The Fungus Phlebiopsis flavidoalba’s Pathogenicity and Virulence Toward the Fluted Scale (Praelongorthezia acapulcoa) Pest of Rice and Sugarcane Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
2.2. Morphological Identification
2.3. DNA Extraction
2.4. Molecular Identification
2.5. Pathogenicity and Virulence
2.6. Statistical Analysis
3. Results
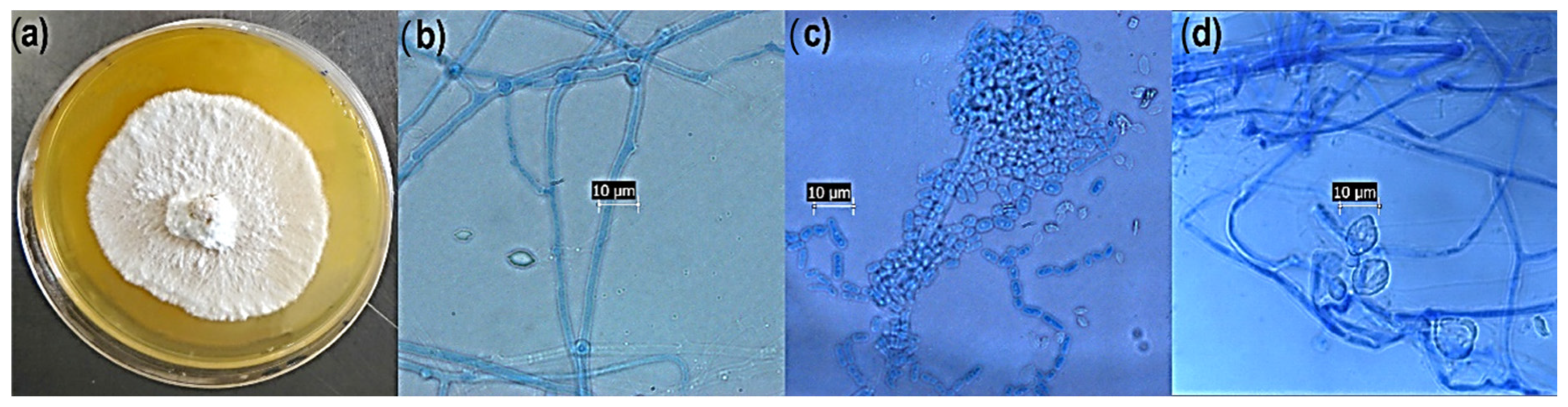
3.1. Morphological Identification
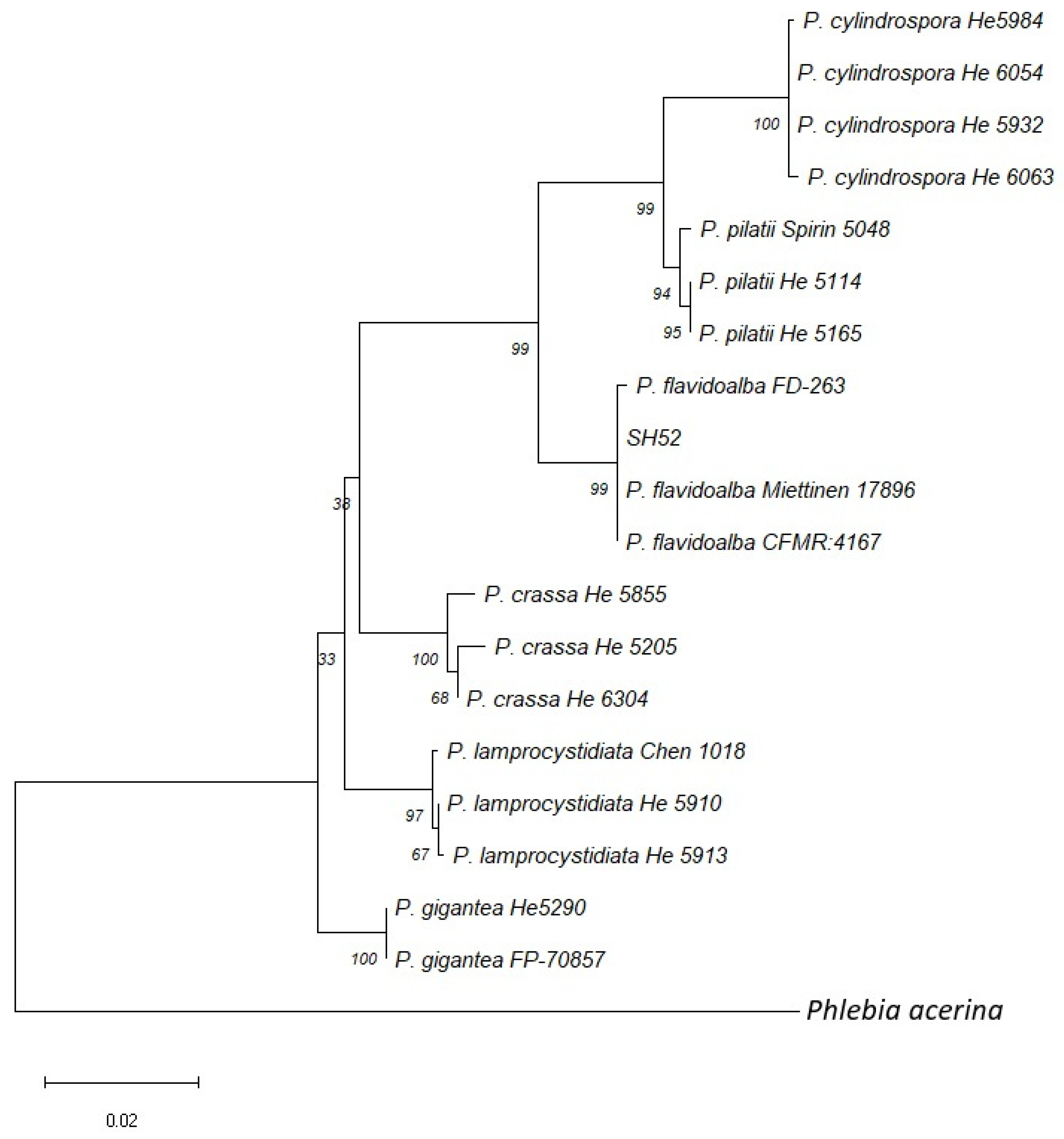
3.2. Molecular Identification and Phylogenetic Analysis
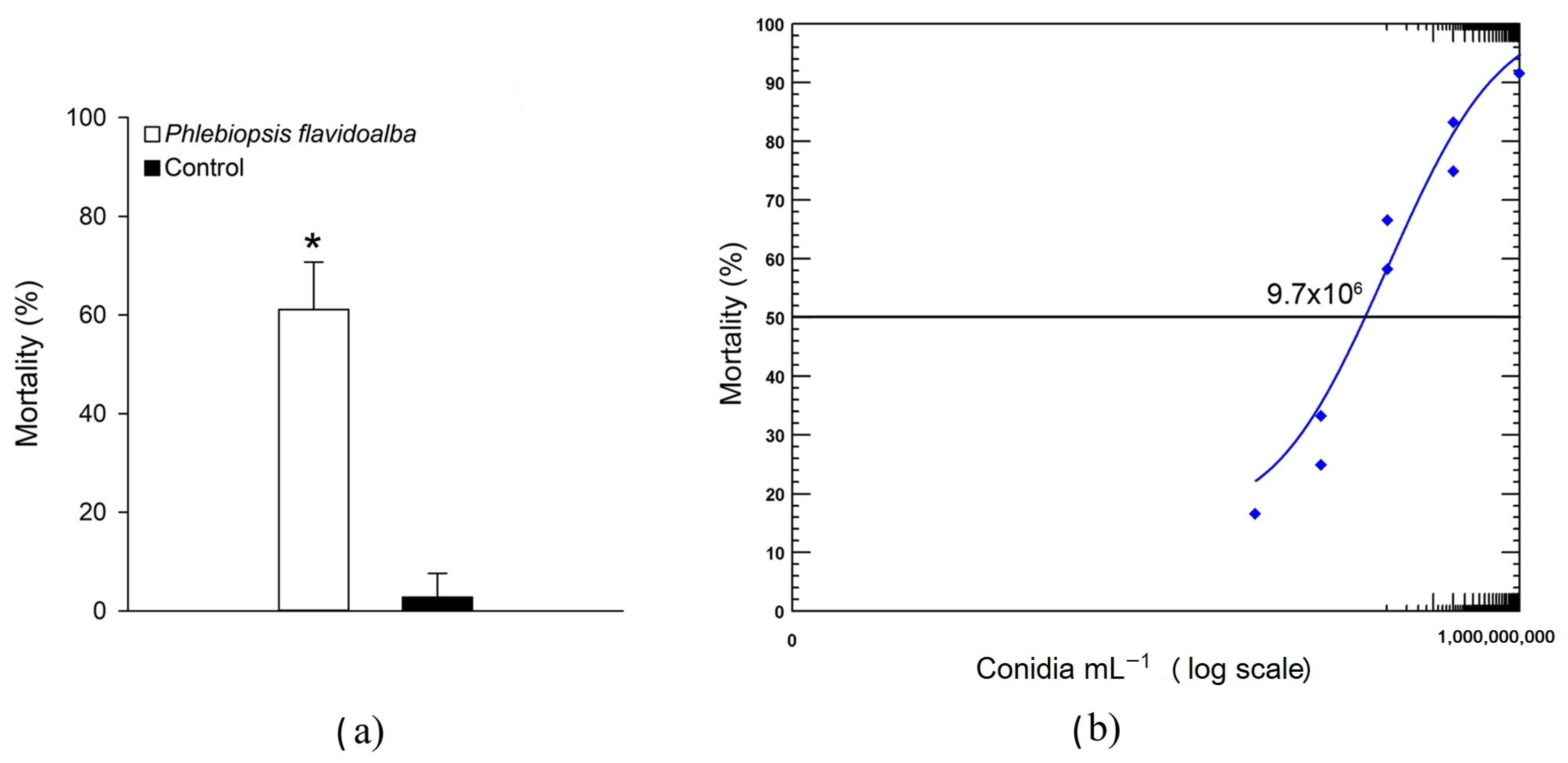
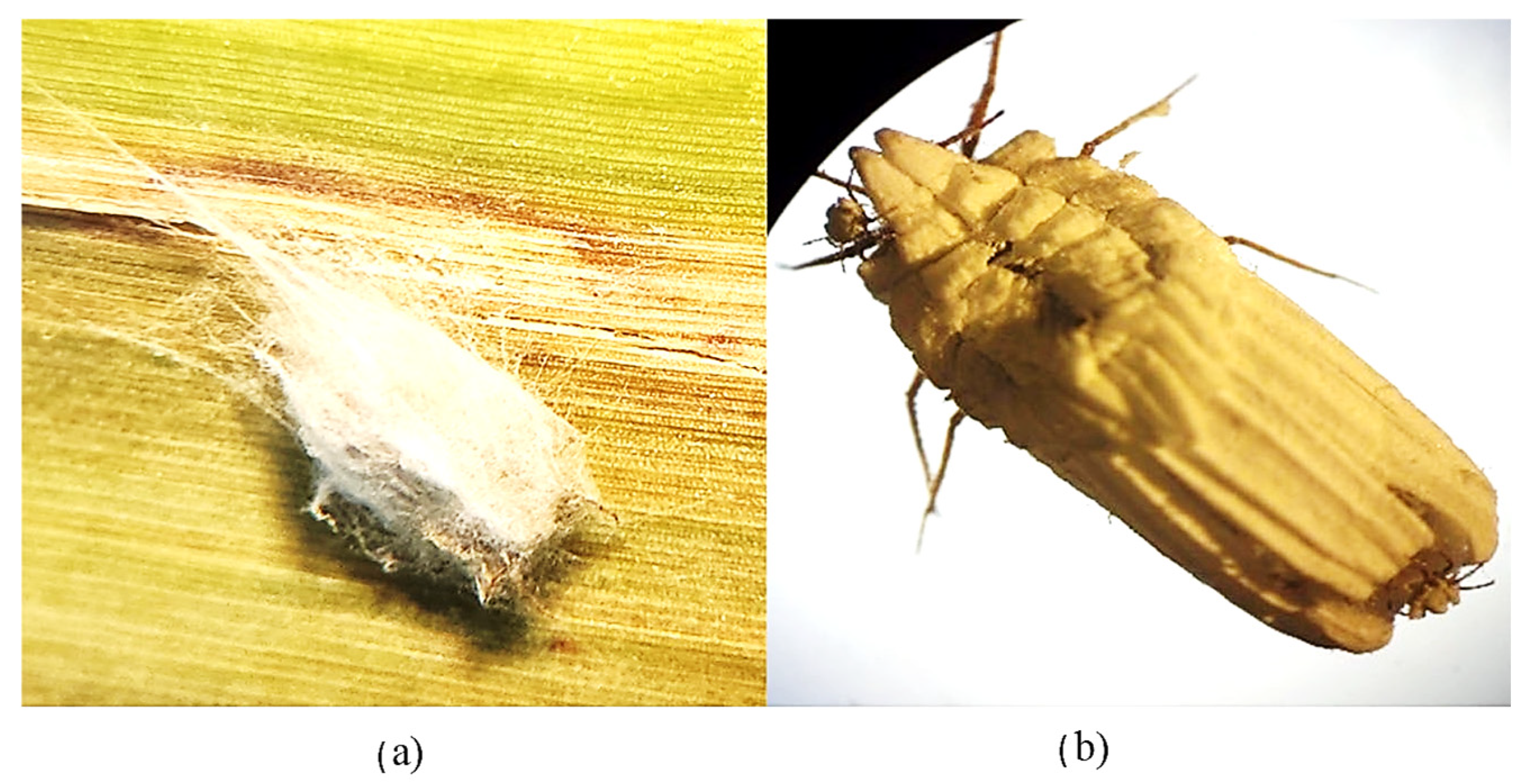
3.3. Pathogenicity and Virulence of Phlebiopsis flavidoalba SH52 Toward Praelongorthezia acapulcoa
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, R. Sugarcane: Production Systems, Uses and Economic Importance; Nova Publishers: Hauppauge, NY, USA, 2017; ISBN 9781536108989.2017. [Google Scholar]
- Carapia Ruiz, V.E. Pralengorthezia Acapulcoa (MORRISON) (HEMIPTERA: ORTHEZIIDAE) UNA NUEVA PLAGA PARA LA CAÑA DE AZUCAR. Investig. Agropecu. 2009, 6, 163–172. [Google Scholar]
- Colombia Ministerio de Agricultura y Desarrollo Rural; Corpoica; Universidad de Antioquia. Cítricos Cultivo, Poscosecha e Industrialización; Corporación Universitaria Lasallista: Caldas, Colombia, 2012; ISBN 9789588406176.
- Chomnunti, P.; Hongsanan, S.; Aguirre-Hudson, B.; Tian, Q.; Peršoh, D.; Dhami, M.K.; Alias, A.S.; Xu, J.; Liu, X.; Stadler, M.; et al. The Sooty Moulds. Fungal Divers. 2014, 66, 1–36. [Google Scholar] [CrossRef]
- Konczné Benedicty, Z.; Kozár, F. Revision of Newsteadia (Homoptera: Coccoidea) of the Nearctic and Neotropic regions, with descriptions of new species. Acta Phytopathol. Entomol. Hung. 2001, 36, 123–142. [Google Scholar] [CrossRef]
- Afifah, L.; Aena, A.C.; Saputro, N.W.; Kurniati, A.; Maryana, R.; Lestari, A.; Abadi, S.; Enri, U. Maize Media Enhance the Conidia Production of Entomopathogenic Fungi Lecanicillium Lecanii Also Its Effective to Control the Weevil Cylas Formicarius (Fabricius) (Coleoptera: Brentidae). Agrivita 2022, 44, 513–525. [Google Scholar] [CrossRef]
- Serna-Domínguez, M.G.; Andrade-Michel, G.Y.; Rosas-Valdez, R.; Castro-Félix, P.; Arredondo-Bernal, H.C.; Gallou, A. High Genetic Diversity of the Entomopathogenic Fungus Beauveria bassiana in Colima, Mexico. J. Invertebr. Pathol. 2019, 163, 67–74. [Google Scholar] [CrossRef]
- Kidanu, S.; Hagos, L. Entomopathogenic Fungi as a Biological Pest Management Option: A Review. Int. J. Res. Stud. Agric. Sci. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Biji, C.P.; Sudheendrakumar, V.V.; Shabna, S. Fungal Endophytes, Phlebiopsis gigantea (Fr.) Jillich and Phanerochaete sordida (P. Karst.) J. Erikss and Ryvarden: New Aspirants in Biopesticide Scenario. J. Biol. Control 2011, 25, 114–117. [Google Scholar] [CrossRef]
- Gökçe, A.; Er, M.K. First Description of the Disease by Conidiobolus Osmodes on Tipula Paludosa Larvae with the Report of a Natural Epizootic. J. Invertebr. Pathol. 2003, 84, 83–89. [Google Scholar] [CrossRef]
- Zemek, R.; Konopická, J.; Jozová, E.; Skoková Habuštová, O. Virulence of Beauveria bassiana Strains Isolated from Cadavers of Colorado Potato Beetle, Leptinotarsa decemlineata. Insects 2021, 12, 1077. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological Factors in the Inundative Use of Fungal Entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Milijaš Jotić, M.; Panevska, A.; Iacovache, I.; Kostanjšek, R.; Mravinec, M.; Skočaj, M.; Zuber, B.; Pavšič, A.; Razinger, J.; Modic, Š.; et al. Dissecting Out the Molecular Mechanism of Insecticidal Activity of Ostreolysin A6/Pleurotolysin B Complexes on Western Corn Rootworm. Toxins 2021, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal Identification Using Molecular Tools: A Primer for the Natural Products Research Community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; He, S.H.; Nakasone, K.K.; Wasantha Kumara, K.L.; Chen, C.C.; Liu, S.L.; Ma, H.X.; Huang, M.R. Global Phylogeny and Taxonomy of the Wood-Decaying Fungal Genus Phlebiopsis (Polyporales, Basidiomycota). Front. Microbiol. 2021, 12, 622460. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.Y.; Wu, S.H. Species Diversity, Taxonomy and Multi-Gene Phylogeny of Phlebioid Clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Divers. 2021, 111, 337–442. [Google Scholar] [CrossRef]
- Domínguez-Galdámez, O.M.; Oliva-Llaven, M.Á.; Aguilar-Tipacamú, G.; Mendoza-Nazar, P.; Ruiz-Sesma, B.; Bautista-Trujillo, G.U.; Antonio, F. Evaluation of Beauveria sp. Strains, Conidial Concentration and Immersion Times on Mortality Rate of Bovine Tick (Boophilus sp). J. Appl. Biol. Biotechnol. 2016, 4, 64–68. [Google Scholar] [CrossRef]
- Demirci, S.N.Ş.; Altuntaş, H. Entomopathogenic Potential of Purpureocillium lilacinum against the Model Insect Galleria mellonella (Lepidoptera: Pyralidae). Environ. Exp. Biol. 2019, 17, 71–74. [Google Scholar] [CrossRef]
- Lei, C.; Sun, X. Comparing Lethal Dose Ratios Using Probit Regression with Arbitrary Slopes. BMC Pharmacol. Toxicol. 2018, 19, 61. [Google Scholar] [CrossRef]
- Carapia-Ruiz, V.E.; Romero-Hernández, J.P.; Hernández-Velázquez, V.M. Primer Reporte de la Escama Bandera Pralengorthezia acapulcoa1 en Arroz, en Morelos, México. Southwest. Entomol. 2019, 44, 349–351. [Google Scholar] [CrossRef]
- Wang, J.; Lovett, B.; St Leger, R.J. The Secretome and Chemistry of Metarhizium; a Genus of Entomopathogenic Fungi. Fungal Ecol. 2018, 38, 7–11. [Google Scholar] [CrossRef]
- Sánchez-Corzo, L.D.; Álvarez-Gutiérrez, P.E.; Meza-Gordillo, R.; Villalobos-Maldonado, J.J.; Enciso-Pinto, S.; Enciso-Sáenz, S. Lignocellulolytic Enzyme Production from Wood Rot Fungi Collected in Chiapas, Mexico, and Their Growth on Lignocellulosic Material. J. Fungi 2021, 7, 450. [Google Scholar] [CrossRef]
- Araújo, J.P.M.; Hughes, D.P. Diversity of Entomopathogens Fungi: Which Groups Conquered the Insect Body? Adv. Genet. 2016, 94, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bauer, L.S. Susceptibility of Agrilus planipennis (Coleoptera: Buprestidae) to Beauveria bassiana and Metarhizium anisopliae. J. Econ. Entomol. 2006, 99, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, G.; Téllez-Téllez, M.; Rodríguez, A.; Obregón-Barbosa, V.; De Lourdes Acosta-Urdapilleta, M.; Villegas, E. Enzymatic, Antioxidant, Antimicrobial, and Insecticidal Activities of Pleurotus pulmonarius and Pycnoporus cinnabarinus Grown Separately in an Airlift Reactor. Bioresources 2016, 11, 4186–4200. [Google Scholar] [CrossRef]
- Asma Noshad, A.N.; Mudassar Iqbal, M.I.; Zafar Iqbal, Z.I.; Hamida Bibi, H.B.; Saifullah, S.; Salma Bibi, S.B.; Shah, H.U. Aphidicidal potential of ethyl acetate extract from Pleurotus ostreatus. Sarhad J. Agric. 2015, 31, 101–110. [Google Scholar] [CrossRef]
- Selvaraj, K.; Kaushik, H.D. Greenhouse Evaluation of Beauveria bassiana (Balsamo) Vuillemin against Aphis craccivora (Das) on Fenugreek. J. Appl. Nat. Sci. 2014, 6, 852–856. [Google Scholar] [CrossRef]
- Zhang, X.C.; Li, X.X.; Gong, Y.W.; Li, Y.R.; Zhang, K.L.; Huang, Y.H.; Zhang, F. Isolation, Identification, and Virulence of a New Metarhizium anisopliae Strain on the German Cockroach. J. Econ. Entomol. 2018, 111, 2611–2616. [Google Scholar] [CrossRef]
| Region | Primer Name | Nucleotide Sequence (5′ → 3′) | Reference |
|---|---|---|---|
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | [15] |
| ITS4 | TCCTCCGCTTATTGATATGC | ||
| Tef 1 | 983F | GCYCCYGGHCAYCGTGAYTTYAT | [16] |
| 2218R | ATGACACCRACRGCRACRGTYTG | ||
| 28S | LROR | GTACCCGCTGAACTTAAGC | [15] |
| LR5 | CCTGAGGGAAACTTCG |
| Species | ITS | 28S |
|---|---|---|
| Phlebiopsis flavidoalba GC 1807-47 | MZ637050 | MZ637254 |
| P. flavidoalba FD-263 | KP135402 | KP135271 |
| P. flavidoalba Miettinen 17896 | KP135397 | KX752607 |
| P. flavidoalba CFMR: 4167 | KX065957 | KX065991 |
| Phlebiopsis gigantea He 5290 | MT386381 | MT447416 |
| P. gigantea FP-70857 | KP135390 | KP135272 |
| Phlebiopsis cylindrospora He 5932 | MT386404 | MT447445 |
| P. cylindrospora He 5984 | MT386404 | MT447445 |
| P. cylindrospora He 6054 | MT561716 | MT598030 |
| P. cylindrospora He 6063 | MT561717 | MT598031 |
| Phlebiopsis crassa He 5205 | MT452523 | MT447448 |
| P. crassa He 5205 | MT452523 | MT447448 |
| P. crassa He 5855 | MT452525 | MT447450 |
| P. crassa He 6304 | MT561714 | MT598029 |
| Phlebiopsis lamprocystidiata He 5910 | MT386383 | MT447419 |
| P. lamprocystidiata He 5913 | MT386384 | MT447420 |
| P. lamprocystidiata Chen 1018 | MT561709 | GQ470647 |
| Phlebiopsis pilatii He 5114 | MT386385 | MT447421 |
| P. pilatii He 5165 | MT386386 | MT447422 |
| P. pilatii Spirin 5048 | KX752590 | KX752590 |
| Phlebia acerina MY51 | MK592753 | PP478794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Hernández, S.; Muñiz-Paredes, F.; Peña-Chora, G.; Hernández-Velázquez, V.M. The Fungus Phlebiopsis flavidoalba’s Pathogenicity and Virulence Toward the Fluted Scale (Praelongorthezia acapulcoa) Pest of Rice and Sugarcane Crops. Microbiol. Res. 2024, 15, 2414-2424. https://doi.org/10.3390/microbiolres15040162
Hernández-Hernández S, Muñiz-Paredes F, Peña-Chora G, Hernández-Velázquez VM. The Fungus Phlebiopsis flavidoalba’s Pathogenicity and Virulence Toward the Fluted Scale (Praelongorthezia acapulcoa) Pest of Rice and Sugarcane Crops. Microbiology Research. 2024; 15(4):2414-2424. https://doi.org/10.3390/microbiolres15040162
Chicago/Turabian StyleHernández-Hernández, Silvia, Facundo Muñiz-Paredes, Guadalupe Peña-Chora, and Víctor Manuel Hernández-Velázquez. 2024. "The Fungus Phlebiopsis flavidoalba’s Pathogenicity and Virulence Toward the Fluted Scale (Praelongorthezia acapulcoa) Pest of Rice and Sugarcane Crops" Microbiology Research 15, no. 4: 2414-2424. https://doi.org/10.3390/microbiolres15040162
APA StyleHernández-Hernández, S., Muñiz-Paredes, F., Peña-Chora, G., & Hernández-Velázquez, V. M. (2024). The Fungus Phlebiopsis flavidoalba’s Pathogenicity and Virulence Toward the Fluted Scale (Praelongorthezia acapulcoa) Pest of Rice and Sugarcane Crops. Microbiology Research, 15(4), 2414-2424. https://doi.org/10.3390/microbiolres15040162







