Alternative Approaches to Control Phytopathogenic Diseases. Microbiology Research. 2025; 16(5):105. https://doi.org/10.3390/microbiolres16050105
Chicago/Turabian StyleBakr, Ramadan, Ali Abdelmoteleb, Vianey Mendez-Trujillo, Daniel Gonzalez-Mendoza, and Omar Hewedy. 2025. "The Potential of Beneficial Microbes for Sustainable Alternative Approaches to Control Phytopathogenic Diseases" Microbiology Research 16, no. 5: 105. https://doi.org/10.3390/microbiolres16050105
APA StyleBakr, R., Abdelmoteleb, A., Mendez-Trujillo, V., Gonzalez-Mendoza, D., & Hewedy, O. (2025). The Potential of Beneficial Microbes for Sustainable Alternative Approaches to Control Phytopathogenic Diseases. Microbiology Research, 16(5), 105. https://doi.org/10.3390/microbiolres16050105







