Antiprotozoal Activity and Selectivity Index of Organic Salts of Albendazole and Mebendazole
Abstract
1. Introduction
2. Materials and Methods
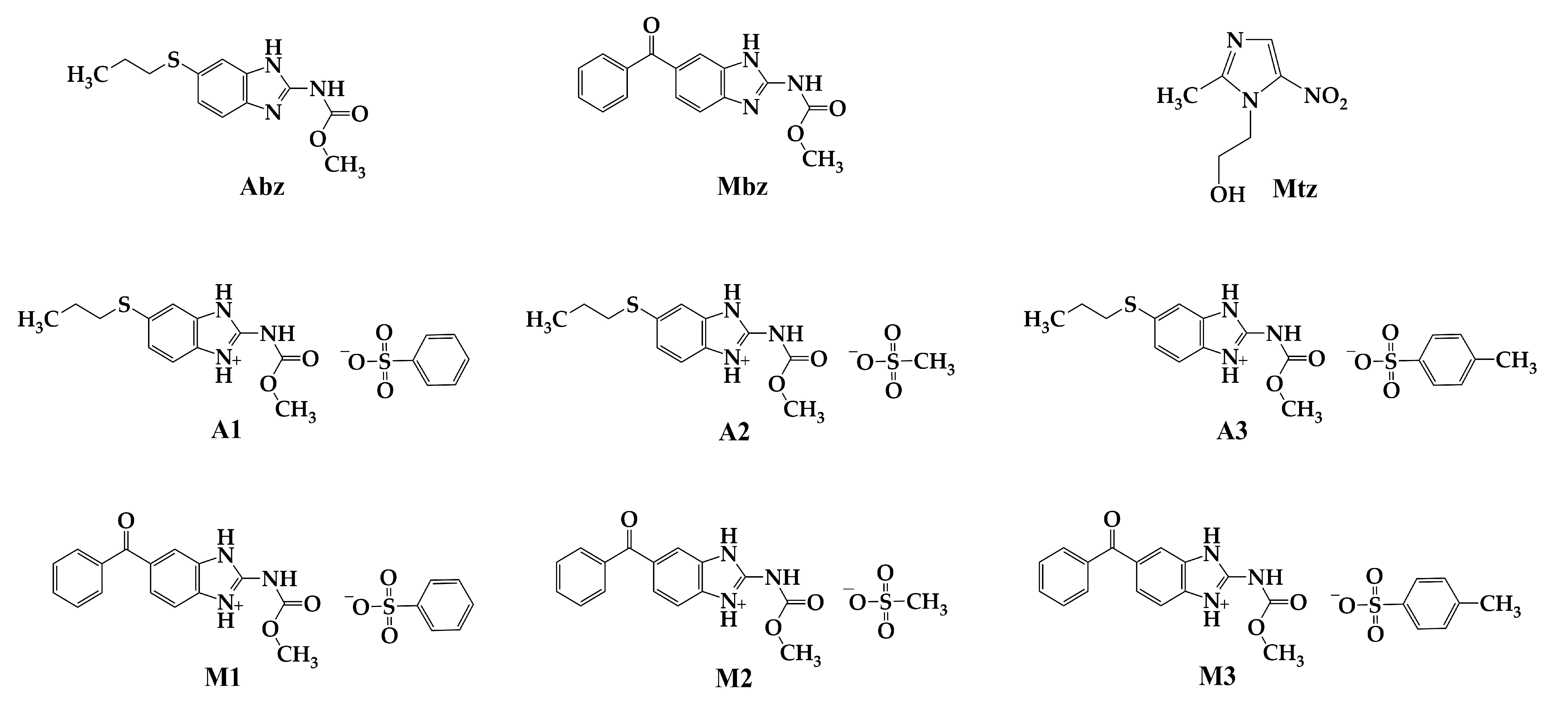
2.1. General Procedure to Prepare Abz and Mbz Sulfonic Salts
2.1.1. Reagents and Solvents
2.1.2. Equipment and Conditions for Chemical Characterization
2.1.3. General Method for Salts Preparation
2.1.4. Chemical Characterization of Salt A1 (Albendazole Besylate)
2.1.5. Chemical Characterization of Salt A2 (Albendazole Mesylate)
2.1.6. Chemical Characterization of Salt A3 (Albendazole Tosylate)
2.1.7. Chemical Characterization of Salt M1 (Mebendazole Besylate)
2.1.8. Chemical Characterization of Salt M2 (Mebendazole Mesylate)
2.1.9. Chemical Characterization of Salt M3 (Mebendazole Tosylate)
2.2. In Vitro Antiprotozoal Activity Assays
2.2.1. Stock Solutions
2.2.2. Trophozoite Culture Conditions
2.2.3. In Vitro Antiparasitic Activity Tests
2.3. In Vitro Cytotoxicity Assays
2.3.1. Cell Line Culture Conditions
2.3.2. Cytotoxicity Assays
2.3.3. Selectivity Index (SI)
2.4. Statistical Analysis
3. Results
3.1. In Vitro Antiparasitic Activity
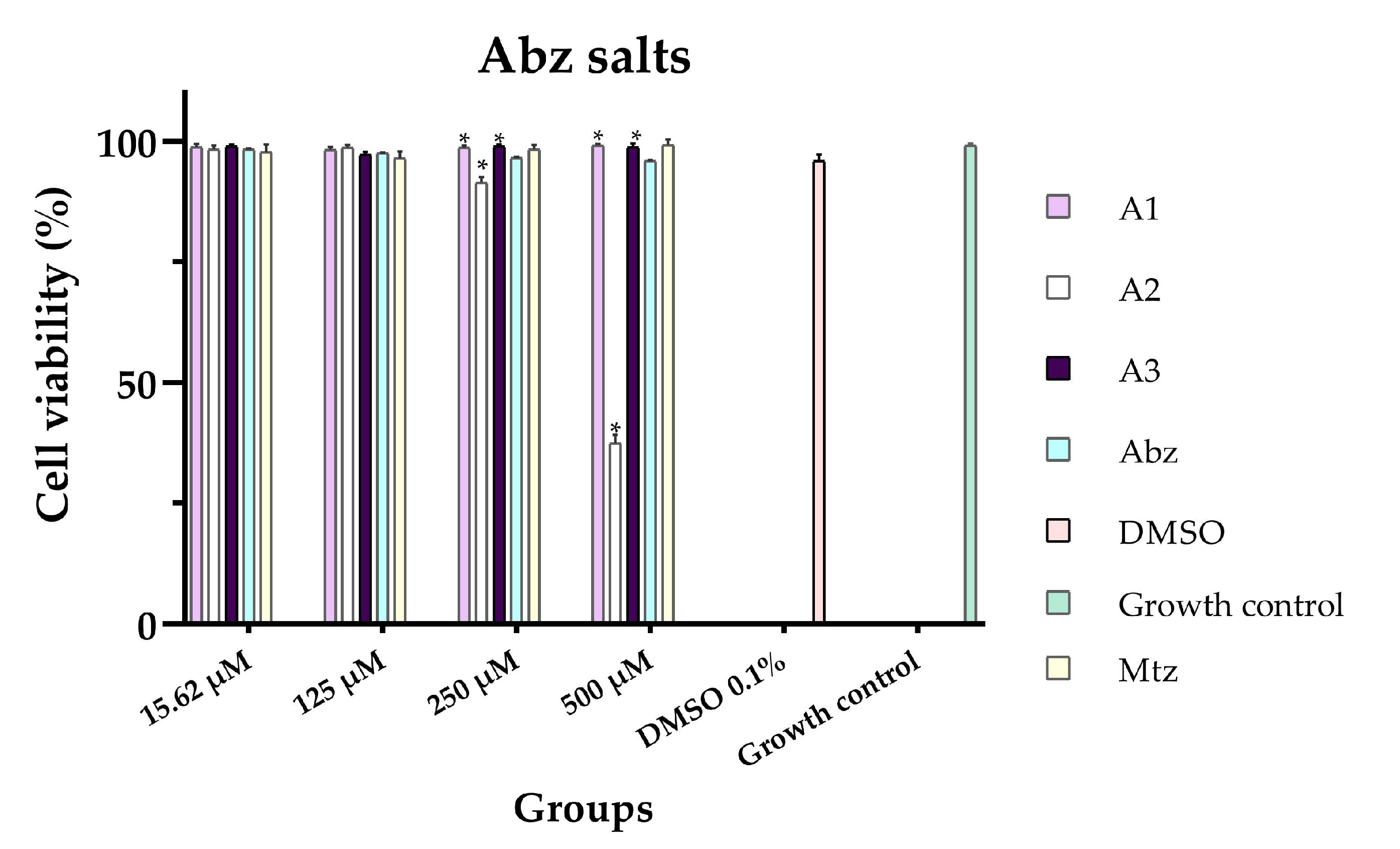
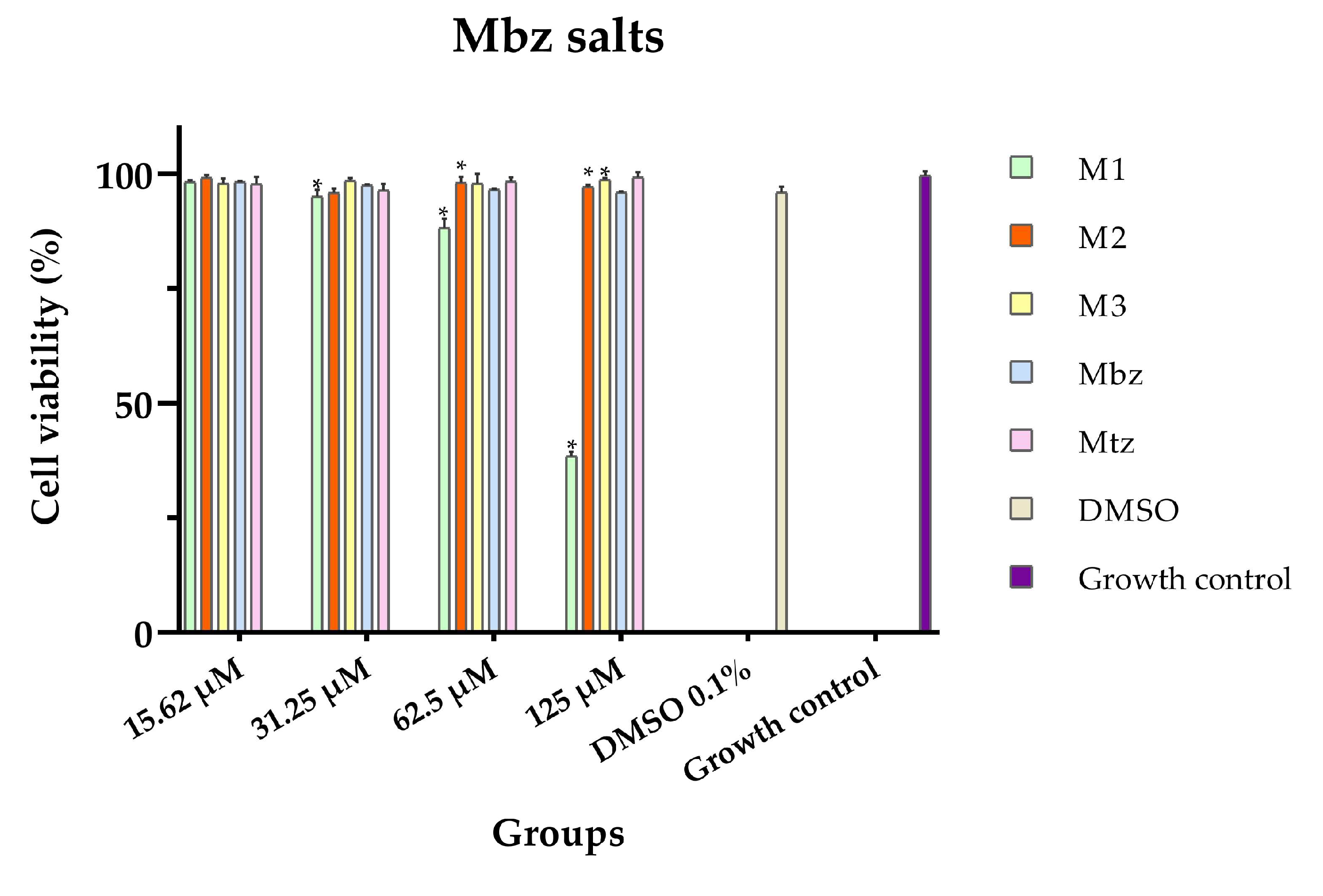
3.2. In Vitro Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABZ | Albendazole |
| API | Active pharmaceutical ingredient |
| CC50 | Cytotoxic concentration 50% |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| IC50 | Inhibitory concentration 50% |
| MBZ | Mebendazole |
| MTZ | Metronidazole |
| NMR | Nuclear Magnetic Resonance |
| OTC | Over the counter |
| PXRD | Powder X-ray diffraction |
| SI | Selectivity index |
| %CV | Cell viability percentage |
References
- Flores-Carrillo, P.; Velázquez-López, J.M.; Aguayo-Ortiz, R.; Hernández-Campos, A.; Trejo-Soto, P.J.; Yépez-Mulia, L.; Castillo, R. Synthesis, antiprotozoal activity, and chemoinformatic analysis of 2-(methylthio)-1H-benzimidazole-5-carboxamide derivatives: Identification of new selective giardicidal and trichomonicidal compounds. Eur. J. Med. Chem. 2017, 137, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; De la Garza, M. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba Histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.D. Giardia duodenalis: Biology and Pathogenesis. Clin. Microbiol. Rev. 2021, 34, 4. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Abdel-Rahman, I.A.M.; Fouad, S.S.; Allemailem, K.S.; Istivan, T.; Ahmed, S.F.M.; Hasan, A.S.; Osman, H.A.; Elshabrawy, H.A. Pomegranate Peel Extract Is a Potential Alternative Therapeutic for Giardiasis. Antibiotics 2021, 10, 705. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Augostini, P.; Asbel, L.E.; Bernstein, K.T.; Kerani, R.P.; Mettenbrink, C.J.; Pathela, P.; Schwebke, J.R.; Secor, W.E.; Workowski, K.A.; et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg. Infect. Dis. 2012, 18, 939–943. [Google Scholar] [CrossRef]
- Hashemi, N.; Ommi, D.; Kheyri, P.; Khamesipour, F.; Setzer, W.N.; Benchimol, M. A review study on the anti-trichomonas activities of medicinal plants. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 92–104. [Google Scholar] [CrossRef]
- Daneman, N.; Cheng, Y.; Gomes, T.; Guan, J.; Mamdani, M.M.; Saxena, F.E.; Juurlink, D.N. Metronidazole-associated Neurologic Events: A Nested Case-control Study. Clin. Infect. Dis. 2021, 72, 2095–2100. [Google Scholar]
- Wongstitwilairoong, B.; Anothaisintawee, T.; Ruamsap, N.; Lertsethtakarn, P.; Kietsiri, P.; Oransathid, W.; Oransathid, W.; Gonwong, S.; Silapong, S.; Suksawad, U.; et al. Prevalence of Intestinal Parasitic Infections, Genotypes, and Drug Susceptibility of Giardia lamblia among Preschool and School-Aged Children: A Cross-Sectional Study in Thailand. Trop. Med. Infect. Dis. 2023, 8, 394. [Google Scholar] [CrossRef]
- Argüello-García, R.; Leitsch, D.; Skinner-Adams, T.; Ortega-Pierres, M.G. Drug resistance in Giardia: Mechanisms and alternative treatments for Giardiasis. Adv. Parasitol. 2020, 107, 201–282. [Google Scholar] [CrossRef]
- Singh, A.; Banerjee, T.; Shukla, S.K.; Upadhyay, S.; Verma, A. Creep in nitroimidazole inhibitory concentration among the Entamoeba histolytic isolates causing amoebic liver abscess and screening of andrographolide as a repurposing drug. Sci. Rep. 2023, 13, 12192. [Google Scholar] [CrossRef]
- Mabaso, N.; Abbai, N. Distribution of genotypes in relation to metronidazole susceptibility patterns in Trichomonas vaginalis isolated from South African pregnant women. Parasitol. Res. 2021, 120, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Mørch, K.; Hanevik, K.; Robertson, L.J.; Strand, E.A.; Langeland, N. Treatment-ladder and genetic characterisation of parasites in refractory giardiasis after an outbreak in Norway. J. Infect. 2008, 56, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Anichina, K.; Mavrova, A.; Vuchev, D. Benzimidazoles Containing Piperazine Skeleton at C-2 Position as Promising Tubulin Modulators with Anthelmintic and Antineoplastic Activity. Pharmaceuticals 2023, 16, 1518. [Google Scholar] [CrossRef]
- Benchimol, M.; Gadelha, A.P.; De Souza, W. Ultrastructural Alterations of the Human Pathogen Giardia intestinalis after Drug Treatment. Pathogens 2023, 12, 810. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, M.; Yan, Y.; Chen, D.; Xie, S. Nanocrystal Suspensions for Enhancing the Oral Absorption of Albendazole. Nanomaterials 2022, 12, 3032. [Google Scholar] [CrossRef]
- Ding, Y.; Zhiyuan, Z.; Ding, C.; Shufeng, X.; Zhe, X. The Use of Cyclodextrin Inclusion Complexes to Increase the Solubility and Pharmacokinetic Profile of Albendazole. Molecules 2023, 28, 7295. [Google Scholar] [CrossRef]
- Soleymani, N.; Sadr, S.; Santucciu, C.; Rahdar, A.; Masala, G.; Borji, H. Evaluation of the In-Vitro Effects of Albendazole, Mebendazole, and Praziquantel Nanocapsules against Protoscolices of Hydatid Cyst. Pathogens 2024, 13, 790. [Google Scholar] [CrossRef]
- Suzuki, K.; Kawakami, K.; Fukiage, M.; Oikawa, M.; Nishida, Y.; Matsuda, M.; Fujita, T. Relevance of Liquid-Liquid Phase Separation of Supersaturated Solution in Oral Absorption of Albendazole from Amorphous Solid Dispersions. Pharmaceutics 2020, 13, 220. [Google Scholar] [CrossRef]
- Fateh, R.; Norouzi, R.; Mirzaei, E.; Nissapatron, V.; Nawaz, M.; Khalifeh-Gholi, M.; Hamta, A.; Adnani Sadati, S.J.; Siyadatpanah, A.; Fattahi Bafghi, A. In vitro evaluation of albendazole nanocrystals against Echinococcus granulosus protoscolices. Ann. Parasitol. 2021, 67, 203–212. [Google Scholar] [CrossRef]
- Castro Alpízar, J.A.; Vargas Monge, R.; Madrigal Redondo, G.; Pacheco Molina, J.A. Development of novel microstructured lipid carriers for dissolution rate enhancement of albendazole. Int. J. Appl. Pharm. 2020, 12, 173–178. [Google Scholar]
- Eriksen, J.B.; Christensen, S.B.; Bauer-Brandl, A.; Brandl, M. Dissolution/permeation of albendazole in the presence of cyclodextrin and bile salts: A mechanistic in-vitro study into factors governing oral bioavailability. J. Pharm. Sci. 2021, 111, 1667–1673. [Google Scholar] [PubMed]
- Pacheco, P.A.; Rodrigues, L.N.C.; Ferreira, J.F.S.; Gomes, A.C.P.; Veríssimo, C.J.; Louvandini, H.; Costa, R.L.D.; Katiki, L.M. Inclusion complex and nanoclusters of cyclodextrin to increase the solubility and efficacy of albendazole. Parasitol. Res. 2018, 117, 705–712. [Google Scholar]
- Meena, A.K.; Sharma, K.; Kandaswamy, M.; Rajagopal, S.; Mullangi, R. Formulation development of an albendazole self-emulsifying drug delivery system (SEDDS) with enhanced systemic exposure. Acta Pharm. 2012, 62, 563–580. [Google Scholar] [PubMed]
- Bolla, G.; Nangia, A. Novel pharmaceutical salts of albendazole. CrystEngComm 2018, 20, 6394–6405. [Google Scholar]
- Elder, D.P.; Delaney, E.; Teasdale, A.; Eyley, S.; Reif, V.D.; Jacq, K.; Facchine, K.L.; Oestrich, R.S.; Sandra, P.; David, F. The utility of sulfonate salts in drug development. J. Pharm. Sci. 2010, 99, 2948–2961. [Google Scholar] [CrossRef]
- Thackaberry, E.A. Non-clinical toxicological considerations for pharmaceutical salt selection. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 1419–1433. [Google Scholar] [CrossRef]
- Verbeek, R.K.; Kanfer, I.; Walker, R.B. Generic substitution: The use of medicinal products containing different salts and implications for safety and efficacy. Eur. J. Pharm. Sci. 2006, 28, 1–6. [Google Scholar]
- Mesallati, H.; Umerska, A.; Tajber, L. Fluoroquinolone Amorphous Polymeric Salts and Dispersions for Veterinary Uses. Pharmaceutics 2019, 11, 268. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin G and Amoxicillin hydrolysate Derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef]
- Duque-Montaño, B.E.; Gómez-Caro, L.C.; Sanchez-Sanchez, M.; Monge, A.; Hernández-Baltazar, E.; Rivera, G.; Torres-Angeles, O. Synthesis and in vitro evaluation of new ethyl and methyl quinoxaline-7-carboxylate 1,4-di-N-oxide against Entamoeba histolytic. Bioorganic Med. Chem. 2013, 21, 4550–4558. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ochoa, B.; Martínez-Rosas, V.; Morales-Luna, L.; Calderón-Jaimes, E.; Rocha-Ramírez, L.M.; Ortega-Cuellar, D.; Rufino-González, Y.; González-Valdez, A.; Arreguin-Espinosa, R.; Enríquez-Flores, S.; et al. Pyridyl Methylsulfinyl Benzimidazole Derivatives as Promising Agents against Giardia lamblia and Trichomonas vaginalis. Molecules 2022, 27, 8902. [Google Scholar] [CrossRef] [PubMed]
- Carapina da Silva, C.; Silveira, P.R.; Nascimento das Neves, R.; Dié Alves, M.S.; Sena-Lopes, A.; Moura, S.; Borsuk, S.; Pereira de Pereira, C.M. Antiparasitic activity of synthetic curcumin monocarbonyl analogues against Trichomonas vaginalis. Biomed. Pharmacother. 2019, 111, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, V.I.G. Actividad Anti-Giardia In Vitro de los Compuestos de Foeniculum Vulgare y Citrus Aurantifolia. Master’s Thesis, Autonomous University of Nuevo León, San Nicolás de los Garza, Mexico, 2015. [Google Scholar]
- Quispe, A.; Zavala, D.; Rojas, J.; Posso, M.; Vaisberg, A. Efecto citotóxico selectivo in vitro de Muricin H (Acetogenina de Annona muricata) en cultivos celulares de cáncer de pulmón. Rev. Peru. Med. Exp. Y Salud Pública 2006, 23, 265–269. [Google Scholar]
- Ortiz, L.J.C.; Balderrabano, L.A. Importancia de las sales orgánicas en la industria farmacéutica. Rev. Mex. Cienc. Farm. 2017, 48, 18–42. [Google Scholar]
- Silva, D.; Lopes, M.V.C.; Petrovski, Ž.; Santos, M.M.; Santos, J.P.; Yamada-Ogatta, S.F.; Bispo, M.L.F.; de Souza, M.V.N.; Duarte, A.R.C.; Lourenço, M.C.S.; et al. Novel Organic Salts Based on Mefloquine: Synthesis, Solubility, Permeability, and In Vitro Activity against Mycobacterium tuberculosis. Molecules 2022, 27, 5167. [Google Scholar] [CrossRef]
- Madeira, D.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Santos, M.M.; Branco, L.C. Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials. Proceedings 2021, 78, 3. [Google Scholar] [CrossRef]
- Jimenez, V.; Mesones, S. Down the membrane hole: Ion channels in protozoan parasites. PLoS Pathog. 2022, 18, e1011004. [Google Scholar] [CrossRef]
- Ferraz, R.; Santarém, N.; Santos, A.F.M.; Jacinto, M.L.; Cordeiro-da-Silva, A.; Prudêncio, C.; Noronha, J.P.; Branco, L.C.; Petrovski, Ž. Synthesis and Biological Evaluation of Amphotericin B Formulations Based on Organic Salts and Ionic Liquids against Leishmania infantum. Antibiotics 2022, 11, 1841. [Google Scholar] [CrossRef]
- Saal, C.; Becker, A. Pharmaceutical salts: A summary on doses of salt formers from the Orange Book. Eur. J. Pharm. Sci. 2017, 49, 614–623. [Google Scholar] [CrossRef]
- Florindo, C.; Costa, A.; Matos, C.; Nunes, S.L.; Matias, A.N.; Duarte, C.M.M.; Rebelo, L.P.N.; Branco, L.C.; Marrucho, I.M. Novel organic salts based on fluoroquinolone drugs: Synthesis, bioavailability and toxicological profiles. Int. J. Pharm. 2014, 469, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.D.R.; Phillipson, J.D.; Croft, S.L.; Solis, P.N.; Marshall, S.J.; Ghazanfar, S. Screening of plant extracts for antiprotozoal and cytotoxic activities. J. Ethnopharmacol. 2003, 89, 185–191. [Google Scholar] [CrossRef] [PubMed]
| Antiparasitic Activity | ||
|---|---|---|
| E. histolytica | G. lamblia | T. vaginalis |
| Salt/Compound | ||
| IC50 [µM] | ||
| Confidence interval | ||
| Mtz 1 | A3 b | Mtz 3 |
| 16.08 ± 0.69 | 51.31 ± 1.23 | 16.16 ± 0.66 |
| (15.40–16.77) a | (50.09–52.52) a | (15.50–16.81) a |
| A2 b | M3 c | M1 c |
| 37.95 ± 0.72 | 77.98 ± 0.70 | 24.17 ± 0.15 |
| (37.24–38.66) a | (77.28–78.67) a | (24.01–24.33) a |
| A3 b | A2 b | Mbz 2 |
| 39.93 ± 0.81 | 78.05 ± 0.43 | 36.59 ± 0.69 |
| (39.14–40.73) a | (77.62–78.05) a | (35.91– 37.28) a |
| M3 c | M2 c | M2 c |
| 44.34 ± 0.45 | 79.62 ± 0.99 | 49.86 ± 0.80 |
| (43.89–44.79) a | (78.64–80.59) a | (49.07–50.64) a |
| M2 | Mtz3 | M3 c |
| 57.72 ± 0.70 | 97.63 ± 1.43 | 62.59 ± 1.44 |
| (57.03–58.41) a | (96.22–99.04) a | (61.18–64.01) a |
| Mbz 2 | A1 b | A2 b |
| 59.81 ± 1.18 | 138.02 ± 1.41 | 125.53 ± 1.80 |
| (58.65–60.98) a | (136.64–139.41) a | (123.76–127.30) a |
| Abz 3 | Mbz 2 | Abz1 |
| 73.51 ± 1.09 | 262.74 ± 2.49 | 211.71 ± 2.18 |
| (72.44–74.59) a | (260.29–265.19) a | (209.57–213.85) a |
| M1 c | Abz3 | A1 |
| 128.05 ± 2.69 | 270.66 ± 1.89 | N. E. 4 |
| (125.41–130.69) a | (263.89–277.44) a | |
| A1 | M1 | A3 |
| N. E. 4 | N. E. 4 | N. E. 4 |
| Cytotoxic Activity | |
|---|---|
| Salt/Compound | CC50 (µM) Confidence Interval |
| A1 | >500 |
| A2 | 435.46 ± 2.17 (433.33–437.59) a |
| A3 | >500 |
| M1 | 104.04 ± 3.85 (100.26–107.82) a |
| M2 | >500 |
| M3 | >500 |
| Abz | >500 |
| Mbz | >500 |
| Mtz | >500 |
| Selectivity Index | |||
|---|---|---|---|
| Salt/Compound | E. histolytica | G. lamblia | T. vaginalis |
| A1 | N. E. | 3.62 | N. E. |
| A2 | 11.47 | 5.57 | 3.46 |
| A3 | 12.52 | 9.74 | N. E. |
| M1 | 0.81 | N. E. | 4.30 |
| M2 | 8.66 | 6.27 | 10.02 |
| M3 | 11.27 | 6.41 | 7.98 |
| Abz | 6.80 | 1.84 | 2.36 |
| Mbz | 8.35 | 1.90 | 13.66 |
| Mtz | 31.09 | 5.12 | 30.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barón-Pichardo, M.G.; Gómez-García, J.; Durán-Martínez, D.; Torres-Angeles, O.; Rivera-Islas, J.; Duque-Montaño, B.E. Antiprotozoal Activity and Selectivity Index of Organic Salts of Albendazole and Mebendazole. Microbiol. Res. 2025, 16, 77. https://doi.org/10.3390/microbiolres16040077
Barón-Pichardo MG, Gómez-García J, Durán-Martínez D, Torres-Angeles O, Rivera-Islas J, Duque-Montaño BE. Antiprotozoal Activity and Selectivity Index of Organic Salts of Albendazole and Mebendazole. Microbiology Research. 2025; 16(4):77. https://doi.org/10.3390/microbiolres16040077
Chicago/Turabian StyleBarón-Pichardo, Miriam Guadalupe, Janeth Gómez-García, David Durán-Martínez, Oscar Torres-Angeles, Jesús Rivera-Islas, and Blanca Estela Duque-Montaño. 2025. "Antiprotozoal Activity and Selectivity Index of Organic Salts of Albendazole and Mebendazole" Microbiology Research 16, no. 4: 77. https://doi.org/10.3390/microbiolres16040077
APA StyleBarón-Pichardo, M. G., Gómez-García, J., Durán-Martínez, D., Torres-Angeles, O., Rivera-Islas, J., & Duque-Montaño, B. E. (2025). Antiprotozoal Activity and Selectivity Index of Organic Salts of Albendazole and Mebendazole. Microbiology Research, 16(4), 77. https://doi.org/10.3390/microbiolres16040077






